
Optimization of a Cold Atmospheric Plasma Treatment to Selectively
Affect the Viability of Skin Cancer Cells
Sara Pereira
a
, Paulo António Ribeiro
b
and Susana Sério
c
CEFITEC, Departamento de Física, Faculdade de Ciências e Tecnologia, Universidade NOVA de Lisboa,
2829-516, Caparica, Portugal
Keywords: Cold Plasmas, Skin Cells, Plasma Jet Device, Hydrogen Peroxide, Plasma Medicine.
Abstract: Cold atmospheric plasmas (CAPs) are a specific type of non-thermal plasmas mainly composed by reactive
oxygen and nitrogen species (ROS and RNS, respectively), UV radiation and charged particles. In the last
years, liquids treated by CAPs (indirect CAPs treatments) have attracted a significant interest in oncology due
to its ability to kill cancer cells with an effectiveness similar to direct irradiation of the cells by cold plasmas.
It is important to point out that indirect treatments have the advantage of avoiding the effects of UV radiation
and the electrical fields present in plasmas being their effects mainly dependent on the ROS and RNS
produced in the liquid phase. To better understand the mechanisms behind the interaction between CAPs,
treated liquids and cells, it was engineered a plasma jet device and studied the vulnerability of different cell
lines to the culture medium previously exposed to CAPs. For that, it was analysed the concentration of H
2
O
2
produced during the treatments by means of colorimetric assays and evaluated the influence of using different
working parameters such as volume of medium and gap. According to the obtained results it could be observed
that the cancer cell line (Met-1) in study is more sensitive to the liquids treated by CAPs than the non-cancer
one (HGF-1), which in the particular case of this jet, seems to be mainly related with the concentration of
RNS species produced in the liquids during the plasma exposure since the concentration of the H
2
O
2
produced
is very low.
1 INTRODUCTION
Cold atmospheric plasmas (CAPs) are a near-room
temperature plasma, generally produced in laboratory
conditions, by applying an external source of energy
to a neutral gas up to a critical point, at which
electrons dissociate from atoms (Fridman, Chirokov,
and Gutsol 2005; Weltmann and Von Woedtke 2017).
The resulting ionized gas will be mainly composed by
a mixture of reactive oxygen and nitrogen species
(ROS and RNS), UV, visible and infrared light,
electromagnetic fields, electrons and ions (Pipa et al.
2012; Reuter et al. 2009, 2012). It is important to note
that, since these plasmas are laboratory-generated,
their properties, such as their energy and charged
particles density, will be dependent on the features of
the used set-up: applied power and its type, and also
of the feeding gas (Bekeschus et al. 2013; Weltmann
and Von Woedtke 2017).
a
https://orcid.org/0000-0002-8682-5462
b
https://orcid.org/0000-0001-9665-7610
c
https://orcid.org/0000-0002-8086-7792
Since, under in vivo conditions, cells and tissues
are surrounded by a liquid environment, during the
past decade, liquids treated by CAPs (indirect plasma
treatments) have attracted attention in clinical plasma
medicine (Jablonowski and von Woedtke 2015). So
far, a significant number of studies, have shown
similar effectiveness between treated liquids and
direct irradiation of cells (Keidar et al. 2013; Liedtke
et al. 2017; Nakamura et al. 2017; Tanaka et al. 2011;
Wende et al. 2014; Yan et al. 2014, 2017), over a wide
range of cancer cell lines, including melanomas and
carcinomas (Pereira et al. 2019; Yan et al. 2015).
Additionally, indirect treatments have the advantage
of avoiding the effects of UV radiation and of the
electromagnetic fields present in plasmas, being their
effects mainly related with the ROS and RNS species
produced in the liquid phase. Although, the explana-
tion in detail of which species are active in plasma-
treated liquids remains a challenge. Some general
Pereira, S., Ribeiro, P. and Sério, S.
Optimization of a Cold Atmospheric Plasma Treatment to Selectively Affect the Viability of Skin Cancer Cells.
DOI: 10.5220/0009373802010208
In Proceedings of the 8th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2020), pages 201-208
ISBN: 978-989-758-401-5; ISSN: 2184-4364
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
201

conclusions have been taken about the anti-cancer
mechanisms of CAPs. For example, it was shown that
the increase in ROS species will cause damages to the
antioxidant system and consequently lead to DNA
double-strand breaks (Adachi et al. 2015; Sauer,
Wartenberg, and Hescheler 2001). Another conclu-
sion is that the CAPs effects will result in cellular
apoptosis or necrosis in a dose-dependent way.
Moreover, H
2
O
2
and NO produced in treated liquids
are proposed to be key molecules to preferably kill
cancer cells instead of non-cancerous ones
(Bekeschus et al. 2014; Jablonowski and von
Woedtke 2015).
