
Dynamic Detectors of Oriented Spatial Contrast
from Isotropic Fixational Eye Movements
Simone Testa
1
, Giacomo Indiveri
2 a
and Silvio P. Sabatini
1 b
1
Department of Informatics, Bioengineering, Robotics and Systems Engineering, University of Genoa, Genoa, Italy
2
Institute of Neuroinformatics, University of Z
¨
urich and ETH Z
¨
urich, Z
¨
urich, Switzerland
Keywords:
Active Vision, Fixational Eye Movements, Event-based Sensor, Neuromorphic Computing, Receptive Fields,
Spiking Neural Networks.
Abstract:
Good vision proficiency and a complex set of eye movements are frequently coexisting. Even during fixa-
tion, our eyes keep moving in microscopic and erratic fashion, thus avoiding stationary scenes from fading
perceptually by preventing retinal adaptation. We artificially replicate the functionalities of biological vision
by exploiting this active strategy with an event-based camera. The resulting neuromorphic active system re-
distributes the low temporal frequency power of a static image into a range the sensor can detect and encode
in the timing of events. A spectral analysis of its output attested both whitening and amplification effects
already postulated in biology depending on whether or not the stimulus’ contrast matched the 1/k falloff typ-
ical of natural images. Further evaluations revealed that the isotropic statistics of fixational eye movements is
crucial for equalizing the response of the system to all possible stimulus orientations. Finally, the design of a
biologically-realistic spiking neural network allowed the detection of stimulus’ local orientation by anisotropic
spatial summation of synchronous activity with both ON/OFF polarities.
1 INTRODUCTION
Visual perception is a fundamentally active process.
Humans and many other mammals are endowed with
a specific and complex set of eye movements through
which they incessantly scan the environment (Land,
2019). This active method has long been proven to
overcome loss of vision during fixation of static ob-
jects (Ditchburn and Ginsborg, 1952) thanks to a pe-
culiar ensemble of oculomotor mechanisms known
as Fixational Eye Movements (or FEMs) (Martinez-
Conde and Macknik, 2008). In particular, while the
desensitization properties of retinal ganglion cells to
unchanging stimuli would lead to perceptual fading of
stationary objects during retinal stabilization, FEMs
enable refreshing neural responses by inducing tem-
poral transients (Riggs and Ratliff, 1952). The fact vi-
sual systems so strongly depend on temporal changes
suggests that the still-camera model of the eye and
the spatial coding idea is at least lacking. Actually, in
order to extract and code spatial information, a com-
bination of spatial sampling and temporal processing
a
https://orcid.org/0000-0002-7109-1689
b
https://orcid.org/0000-0002-0557-7306
is required (Rucci et al., 2018). The performance gap
between artificial and biological visual systems could
therefore depend on substantial differences about how
information is acquired and encoded. Biological evi-
dences (Gollisch and Meister, 2008) indicate that reti-
nal ganglion cell outputs are massively parallel, data-
driven (asynchronous) and with high temporal resolu-
tion. Here, a temporal encoding scheme is adopted,
where information is carried in the timing of activa-
tion, as opposed to pure spatial encoding schemes,
which are solely based on the identity of activated re-
ceptors.
Similar to a biological retina, a neuromorphic
camera, such as the Dynamic Vision Sensor (DVS),
only responds to temporal transients in the visual
scene, by converting them into a stream of asyn-
chronous events uniquely based on time-variations of
luminance contrast (Lichtsteiner et al., 2008). In ad-
dition to the position and the timing of brightness
change, each event brings information about its po-
larity, i.e. ON or OFF events, that represent dark-to-
light or light-to-dark transitions, respectively. By in-
terfacing these sensors to mixed signal analog-digital
neuromorphic electronic processors, such as the DY-
namic Neuromorphic Asynchronous Processor (DY-
674
Testa, S., Indiveri, G. and Sabatini, S.
Dynamic Detectors of Oriented Spatial Contrast from Isotropic Fixational Eye Movements.
DOI: 10.5220/0009170606740681
In Proceedings of the 15th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2020) - Volume 5: VISAPP, pages
674-681
ISBN: 978-989-758-402-2; ISSN: 2184-4321
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

NAP) (Moradi et al., 2017), real-time and energy-
efficient vision processing systems can be imple-
mented. However, despite the outstanding capabili-
ties of neuromorphic sensors with respect to classical
frame-based cameras, their intrinsic blindness to sta-
tionary images limits their application in several real-
world scenarios, as a continuous relative motion be-
tween the scene and the sensor is required. Hence, a
commonly adopted approach is to use moving stimuli
displayed on a monitor, resulting however in record-
ing artifacts due to video refreshing (Orchard et al.,
2015). Yet, by emulating the FEMs of biological vi-
sion systems, we will be able to extract information
from otherwise undetectable static stimuli and simul-
taneously avoid such artifacts.