In the present work, the indirect treatment of Met-
1 cells (Squamous Cell Carcinoma keratinocytes),
which represents 24% of all skin cancers, was
performed. Squamous Cell Carcinoma represents 20-
30% of the reported cases of non-melanoma skin
cancers and its incidence is increasing over the world
(Graham and Tuchayi 2016; Waldman and Schmults
2019). To understand the mechanisms behind the
interaction between CAPs, treated liquids, and
eukaryotic cells, it was engineered a plasma jet
device, which will be described in this paper, and
study the vulnerability of Met-1 and HGF-1 cells
(human fibroblasts) to indirect CAPs treatment. It
was also investigated if CAPs anti-cancer capacity
was dependent on the parameters that were chosen to
perform the plasma treatments. For that, different
volumes of the medium, distances from the jet to the
liquid to be treated and times of plasma exposure
were tested. The vulnerability of Met-1 cells to CAPs
treatment was then compared to one of the HGF-1
cells (human fibroblasts). In addition, the
concentration of H
2
O
2
produced in the treated liquid
was measured and the vulnerability of Met-1 and
HGF-1 cell lines to H
2
O
2
rich DMEM w/o sodium
pyruvate was studied, to determine if H
2
O
2
is the only
reactive species responsible for the CAPs effects.
2 MATERIALS AND METHODS
2.1 Experimental Set-up
The CAP jet device used in this research was
designed and constructed in our laboratory (Plasmas
and Applications laboratory, CEFITEC, Physics
Department, FCT/UNL, Portugal). It consists of a
hand-held principal unit composed by a borosilicate
capillary with an outer diameter of 6.93 mm and an
inner diameter of 3.76 mm, with two metal electrodes,
a custom made DC power supply (2.5 mA, 20 kV),
and a gas supply unit (Pereira et al 2019). The
Figure 1: Tested electrodes: (a) copper ring, (b) titanium
ring, and (c) copper wire.
electrode on the inside of the capillary is a stainless-
steel needle with a diameter of 2 mm. For the outer
electrode (connected to the high voltage) three
different hypotheses were tested: a copper ring, a
titanium ring and one made from an enameled copper
wire, Figures 1 (a), (b) and (c), respectively. In order
to choose the electrode that allowed a more stable
plasma, spectroscopic analysis of the plasma plume
obtained using each one of the referred electrodes was
performed. According to the obtained results, it was
decided to develop the jet using the third
configuration (Figure 1 (c)). The copper wire coil
used has a 1 mm of thickness and a height of 7 mm,
corresponding to seven turns. Once the optimal
configuration in terms of stability was determined,
the device was coated in order to facilitate safe
handling and system automation.
2.2 Optical Emission Spectroscopy
OES was performed using an optic fiber (FC-UV600-
2, Avantes) coupled to a spectrometer (SPEC STD,
Sarspec’s). The size of the spectrometric quartz glass
lens covered the whole length of the effluent and was
kept at approximately 5 mm perpendicular to the
plasma plume. The instrument has a resolution of 1.7
mm and emission intensities on the range 180-1100
nm were recorded.
2.3 Cell Lines and Cell Culture
Human Squamous Cell Carcinoma (Met-1) cells were
purchased from Ximbio (153539, London) while
human fibroblasts obtained from a gingival biopsy
(HGF-1) were purchased from American Type
Culture Collection (ATCC
®
CRL-2014
TM
,
Barcelona). Both cell lines were cultured in 75 cm
2
flasks (Corning
®
, 4314640) with complete DMEM
[(Dulbecco’s Modified Eagle’s Medium, Sigma,
D5030), supplemented with 1.0 g/L D-glucose
(Gibco, 15023-021), 3.7 g/L sodium bicarbonate
(Sigma-Aldrich, S5761), 1% GlutaMAX
TM
(L-
Biophotonics 2020 - Special Session on Biomedical Optics
202

alanyl-L-glutamine dipeptide, Life Technologies,
35050-038), 1% sodium pyruvate (Gibco, 11360039),
penicillin (100U/ml) and streptomycin (100 µg/mL)
(Invitrogen, 15140122), 10% FBS (Fetal Bovine
Serum, Invitrogen, 10270106)]. When reaching
confluence, the cells had to be transferred into new
flasks by an enzymatic method. Met-1 and HGF-1
cells are adherent cell lines that have to be maintained
under 37°C in a humidified atmosphere of 5% CO
2
(standard conditions).
2.4 Plasma Treatment Conditions
The protocol used for the both cell lines in study was
identical. Met-1 and HGF-1 cells were seeded in a 96-
well plate (83.3924, Sarstedt) with a confluence of
3.5 10
cells/mL and incubated overnight under
standard conditions. A specific volume of complete
DMEM without sodium pyruvate were plasma-
treated in wells of a 12-well plate (83.3921.005,
Sarstedt), for different times. To perform the
treatments, the plasma jet was placed above the upper
edge of the well containing the liquid to be treated and
it rested at the chosen position until the end of the
treatment. The treated liquid was mixed by an argon
flux of 3 standard liters per minute (slm) controlled
by a flowmeter (Dynamal Argon 0-15 L/min, Air
Liquid). 100 or 150 µL of the treated culture medium
were then immediately transferred to the previously
cultured cells in sextuplicate. The same volume of
untreated medium was used as a positive control in
sextupllicate, in all the performed experiments.
Before this, the medium used to culture the cells
overnight was discarded and the cells were washed
with Phosphate Buffer Saline (PBS). The cells were
incubated for 48 hours under standard conditions and
only then the cellular viability was assessed by the
resazurin assay. On the present study, it was decided
to use DMEM without sodium pyruvate, since the last
could act as a scavenger for hydrogen peroxide
(Pereira et al. 2019; Wende et al. 2015).
2.5 pH and Temperature
Measurements
2 mL of cell culture medium were treated as
previously described. Immediately after the
treatment, the pH of the treated solution was
measured using a ph-meter. The temperature of the
treated solution was determined using a multimeter
associated with a k type thermocouple (RS, Portugal).
2.6 Hydrogen Peroxide Determination
in Complete DMEM
The H
2
O
2
concentration of the treated liquid was
analyzed using a Fluorimetric Hydrogen Peroxide
Assay Kit (Sigma-Aldrich, MAK165). After the
plasma treatment, 50 µL/well of the treated liquid
were transferred in triplicate to different wells of a
black 96 well flat-bottom plate. As control, 50 µL of
DMEM without sodium pyruvate were also
transferred to a well on the same plate in triplicate. 50
µL of a previously prepared master mix (assay
reaction solution) was added to all the wells in study
(samples, standards, and controls). The plate was then
incubated at room temperature for 30 minutes
protected from light and the fluorescence intensity
was measured at λ
excitation
=540 nm and λ
emission
=590
nm using a fluorescence plate reader (Tecan infinite
200). Final concentrations were calculated using a
H
2
O
2
standard curve.
2.7 Effects of H
2
O
2
Rich Medium
Met-1 and HGF-1 cells were seeded in a 96-well plate
with a cell confluence of 3.5 10
cells/mL and
cultured in an incubator overnight under standard
conditions. Then, different solutions of H
2
O
2
rich
medium were prepared. The H
2
O
2
solutions were
prepared by mixing a 30% w/w H
2
O
2
solution (Sigma)
into the complete DMEM without sodium pyruvate.
The prepared H
2
O
2
rich medium was then transferred
to the previously cultured cells in sextuplicate. Before
this step, the medium that has been used to culture the
cells overnight was discarded and the cells were
washed with PBS. After that, the cells were incubated
for 48 hours under standard conditions, and only then
the cellular viability was assessed by the resazurin
assay. Positive control of cells in untreated DMEM
without sodium pyruvate and without H
2
O
2
was used
in all the realized experiments.
2.8 Cell Viability: Resazurin Assay
The resazurin assay is based on the ability of the
dehydrogenase enzyme, present in metabolically
active cells, to reduce the resazurin (7-Hydroxy-3H-
phenoxazin-3-one 10-oxide) blue dye into a pink
colored and highly red fluorescent resorufin (3H-
phenoxazin-3-one) product. The amount of resorufin
produced is directly proportional to the mitochondrial
enzyme activity, i.e. to the number of viable present
cells (Anoopkumar-Dukie et al. 2005; Riss et al.