In this paper, non-saccadic FEMs were modeled
by a Brownian motion and physically induced on the
sensor by using a pan-tilt unit (PTU). The resulting
active approach proved to be effective for making
event-based sensors responsive to static scenes, thus
closely matching a strategy that biology has evolved
to perform for similar tasks. The spatial frequency
characterization of our sensing system confirmed be-
haviors postulated in biological studies (Rucci and
Victor, 2015). Further analysis revealed that the spa-
tial statistical distribution of micro arbitrary move-
ments (particularly their isotropy) equalizes sensor
activity with respect to the orientation of visual stim-
uli, preserving the efficacy of subsequent feature ex-
traction stages. Specifically, a Spiking Neural Net-
work (SNN) was designed to properly discriminate
stimulus orientation at a local scale. The detection
mechanism exploits highly synchronized events of
both polarities, emitted by jittery pixels aligned with
oriented edges, collected by means of a push-pull con-
figuration of anisotropic spatial kernels, that resemble
biological receptive fields of simple cells in the pri-
mary visual cortex.
The rest of the paper is organized as follows. Sec-
tion 2 introduces both the set-up considered for FEM
reproduction on the DVS and the experiments con-
ducted, providing a description on how information
is encoded by the system. Section 3 details the spec-
tral analysis of the overall behavior of the active sens-
ing system and the influence of isotropic random-like
motion on the response to differently oriented stimuli.
Section 4 presents the SNN for the detection of local
orientation and, finally, in Section 5 we draw the con-
clusions.
2 MATERIALS AND METHODS
2.1 Active Vision System and Stimuli
Inducing bio-inspired fixational eye movements on
a sensor requires, first of all, a mathematical model
of such motion based on its characteristics in natural
viewing. To this aim, we used a Brownian motion
model to approximate some peculiar components of
FEMs (Kuang et al., 2012). Four different random
seeds of Brownian motions (with number of steps
varying between 30 to 50) have therefore been gener-
ated through Python simulations. The resulting paths
should keep unchanged the erratic aspect, mean fre-
quency and size of biological FEMs, adapting the lat-
ter to the characteristics of neuromorphic sensors.
The overall experimental set-up is shown in
Fig. 1a. It is mainly composed of a neuromorphic
sensing hardware and a remotely controlled motor-
ized unit for the generation of precise pan and tilt ro-
tations of the camera. Specifically, we used a neu-
romorphic sensor DVS128 (having a 128 × 128 pixel
array) with a TV-lens C-mount F1.4-16 (6 mm focal
length) and a PTU-D46-17 from Directed Perception
Inc. (chosen for its resolution, as low as 3.1 arcmin).
In order to obtain reproducible results, all acquisitions
were conducted in controlled lighting conditions, by
executing them in a specifically-dedicated dimly lit
room. Stimuli were generated using PsychoPy and
displayed on a 19
0
LCD Philips monitor with a reso-
lution of 1440 × 900 pixels, and refresh rate of 60 Hz.
The distance between sensor and screen was kept at
50 cm for all the experiments. The event-based data,
provided by the sensor in the Address-Event Repre-
sentation (AER) protocol, have been visualized on a
computer’s screen and saved to disk for off-line pro-
cessing.
The same custom Python script simulating the
Brownian motion model converts the whole FEM-
like path in a specific set of commands encoded in
ASCII format. These commands were sent to the
servo-motors of the PTU by means of a terminal em-
ulator, which serves as interface for the controller, in
order to drive the position of the PTU over time, ac-
cording to the simulated path. As the field of view
(FOV) of a single DVS pixel (∼ 22.9 arcmin, leading
to a maximum discernible spatial frequency of ∼ 1.3
cyc/deg) strongly differs from that of human photo-
receptors (∼ 0.6 arcmin in the fovea), typical sizes of
natural FEMs have been scaled up accordingly. Thus,
the maximum step amplitude for artificial FEM in-
duced on the DVS is ∼ 190 arcmin (corresponding to
5 arcmin of biological motion), while the minimum is
∼ 3.1 arcmin (corresponding to 5 arcsec for biologi-
Dynamic Detectors of Oriented Spatial Contrast from Isotropic Fixational Eye Movements
675

Events
from the DVS
Driving commands
to the PTU
(a)
t=t
1
t=t
2
t=t
3
t
0
t
1
OFF
t
2
t
3
t
1
t
2
ON
t
0
t
1
t
1
t
2
t
2
t
3
t
0
t
3
Resulting events
from selected pixel
t=t
0
(b)
Figure 1: Set-up and consequence of fixational movements. (a) The experimental set-up: the DVS-PTU system is placed in
front of a monitor for active scanning of artificial visual stimuli. Driving commands (in ASCII code) are sent from a host
computer’s serial port to the PTU controller for generation of pan and tilt rotations. Conversely, data coming from the sensor,
in AER protocol, are sent to the computer via USB interface. (b) Example of a natural visual scene and relative ON/OFF events
generated by a single pixel of the DVS during fixational eye movements (green path). The inset shows results of the isotropic
analysis of the movement (green polar histogram) with respect to a perfectly isotropic motion (black circle, representing the
reference probability value of ∼ 8.3%, as 12 directions are considered). Red boxes represent the image portions falling in the
FOV of the pixel at three different times.
cal motion). Lastly, the speed of both rotations was
also controlled, in order to keep the whole motion at
a mean temporal frequency between 40 and 50 Hz.
By varying the seed of the random process, dif-
ferent FEMs have been induced on the sensor while
it is exposed to various stimuli, and the relative data
streams have been recorded. Visual experiments have
been conducted with artificial grating stimuli with
varying contrast, spatial frequency and orientation. A
first set of stimuli was composed of 180 gratings with:
unitary contrast, spatial frequency k evenly spaced be-
tween 0.2 and 1.6 cyc/deg (15 discrete values), and
orientation θ evenly spaced between 0 and 165 deg
(12 discrete values, with a 15 deg step). An additional
set of 180 gratings was considered, having same val-
ues of k and θ but an adjusted contrast for every tested
spatial frequency, according to the 1/k falloff of nat-
ural image spectrum amplitude (Field, 1987).