2004), which can be easily quantified using a
Optimization of a Cold Atmospheric Plasma Treatment to Selectively Affect the Viability of Skin Cancer Cells
203
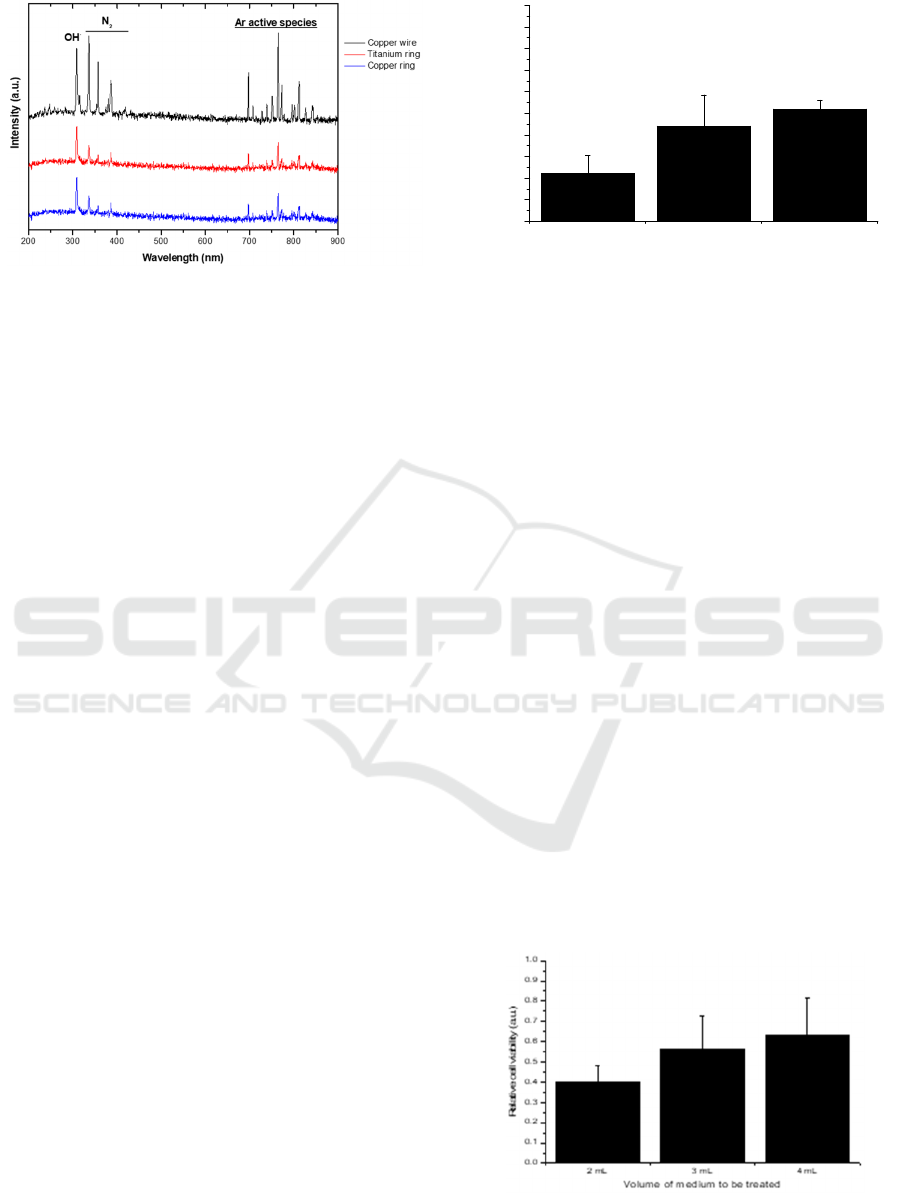
Figure 2: Optical emission spectrum of the plasma plume
for three different electrodes, obtained using an Argon flow
of 3 slm, where the black spectrum represents the electrode
of copper wire, the red the titanium ring and the blue the
copper ring.
microplate reader. To assess cell viability, the culture
medium in the wells was discarded and 100 µL of
resazurin solution (0.04% resazurin, Life
Technologies, USA) were added to all the wells in
study. After an adequate time of incubation in the
dark at standard conditions, the absorbance was
measured in a microplate reader, using a wavelength
of 570 nm and a reference standard of 600 nm.
2.9 Statistical Analysis
All data are expressed as mean ± standard deviation
(SD) of at least three independent experiments with
six replications. The statistical significance of the
differences was evaluated using the Student t-test and
it was recognized as * for p0.05, ** for p0.01 and
*** for p0.005.
3 RESULTS
3.1 OES Obtained for the Different
Tested Electrodes
To verify if the plasma produced using the three
different electrodes (copper ring, titanium ring, and
copper wire) have the same composition, the optical
emission spectrum of the discharge was acquired for
all of them. Observing Figure 2, despite some small
differences in terms of relative intensities, the
produced species seems not be dependent on the outer
electrode used. The obtained emission spectra show
an emission peak belonging to OH
·
radical in the UV-
B region at 308 nm and some peaks between 330 and
400 nm representing the nitrogen (N
2
) emission in the
UV-A range. Since neither oxygen nor nitrogen are
Figure 3: Plasma effects are dependent on the used gap.
present in the working gas used, the appearance of
these emission bands can be attributed to interactions
between the generated plasma and the surrounding
ambient air (Hoentsch et al. 2014). According to
Lukes and Locke 2005, the OH
·
radical in the gas
phase discharge appears as a consequence of the
electron impact of H
2
O molecules in the water vapor
above and near the liquid surface. The near infrared
(700-900 nm) emission peaks mainly represent
excited molecules of Argon.
3.2 Effect of the Plasma Treatment
Conditions on the Cell Viability
In order to understand the relationship between
plasma treatment and cell viability, the plasma
treatment conditions, namelly, the distance between
the jet and the liquid to be treated (gap), the treated
volume, and the time of exposition to plasma were
changed. First, to understand how the chosen gap can
affect cell viability, using a cell concentration of
3.5 10
cells/mL and treatment time of 2 minutes,
the used gap was varied from 2 mm to 7 mm, and to
9 mm. The results, shown in Figure 3, indicate that
the viability of Met-1 cells increases as the distance
from the jet increases. This means that proximity of
the jet enhances its anti-tumor capacity, and for this
Figure 4: Influence of use different volumes during plasma
treatment.