2.2 Spatiotemporal Coding
Figure 1b shows an example of how static spatial in-
formation is temporally encoded in the activity of the
neuromorphic sensor as a result of the induced fix-
ational movements. As a matter of fact, simulated
FEMs ensure that the spatial structure of a static im-
age is encoded both in space and time, as both the po-
sition and the timing of an activated pixel is informa-
tive. Specifically, camera movements transform a sta-
tionary spatial scene into a spatiotemporal luminance
flow, thus redistributing its power in the (non-zero)
temporal frequency domain which is capable of acti-
vating sensors pixels. Spatial luminance discontinu-
ities are now converted into synchronous firing activ-
ity due to the combined effect of microscopic camera
movements and the event-based (i.e., non-redundant)
acquisition process in DVS pixels (see also Section 4).
It is worth noting that a similar role of natural FEMs
VISAPP 2020 - 15th International Conference on Computer Vision Theory and Applications
676
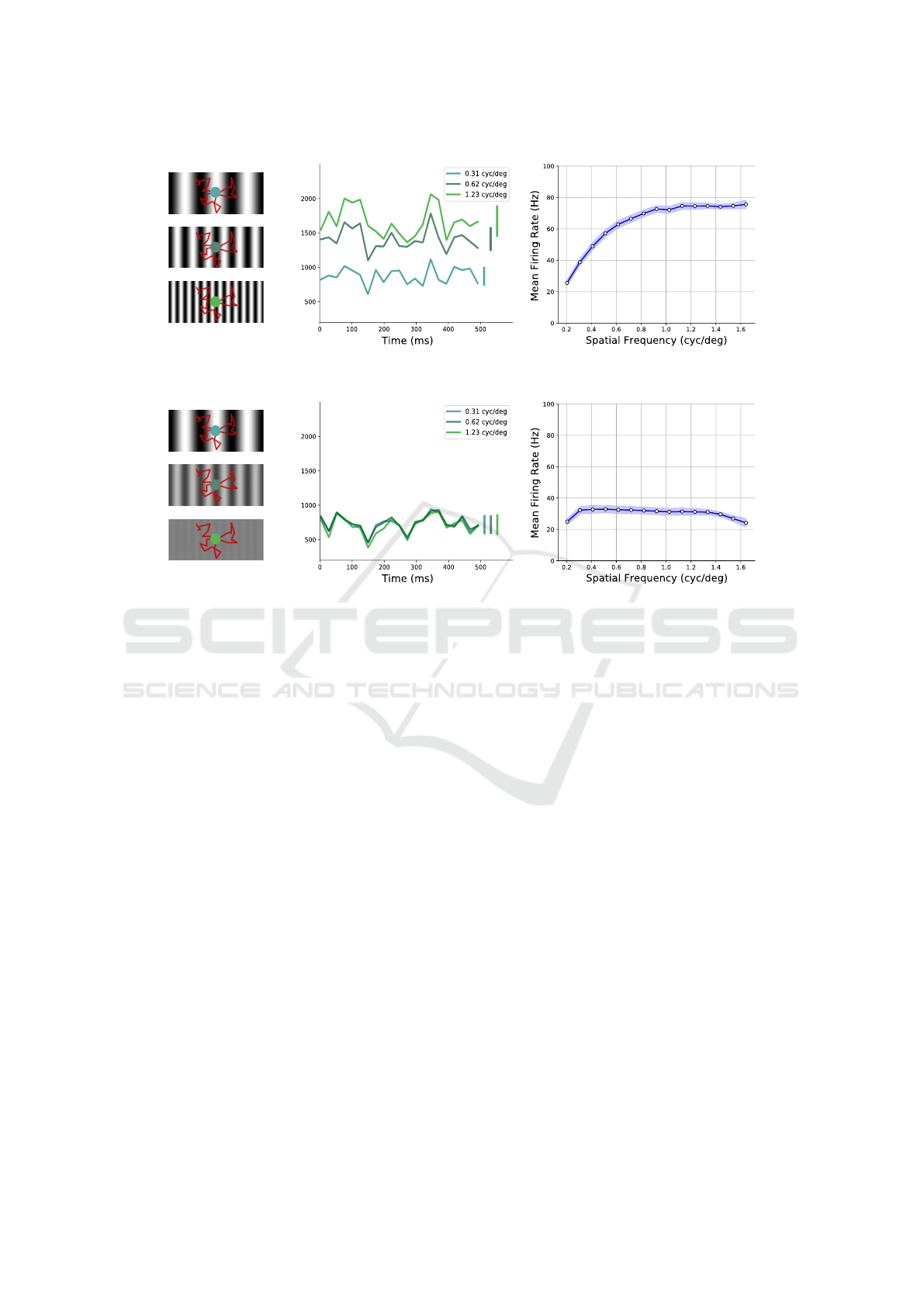
Space
Visual Scene
Time Response Spectral Amplification
Spike Count
(a)
Space
Visual Scene
Time Response Spectral Whitening
Spike Count
(b)
Figure 2: Sensors spectral characterization, for comparison with the results in (Rucci and Victor, 2015). (a) On the left,
examples of unit-valued contrast gratings with 3 different spatial frequencies. FEM-like movements, induced on the sensor,
are superimposed (red path). The central plot presents the resulting activity fluctuations exhibited by the 30 × 30 central pixels
of the DVS during 500 ms of recording: response is measured in number of events occurring in time windows of ∼ 24 ms.