2 mm 7 mm 9 mm
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
***
Relative cell viability (a.u.
)
Gap (mm)
***
Biophotonics 2020 - Special Session on Biomedical Optics
204

Figure 5: Plasma treatments are time dependent.
reason the gap used to carry out the treatments must
be carefully chosen.
Likewise, to see if the effects of plasma treatment
are volume dependent, different volumes of DMEM
w/o sodium pyruvate were treated under the same
conditions. After the treatment 150µL of the treated
medium were transferred to the previously cultured
Met-1 cells, and cell viability was measured 48 hours
after plasma treatment. Three different volumes of
medium were plasma treated: 2, 3 and 4 mL. By the
analysis, of the Figure 4, it is observed that the killing
capacity of CAPs treated medium seems to decrease
as the total volume of treated medium increase.
According to literature, these results can be explained
by the dilution of the reactive species formed during
the plasma exposure in the treated medium(Wende et
al. 2015, 2016; Yan et al. 2015).
Moreover, using the same volume and gap, the
plasma treatment was performed for different times
of exposure. In this case, the assays were performed
for Met-1 and also HGF-1, in order to investigate if
cancerous, and non-cancerous cells have a similar
response to CAPs treatment. According to the
obtained results, Figure 5, Met-1 cells seems to be
much more sensitive to plasma treatment than HGF-
1 cells, whose viability remains almost unaffected.
For Met-1 cells, and a treatment time of 2 minutes,
the viability was already reduced to less than 50%
relative to the control, while for HGF-1 no significant
differences were found between the different tested
times. Here, it can be concluded that cancerous cells
are much more sensitive to CAPs treatment than non-
cancerous cells, and CAPs treatment is time
dependent. Accordingly, to the literature (Chauvin et
al. 2017; Wende et al. 2016), this occurs due to the
increased concentration of short and long reactive
species in the medium as the time of plasma exposure
increase (section 3.2.2).
Figure 6: pH measurements of plasma treated culture
medium. Results of 3 independent experiments, expressed
in terms of mean±SD.
3.3 Study of the Liquid Phase
3.3.1 pH and Temperature Measurements
It is known that plasma treatment may alter the pH of
the treated culture medium and consequently affect
the cell viability. For that reason, it was measured the
pH of the plasma-treated culture medium before and
after the treatments. As shown in Figure 6, a slight
increase in the pH could be observed as the time of
plasma exposure increase. These increase in pH can
be explained by the degassing effect of the carbonate
buffer sodium bicarbonate, present in the treated
medium (DMEM w/o sodium pyruvate)
(Bundscherer et al. 2013, Rumbach et al 2015)
Despite, these small differences are not significant in
a statically point, however biologically they can
contribute to increasing the alkaline stress,
contributing to apoptosis induction.
In terms of temperature, the longest plasma
exposure of 270 seconds yielded a temperature of
31°C. Since the cellular tolerance threshold without
thermal damage is around 40°C, it can be concluded
that this increase is not enough to cause cellular
damage on its own. This indicates that the results
obtained after the cellular assays are not a
consequence of the thermal effects caused by the
plasma treatments, but of the oxidative stress caused
due to the presence of some reactive species, such as
hydroxyl radicals, that are formed by plasma
treatment of liquids.
3.3.2 Effectiveness of H
2
O
2
Produced in
Caps and H
2
O2 Rich Medium
Some authors claim that H
2
O
2
is the main reactive
species responsible for the selective effects of CAPs,
due to its stability and role in a variety of multiple
cellular pathways (Liedtke et al, 2017). In order to
0 60 180 270
4.0
4.5
5.0
5.5
6.0
6.5
7.0
7.5
8.0
8.5
9.0
pH values
Time of plasma exposure (s)
Optimization of a Cold Atmospheric Plasma Treatment to Selectively Affect the Viability of Skin Cancer Cells
205
investigate this fact, it was measured the
concentration of H
2
O
2
produced during the CAPs
treatment and studied the response of MET-1 and
HGF-1 cells to different concentrations of H
2
O
2
rich
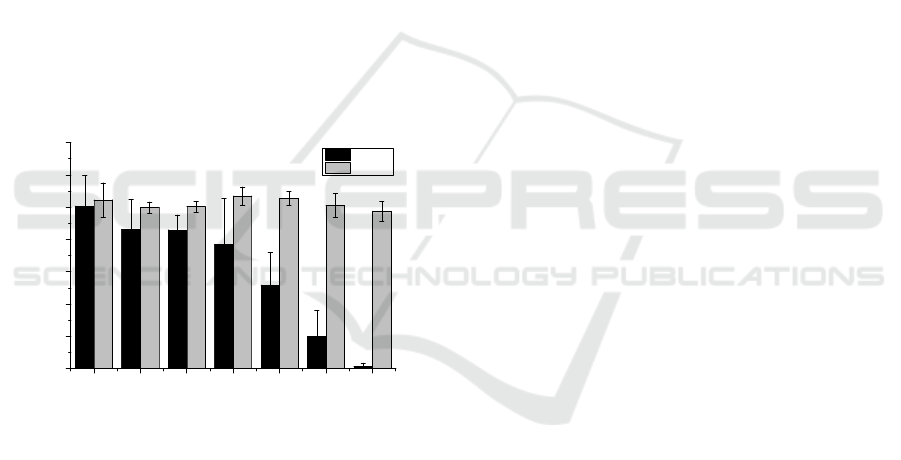
medium. As shown in Figure 7, Met-1 cells are much
more sensitive to H
2
O
2
rich medium than HGF-1 cells
(IC
50
=11.2µM and IC
50
=184µM, respectively with
IC
50
corresponding to the half maximal inhibitory
concentration), whose viability seems to be almost
unaffected by the presence of the H
2
O
2
in the tested
concentrations. However, it is important to note that
the real concentration of H
2
O
2
rich medium is
different than the one calculated for the preparation
of the solutions (being lower) since H
2
O
2
reacts with
the proteins present in the medium, for example via
Fenton reaction (Yan et al. 2015).
These results are very different from the ones
observed in treatments with CAPs medium on these
cell lines. In this last case, it was calculated an IC
50
of
only 2.69µM of H
2
O
2
to Met-1 cells, corresponding
to a treatment between 1 and 2 minutes. These
differences demonstrate that H
2
O
2
is not the only
reactive species responsible for the anti-cancer effects
of CAPs treated medium.
Figure 7: Vulnerability of H
2
O
2
rich medium on HGF-1 and
Met-1 cells. Results are present as mean±SD, and the
significance compared to the last bar (concentration of
0.29µM) is indicated as * p<0.05, ** p<0.01 and
***p<0.005.
4 CONCLUSIONS
The aim of this work was to understand the principles
behind the use of CAPs treated liquids in cancer
treatment, specifically on Squamous Cell Carcinoma.
For that, several parameters were studied in order to
try to optimize the anti-cancer capacity of a custom-
made Argon plasma jet device. The results presented
in this paper, show that the effectiveness of CAPs
treatment is time, volume and distance dependent.
Specifically, a shorter volume of medium and a closer
distance from the jet device to the liquid to be treated,
increase the effectiveness of the treatment. Regarding
time, this must be carefully chosen, since longer
treatments will produce high concentrations of
reactive species and consequently both cancer and
non-cancer cells may suffer irreversible damage. To
prove this anti-cancer capacity, the vulnerability of a
cancerous cell line, Met-1, was compared to the one
of a non-cancerous cell line, HGF-1, for treatments
performed in the same conditions. Despite a
significant reduction in Met-1 viability was
registered, no significant reduction in non-cancerous
HGF-1 cells was observed. To exclude possible
thermal damage and acidification of the medium,
changes on temperature and pH were monitored. In
addition, it was observed that cells respond in a
different way to the H
2
O
2
produced in the medium
during the treatment and to H
2
O
2
rich medium. This
indicates that H
2
O
2
is an active specie contributing to
the anti-cancer ability of CAPs treated medium but is
not the only one.
ACKNOWLEDGEMENTS
This work as supported by Fundação para a Ciência e
Tecnologia (FCT), within Radiation Biology and
Biophysics Doctoral Training Programme (Rabbit,
PD/00193/2012), through the scholarship grant
number PD/BD/114444/2016 (S. Pereira), the project
UID/Multi/04378/2013 (UCIBIO) and the project
UID/FIS/00068/2019 (CEFITEC). The authors
acknowledge Professor Doutor Jorge Carvalho Silva
from Physics Department, FCT/UNL for the use of
TELab-Tissue Engineering Laboratory facilities
REFERENCES
Adachi, Tetsuo, Hiromasa Tanaka, Saho Nonomura,
Hirokazu Hara, Shin Ichi Kondo, and Masaru Hori.
2015. “Plasma-Activated Medium Induces A549 Cell
Injury via a Spiral Apoptotic Cascade Involving the
Mitochondrial-Nuclear Network.” Free Radical
Biology and Medicine.
Anoopkumar-Dukie, S., J. B. Carey, T. Conere, E.
O’Sullivan, F. N. van Pelt, and A. Allshire. 2005.
“Resazurin Assay of Radiation Response in Cultured
Cells.” The British Journal of Radiology 78(934):945–
47.
Bekeschus, S., J. Kolata, C. Winterbourn, A. Kramer, R.