On the right, the amplification of the system to high-frequency grating stimuli with unitary contrast; standard deviation
for different orientations of the gratings is represented by light-blue shaded regions. (b) On the left, gratings having same
frequencies as in (a) but with contrast adjusted according to the 1/k falloff of natural images. Resulting activity fluctuations
of the neuromorphic active system are shown in the central plot, and spectral whitening effect to all frequencies on the right
(shaded regions as in (a), right plot).
in biological early visual processing has been pos-
tulated both in (Greschner et al., 2002) and (Kuang
et al., 2012), for initiating edge extraction and provid-
ing redundancy reduction of spatial information to-
wards an economical representation of the image sig-
nal. Furthermore, phase shifts of activation in nearby
pixels, that are subject to the same movement, should
reflect spatial variations of luminance discontinuities
impinging on nearby receptors, as proposed in the
dynamic theory of vision presented in (Ahissar and
Arieli, 2001).
It is worth noting that, as the DVS is sensitive
to temporal changes of luminance only, anisotropic
movements are undesirable, as they put into an ad-
verse condition all stimuli having the same orienta-
tion of the directional bias of FEMs. Hence, to bet-
ter investigate the effects of the erratic motion on
the perception of such variously oriented stimuli, an
isotropic analysis was conducted. To this aim, the
possible angles that each step of the Brownian mo-
tion could take was limited to multiples of 15 deg (as
for stimuli). Results of the analysis (further described
in section 3.2) for one particular realization of Brow-
nian motion are shown in the polar plot in the inset of
Fig. 1b. We anticipate that oriented luminance transi-
tions would be the feature that benefit of such a prop-
erty of FEM-like movements, supplying the system
with an effective method for unbiased perception of
local elements in the image signal.
3 SYSTEM RESPONSE
CHARACTERIZATION
By taking inspiration from neuroscience studies
(Rucci and Victor, 2015), we characterize the be-
havior of our active sensing system with respect to
stimulus’ spatial frequency - i.e. we examine sen-
Dynamic Detectors of Oriented Spatial Contrast from Isotropic Fixational Eye Movements
677
sor response to individual spatial gratings for differ-
ent contrast conditions (either constant or matching
the statistics of natural images). Certainly, the pro-
cess for event generation in the DVS, the structure
of the visual stimulus, and the specific camera move-
ment adopted, collectively affect such a behavior at
any moment. Therefore, we expect that the activ-
ity elicited in each receptor of our artificial retina
complies with the statistical properties of natural im-
ages in the same way it happens in the biological one
(Kuang et al., 2012). Furthermore, isotropic FEMs
(i.e., for which all directions are visited with equal
probability at each step) should provide an unbiased
orientation information in the output signal. Accord-
ingly, we have analyzed the mean response of the
sensor with respect to this visual feature by system-
atically using gratings of different orientations. It is
worth noting that all the results shown in the follow-
ing are relative to one particular Brownian motion se-
quence, but same results were achieved independently
of the random seed we considered.
3.1 Spectral Analysis
If we compute the mean firing rate of sensors pix-
els, for each spatial frequency k tested and by aver-
aging over all the orientations, we get a measure of
the overall spectral response of the system. In case
of stimuli with maximum contrast, we can notice that
pixels’ mean activity increases with spatial frequency
(see Fig. 2a rightmost panel): stimuli with higher fre-
quencies elicit stronger responses in the system and a
plateau value is reached approximately when spatial
frequency k approaches the spatial resolution limit of
the pixel array. As a matter of fact, by randomly mov-
ing around, as k increases, each receptor scans an in-
creasing number of light-dark transitions, thus elicit-
ing an increasing number of events. Remarkably, by
adjusting the gratings’ contrast according to the 1/k
falloff of natural image amplitude spectrum (Field,
1987), the frequency response with the same drift tra-
jectory gives a roughly constant profile over the whole
range of discernible spatial frequencies (see Fig. 2b
rightmost panel). Hence, the active system tends to
intrinsically oppose to the 1/k trend of natural image
distribution across spatial frequencies, thus counter-
balancing the latter and enabling a whitened response.