Turner, K. D. Weltmann, B. Bröker, and K. Masur.
2014. “Hydrogen Peroxide: A Central Player in
Physical Plasma-Induced Oxidative Stress in Human
Blood Cells.” Free Radical Research.
0.59 1.17 2.34 4.69 9.38 18.75 37.5
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
Relative cell viability (a.u.)
H
2
O
2
concentration (
M)
Met-1
HGF-1
Biophotonics 2020 - Special Session on Biomedical Optics
206

Bekeschus, Sander, Kai Masur, Julia Kolata, Kristian
Wende, Anke Schmidt, Lena Bundscherer, Annemarie
Barton, Axel Kramer, Barbara Bröker, and Klaus-
Dieter Weltmann. 2013. “Human Mononuclear Cell
Survival and Proliferation Is Modulated by Cold
Atmospheric Plasma Jet.” Plasma Processes and
Polymers 10(8):706–13.
Bundscherer, Lena, Sander Bekeschus, Helena Tresp,
Sybille Hasse, Stephan Reuter, Klaus-Dieter
Weltmann, Ulrike Lindequist, and Kai Masur. 2013.
“Viability of Human Blood Leukocytes Compared with
Their Respective Cell Lines after Plasma Treatment.”
Plasma Medicine 3(1–2):71–80.
Chauvin, Julie, Florian Judée, Mohammed Yousfi, Patricia
Vicendo, and Nofel Merbahi. 2017. “Analysis of
Reactive Oxygen and Nitrogen Species Generated in
Three Liquid Media by Low Temperature Helium
Plasma Jet.” Scientific Reports.
Fridman, A., A. Chirokov, and A. Gutsol. 2005. “Non-
Thermal Atmospheric Pressure Discharges.” Journal of
Physics D: Applied Physics 38(2):R1–24.
Graham, Gloria F. and Sara Moradi Tuchayi. 2016.
“Squamous Cell Carcinoma.” in Dermatological
Cryosurgery and Cryotherapy.
Hoentsch, Maxi, René Bussiahn, Henrike Rebl, Claudia
Bergemann, Martin Eggert, Marcus Frank, Thomas
Von Woedtke, and Barbara Nebe. 2014. “Persistent
Effectivity of Gas Plasma-Treated, Long Time-Stored
Liquid on Epithelial Cell Adhesion Capacity and
Membrane Morphology.” PLoS ONE.
Jablonowski, Helena and Thomas von Woedtke. 2015.
“Rliquid Interaction: Wesearch on Plasma Medicine-
Relevant Plasma–Hat Happened in the Past Five
Years?” Clinical Plasma Medicine 3(2):42–52.
Keidar, M., R. Walk, A. Shashurin, P. Srinivasan, A.
Sandler, S. Dasgupta, R. Ravi, R. Guerrero-Preston,
and B. Trink. 2011. “Cold Plasma Selectivity and the
Possibility of a Paradigm Shift in Cancer Therapy.”
British Journal of Cancer 105(9):1295–1301.
Keidar, Michael, Alex Shashurin, Olga Volotskova, Mary
Ann Stepp, Priya Srinivasan, Anthony Sandler, and
Barry Trink. 2013. “Cold Atmospheric Plasma in
Cancer Therapy.” Physics of Plasmas 20(5):057101.
Liedtke, Kim Rouven, Sander Bekeschus, André Kaeding,
Christine Hackbarth, Jens-Peter Kuehn, Claus-Dieter
Heidecke, Wolfram von Bernstorff, Thomas von
Woedtke, and Lars Ivo Partecke. 2017. “Non-Thermal
Plasma-Treated Solution Demonstrates Antitumor
Activity against Pancreatic Cancer Cells in Vitro and in
Vivo.” Scientific Reports 7(1):8319.
Lukes, Petr and Bruce R. Locke. 2005. “Plasmachemical
Oxidation Processes in a Hybrid Gas-Liquid Electrical
Discharge Reactor.” Journal of Physics D: Applied
Physics.
Nakamura, Kae, Yang Peng, Fumi Utsumi, Hiromasa
Tanaka, Masaaki Mizuno, Shinya Toyokuni, Masaru
Hori, Fumitaka Kikkawa, and Hiroaki Kajiyama. 2017.
“Novel Intraperitoneal Treatment With Non-Thermal
Plasma-Activated Medium Inhibits Metastatic Potential
of Ovarian Cancer Cells.” Scientific Reports.
Pereira, S., E. Pinto, P. A. Ribeiro, and S. Sério. 2019.
“Study of a Cold Atmospheric Pressure Plasma Jet
Device for Indirect Treatment of Squamous Cell
Carcinoma.” Clinical Plasma Medicine 13(August
2018):9–14.
Pipa, A. V., S. Reuter, R. Foest, and K. D. Weltmann. 2012.