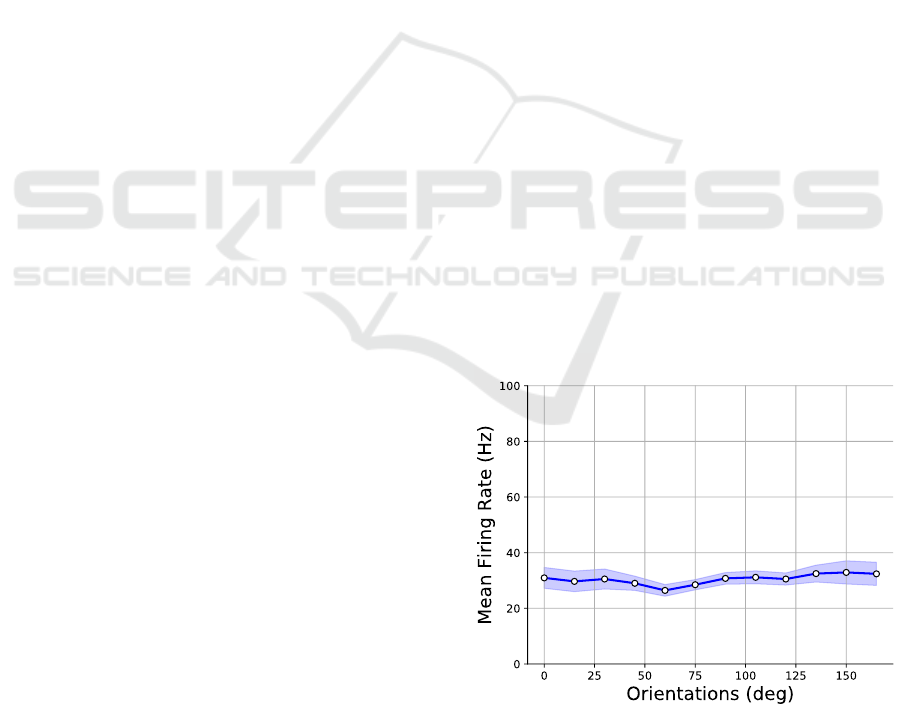
3.2 Orientation Analysis
First, we computed the circular statistics of the Eu-
clidean distances travelled in each of the 12 direc-
tions considered for the stimuli, relative to the whole
path length. The aim was to quantify the isotropy of
FEM-like movements of our active system, in relation
with the effect that each step of the whole movement
causes on the perception of a specific orientation of
the stimulus (that is why the isotropy was computed in
the 0 − π range rather than on the full circle). Results
for a FEM-like motion sequence of 35 steps (e.g., see
the inset of Fig. 1b, where the 12 probability values
were remapped on a full circle for a more intuitive
graphical representation) indicate that the maximum
deviation from circularity is of 3%, thus evidencing
a good approximation of an isotropic behavior. It is
worth noting that the longer is the duration of active
fixations the better is the isotropy we achieve. As a
matter of fact, by considering 80 Brownian motion se-
quences with increasing number of steps (up to 400),
the maximum discrepancy from isotropy decreases
with this number (reaching a minimum of ∼ 1%), in
line with the fact that vision improves for longer fix-
ational periods. By analogy with previous analysis,
we have then computed the mean firing rate of sen-
sor’s pixels as a function of stimulus’ orientation θ,
by averaging across spatial frequencies. As the sensor
moves in isotropic fashion, we expect that the mean
response of the system does not change with respect
to different oriented gratings, as evidenced in Fig. 3,
which refers to 1/k falloff contrast gratings. Simi-
lar behavior has been obtained for constant contrast
gratings as well (not shown). This equalized activity,
in the form of spike sequences from each DVS pixel,
provides a convenient input to a subsequent stage for
unbiased detection of such orientation. Thus, we have
designed and tested a bio-inspired spiking neural net-
work for the detection of static objects’ orientations
on a local scale during active fixation.
Figure 3: Average sensor response (mean pixel firing rate)
to different oriented gratings (blue curve). The light-blue
shaded region represents the standard deviation of pixels fir-
ing rate for different spatial frequencies.
VISAPP 2020 - 15th International Conference on Computer Vision Theory and Applications
678
4 ORIENTATION DETECTION
Orientation detectors consist of spiking neurons orga-
nized in a specific network architecture and fed with
the event-based data provided by the neuromorphic
camera. A spiking neural network is a biologically-
realistic computational primitive where neurons and
synapses are modeled as differential equations, repre-
senting the dynamics of membrane and synaptic cur-
rents (or voltages) respectively (Maass, 1997). There-
fore, SNNs are powerful instruments for exploiting
both space and time information of events from a
jittery sensor. Besides, they can be implemented
onto neuromorphic electronic processors (namely, the
DYNAP) for bio-inspired, highly-efficient and real-
time computing systems. To this purpose, we had to
take into account hardware limitations both in the de-
sign and simulation phases. Accordingly, the neuron
model used for software simulations of the network is
the Differential Pair Integrator (DPI) (Indiveri et al.,
2011), a variant of the generalized integrate-and-fire
model, which is implemented by silicon neurons in
the DYNAP. Likewise, the DPI model of synapses
(Bartolozzi and Indiveri, 2007) was considered.
4.1 Network Architecture
The custom-designed detectors aim to mimic the
functionality of simple cells in the primary visual cor-
tex (V1). At any given spatial scale, detection is
achievable by means of a 2D spatial kernel - i.e. a
receptive field (RF) profile that defines the (feedfor-
ward) synaptic weights between the DVS pixels and
a single V1-like spiking neuron. Neurons’ selectivity
to specifically-oriented edges relates to the direction
of kernel’s elongation (θ), i.e. by the anisotropic spa-
tial summation of events provided by the sensor. In
particular, we used difference of 2D offset elongated
Gaussians that model two sub-fields organized in a
push-pull configuration (i.e. adjacent excitatory and
inhibitory regions) for antagonistic effects of ON and
OFF events, as postulated by experimental evidence -
e.g., see (Hirsch and Martinez, 2006). We specifically
used RF sub-fields with approximate size of 10 × 3
pixels and orientations (θ) evenly spaced between 0
and 165 deg (as we have done for the stimuli). The
kernel was defined as:
h(x
θ
,y
θ
) = e
−
(x
θ
+σ)
2
+(y
θ
/p)
2
2σ
2
− e
−
(x
θ
−σ)
2
+(y
θ
/p)
2
2σ
2
(1)
where x
θ
,y
θ
define a rotated reference frame, with re-
spect to x,y, σ represents the standard deviation (in
number of pixels) along x
θ
, and p > 1 defines the
elongation of the RF along y
θ
. The specific values
of the parameters are σ = 1.08 and p = 4. It is worth
128
128 30
30
9
+
+
ONOFF
Hypercolumn
-
Fovea
Pixel Array
OFFON
9
9
-
-
-
Figure 4: Schematic representation of network organiza-
tion. The 30 × 30 central region (fovea) of the whole DVS
pixel array provides input to the SNN. Each neuron of
a 9 × 9 orientation column takes excitatory synaptic con-
nections from the fovea through specific anisotropic spa-
tial kernels acting as receptive fields (RFs). These RFs
are characterized by two adjacent sub-regions selective to
ON and OFF events respectively, and such distinction is
interchanged for neurons in the two hypercolumns. The
push-pull configuration is achieved via reciprocal inhibitory
connections between corresponding neurons in the hyper-
columns. Within a given hypercolumn, recurrent inhibition
between different orientation columns is also considered.
noting that, in order to restrict the number of neurons
in the network to a size that can be contained on the
DYNAP board, only the central 30 × 30 pixel region
of the DVS was considered as input of the network,
which is comparable to the biological fovea. Fur-
thermore, in order to satisfy the constraints imposed
by the DYNAP on the maximum number of connec-
tions available for each neuron, synaptic weights hav-
ing absolute values lower than 0.2 were set to 0, thus
limiting the actual size of the kernel.
A schematic (non-exhaustive) overview of net-
work organization can be found in Fig. 4. The whole
SNN consists of two neuronal populations, which we
call “hypercolumns”, each one comprising 12 “ori-
entation columns” (for the sake of clarity, only 4 are
shown for each hypercolumn in Fig. 4). A single col-
umn constitutes a group of neurons having same tun-
ing selectivity to a specific orientation of the stimulus
as a matter of fact, the name relates to the columnar-
like structures by which cortical orientation-selective
cells are known to be organized in V1 (Hubel and
Wiesel, 1974). The center of each RF was shifted by 3
pixels for nearby neurons belonging to the same col-
umn (i.e. their RFs share the same θ but have different
centers on the receptor array). Thereby, each orienta-
tion column includes 81 neurons, i.e. 9×9, starting
from position (3,3) of the DVS fovea and ending in
(27,27). The set of all orientation columns, internal to
a same hypercolumn, constitutes a total of 972 neu-
rons (81×12), which can then be contained in a sin-
gle chip of the DYNAP board. Finally, the distinction
between the two hypercolumns is given by the rel-
ative organization of their neuronal receptive fields.
Particularly, if one sub-region of a RF is selective to
Dynamic Detectors of Oriented Spatial Contrast from Isotropic Fixational Eye Movements
679
ON events, the adjacent sub-field will be selective to
OFF events only, and this selectivity is interchanged
between neurons in the two hypercolumns. The push-
pull configuration is then achieved by means of re-
ciprocal inhibition between neurons sharing the same
location in the two hypercolumns (therefore sensitive
to opponent contrast polarity). Furthermore, recurrent
connectivity within both hypercolumns was consid-
ered, with the aim of optimizing discrimination by lat-
eral inhibition (Blakemore et al., 1970) between neu-
rons having different orientation selectivity but RFs
centered in the same location of the pixel array. The
weight of such connections was, in absolute value, 10
times higher than that used for feedforward synapses.
Note that, in order to optimize network stabilization,
an inhibitory neuron (not shown in Fig. 4), spiking
according to a Poisson process (with a 80 Hz firing
rate), was also connected to all other neurons.