“Controlling the NO Production of an Atmospheric
Pressure Plasma Jet.” Journal of Physics D: Applied
Physics 45(8):085201.
Reuter, S., K. Niemi, V. Schulz-von der Gathen, and H. F.
Döbele. 2009. “Generation of Atomic Oxygen in the
Effluent of an Atmospheric Pressure Plasma Jet.”
Plasma Sources Science and Technology 18(1):015006.
Reuter, Stephan, Helena Tresp, Kristian Wende, Malte U.
Hammer, Jörn Winter, Kai Masur, Ansgar Schmidt-
Bleker, and Klaus-Dieter Weltmann. 2012. “From
RONS to ROS: Tailoring Plasma Jet Treatment of Skin
Cells.” IEEE Transactions on Plasma Science
40(11):2986–93.
Rhee, Sue Goo, Sang Won Kang, Woojin Jeong, Tong-Shin
Chang, Kap-Seok Yang, and Hyun Ae Woo. 2005.
“Intracellular Messenger Function of Hydrogen
Peroxide and Its Regulation by Peroxiredoxins.”
Current Opinion in Cell Biology 17(2):183–89.
Riss, Terry L., Richard A. Moravec, Andrew L. Niles,
Sarah Duellman, Hélène A. Benink, Tracy J. Worzella,
and Lisa Minor. 2004. Cell Viability Assays.
Rumbach, Paul, David M. Bartels, R. Mohan Sankaran,
and David B. Go. 2015. “The Solvation of Electrons
by an Atmospheric-Pressure Plasma.” Nature
Communications.
Sauer, Heinrich, Maria Wartenberg, and Juergen Hescheler.
2001. “Reactive Oxygen Species as Intracellular
Messengers During Cell Growth and Differentiation.”
Cellular Physiology and Biochemistry 11(4):173–86.
Tanaka, Hiromasa, Masaaki Mizuno, Kenji Ishikawa, Kae
Nakamura, Hiroaki Kajiyama, Hiroyuki Kano,
Fumitaka Kikkawa, and Masaru Hori. 2011. “Plasma-
Activated Medium Selectively Kills Glioblastoma
Brain Tumor Cells by down-Regulating a Survival
Signaling Molecule, AKT Kinase.” Plasma Medicine.
Waldman, Abigail and Chrysalyne Schmults. 2019.
“Cutaneous Squamous Cell Carcinoma.” Hematology/
Oncology Clinics of North America.
Weltmann, K. D. and Th Von Woedtke. 2017. “Plasma
Medicine - Current State of Research and Medical
Application.” Plasma Physics and Controlled Fusion.
Wende, K., S. Bekeschus, A. Schmidt, L. Jatsch, S. Hasse,
K. D. Weltmann, K. Masur, and T. von Woedtke. 2016.
“Risk Assessment of a Cold Argon Plasma Jet in
Respect to Its Mutagenicity.” Mutation Research -
Genetic Toxicology and Environmental Mutagenesis.
Wende, Kristian, Stephan Reuter, Thomas Von Woedtke,
Klaus Dieter Weltmann, and Kai Masur. 2014. “Redox-
Based Assay for Assessment of Biological Impact of
Plasma Treatment.” Plasma Processes and Polymers.
Wende, Kristian, Paul Williams, Joe Dalluge, Wouter Van
Gaens, Hamada Aboubakr, John Bischof, Thomas von
Woedtke, Sagar M. Goyal, Klaus-Dieter Weltmann,
Annemie Bogaerts, Kai Masur, and Peter J.
Optimization of a Cold Atmospheric Plasma Treatment to Selectively Affect the Viability of Skin Cancer Cells
207

Bruggeman. 2015. “Identification of the Biologically
Active Liquid Chemistry Induced by a Nonthermal
Atmospheric Pressure Plasma Jet.” Biointerphases.
Yan, Dayun, Haitao Cui, Wei Zhu, Niki Nourmohammadi,
Julian Milberg, Lijie G. Zhang, Jonathan H. Sherman,
and Michael Keidar. 2017. “The Specific
Vulnerabilities of Cancer Cells to the Cold
Atmospheric Plasma-Stimulated Solutions.” Scientific
Reports.
Yan, Dayun, Jonathan H. Sherman, Xiaoqian Cheng,
Edward Ratovitski, Jerome Canady, and Michael
Keidar. 2014. “Controlling Plasma Stimulated Media in
Cancer Treatment Application.” Applied Physics
Letters.
Yan, Dayun, Annie Talbot, Niki Nourmohammadi,
Xiaoqian Cheng, Jerome Canady, Jonathan Sherman,
and Michael Keidar. 2015. “Principles of Using Cold
Atmospheric Plasma Stimulated Media for Cancer
Treatment.” Scientific Reports 5(1):18339.
Biophotonics 2020 - Special Session on Biomedical Optics
208