4.2 Simulation Results
Python simulations of the SNN were performed on
the event-based data corresponding to the second set
of grating stimuli, having a spatial frequency of ∼
0.5 cyc/deg and all possible orientations. It is worth
noting that the movement of the sensor induces syn-
chronized activity of DVS receptors, as also postu-
lated for biological FEMs (Greschner et al., 2002;
Kuang et al., 2012), and the spatial structure of the
static image will therefore be temporally encoded in
the relative activity of nearby pixels. Hence, by ex-
ploiting this attribute thanks to the designed kernels
TH
Spike
Elicited
No
Response
Membrane Potential
TH
Time
1
1
2
2
3
3
1
2
3
1
2
3
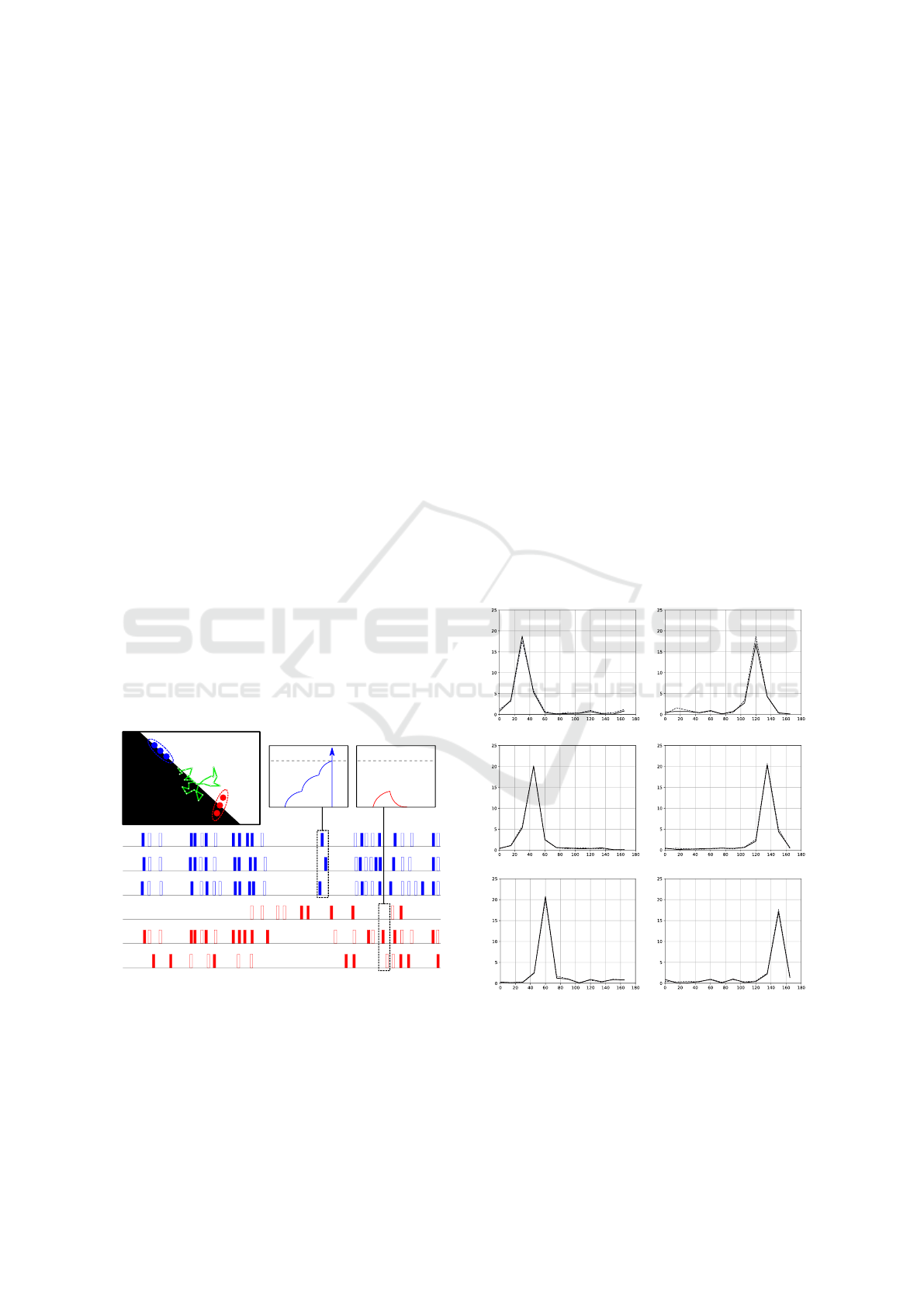
Figure 5: (Top) A cartoon illustrating the spiking dependent
spatial summation of ON and OFF events. A simple visual
scene is shown on the left, where 3 pixels aligned (blue) or
not (red) with the edge are displayed. The FEM-like path is
superimposed (green). (Bottom) An example of membrane
potential in neurons taking inputs from blue or red retinal
neurons is also shown: only blue pixels, thanks to their syn-
chronized activity (for both ON/OFF polarities), are able to
elicit a response on the target V1 model neuron.
and the timing properties of neurons, we can achieve
optimal discrimination performances. Hence, at any
given time, only the membrane potential of neurons
that integrate synchronized events (i.e. from pix-
els that are aligned with the edge) will exceed the
threshold for generating a spike, and thus an output
response. The same behavior cannot be observed
in neurons collecting events from pixels that are not
aligned with the edge, as these activities are not syn-
chronous. Figure 5 illustrates such a mechanism.
To present detection results of our network, we
have computed the mean activity of all neurons within
a single column, when exposed to a set of differently
oriented gratings. Accordingly, we derived the tuning
curve of such neuronal group. Figure. 6 shows, on
6 different plots, the simulation results for the same
number of distinct orientation columns in both hyper-
columns. As expected, the tuning curves have a peak
in correspondence of the RFs θ value. Hence, we can
infer that the designed SNN is capable of a reliable
estimation of local orientations. Finally, it is worth
noting that the trend is very similar for the two curves
in all plots. This could be due to the fact that random-
like movements of a receptor across an edge trigger
almost the same amount of ON and OFF events.
Stimulus Orientation (deg)
Mean Firing Rate (Hz)
Mean Firing Rate (Hz)
Mean Firing Rate (Hz)
Mean Firing Rate (Hz)
Stimulus Orientation (deg)
Stimulus Orientation (deg)
Mean Firing Rate (Hz)
Mean Firing Rate (Hz)
Stimulus Orientation (deg)
Stimulus Orientation (deg)
Stimulus Orientation (deg)
Figure 6: Resulting tuning curves. They describe, for both
hypercolumns (dashed and solid black curves), the firing ac-
tivity, with respect to all tested orientations of the stimuli,
corresponding to 6 different orientation columns (with tun-
ing of 30, 45 and 60 deg from top to bottom on the left, and
120, 135 and 150 deg on the right).
VISAPP 2020 - 15th International Conference on Computer Vision Theory and Applications
680

5 CONCLUSIONS
Bio-inspired fixational eye movements can transform
a static scene into a spatiotemporal input luminance
signal to the event-based camera. As a consequence,
the low temporal frequency power of a static scene
is shifted into a range that the DVS can properly de-
tect. Besides preventing “perceptual fading” of static
scenes, we show that FEMs can play a central role in
event-based vision by providing an efficient strategy
for acquiring and processing information from nat-
ural images, both enhancing the perception of fine
spatial details in the scene, and facilitating or im-
proving the extraction of important features. Par-
ticularly, due to camera motion, edges in the visual
scene will provoke highly time-correlated activity of
nearby pixels. Due to the randomness of such motion,
events with both polarities can be elicited over time
in each pixel as a result of a same spatial luminance
discontinuity. Therefore, synchronized events with
both polarities eventually encode the spatial structure
of the underlying static image. The push-pull con-
figuration, at which the network operates, exploits
the distinction between events’ polarities, inducing
appropriate excitation or inhibition of ON and OFF
events, for optimizing detection performances. The
whole artificial neural architecture proposed is fully
bio-inspired, both at single unit (neuron model) and at
network level, and is entirely conceived to satisfy the
constrains imposed by ultra low-power mixed signal
analog-digital neuromorphic processors for a future
hardware implementation.
ACKNOWLEDGEMENTS
This project has received funding from the European
Research Council under the Grant Agreement No.
724295 (NeuroAgents).
REFERENCES
Ahissar, E. and Arieli, A. (2001). Figuring space by time.
Neuron, 32(2):185–201.
Bartolozzi, C. and Indiveri, G. (2007). Synaptic dynamics
in analog vlsi. Neural computation, 19:2581–2603.
Blakemore, C., Carpenter, R. H., and Georgeson, M. A.
(1970). Lateral inhibition between orientation detec-
tors in the human visual system. Nature, 228:37–39.
Ditchburn, R. and Ginsborg, B. (1952). Vision with a stabi-
lized retinal image. Nature, 170(4314):36.
Field, D. J. (1987). Relations between the statistics of natu-
ral images and the response properties of cortical cells.
Journal of the Opt. Soc. of America, 4:2379–2394.
Gollisch, T. and Meister, M. (2008). Rapid neural coding
in the retina with relative spike latencies. Science,
319(5866):1108–1111.
Greschner, M., Bongard, M., Rujan, P., and Ammerm
¨
uller,
J. (2002). Retinal ganglion cell synchronization by
fixational eye movements improves feature estima-
tion. Nature neuroscience, 5(4):341.
Hirsch, J. A. and Martinez, L. M. (2006). Circuits that
build visual cortical receptive fields. Trends in neu-
rosciences, 29(1):30–39.
Hubel, D. H. and Wiesel, T. N. (1974). Sequence regular-
ity and geometry of orientation columns in the mon-
key striate cortex. Journal of Comparative Neurology,
158(3):267–293.
Indiveri, G., Linares-Barranco, B., Hamilton, T. J.,
Van Schaik, A., Etienne-Cummings, R., Delbruck, T.,
Liu, S.-C., Dudek, P., H
¨
afliger, P., Renaud, S., et al.
(2011). Neuromorphic silicon neuron circuits. Fron-
tiers in neuroscience, 5:73.
Kuang, X., Poletti, M., Victor, J. D., and Rucci, M. (2012).
Temporal encoding of spatial information during ac-
tive visual fixation. Current Biology, 22(6):510–514.
Land, M. (2019). Eye movements in man and other animals.
Vision research, 162:1–7.
Lichtsteiner, P., Posch, C., and Delbruck, T. (2008). A
128×128 120 dB 15µs latency asynchronous tempo-
ral contrast vision sensor. IEEE journal of solid-state
circuits, 43(2):566–576.
Maass, W. (1997). Networks of spiking neurons: the third
generation of neural network models. Neural net-
works, 10(9):1659–1671.
Martinez-Conde, S. and Macknik, S. L. (2008). Fixational
eye movements across vertebrates: comparative dy-
namics, physiology, and perception. Journal of Vision,
8:1–18.
Moradi, S., Qiao, N., Stefanini, F., and Indiveri, G. (2017).
A scalable multicore architecture with heterogeneous
memory structures for dynamic neuromorphic asyn-
chronous processors (DYNAPs). IEEE transactions
on biomedical circuits and systems, 12(1):106–122.
Orchard, G., Jayawant, A., Cohen, G. K., and Thakor, N.
(2015). Converting static image datasets to spiking
neuromorphic datasets using saccades. Frontiers in
neuroscience, 9:437.
Riggs, L. and Ratliff, F. (1952). The effects of counteracting
the normal movements of the eye. 42:872–873.
Rucci, M., Ahissar, E., and Burr, D. (2018). Temporal
coding of visual space. Trends in cognitive sciences,
22(10):883–895.
Rucci, M. and Victor, J. D. (2015). The unsteady eye: an
information-processing stage, not a bug. Trends in
neurosciences, 38(4):195–206.
Dynamic Detectors of Oriented Spatial Contrast from Isotropic Fixational Eye Movements
681
