
The Influence of Variation of Electrical Current and Electroplating
Process Time on Coating Thickness, Glossiness and Adhesion of
Copper on Low Carbon Steel
Deny Hendra Cipta and Ismail Ramli
Heavy Equipment Engineering Study Program, Nunukan State Polytechnic, Nunukan, Indonesia
Keywords: Copper, Electroplating, Thickness, Glossiness, Adhesion.
Abstract: The electroplating process is widely used for various purposes, such as; decorative applications, improving
the base material properties such as wear resistance, electrical conductivity, corrosion resistance, and the
dimensions and geometry of a work piece. In this study, testing of the copper electroplating process on low
carbon steel with variations in current strength and processing time was carried out. Electric currents used are
5 Ampere, 10 Ampere, and 15 Ampere. The processing time is 20 seconds, 40 seconds, and 60 seconds. An
acidic copper solution was used as the electrolyte, with a nickel layer as the base coating layer. The plating
results were then tested for thickness, glossiness, and adhesion. Test results show the effects of electrical
current and time on coating quality. 5 Ampere current does not give a significant difference for the value of
thickness, glossiness, or adhesion. While the 15 Ampere current shows symptoms of a too-large current
density, such as dark color and low adhesion. The study shows that paired variations of 10 Ampere current
strength with 40 seconds process time gives the best results, with a thickness of 0.78 microns, a gloss value
of 46 percent, and a coating adhesion of 71.6 percent.
1 INTRODUCTION
The electroplating process is one of the finishing
processes that is often needed in the metalwork
process. Both in the metal industry and machinery
production. The final finishing process varies, some
are simply polished to make it smooth and shiny, can
also be coated with other metals with the aim of
improving the properties of the base metal, some are
painted or varnished or coated with ceramics.
Finishing is also preferred for metals that are easily
corroded. One common method to achieve this
purpose is throuh electroplating process (Kumar et
al., 2015; Purwanto and Huda, 2005).
Copper (Cu) is one of commonly substance used
for electroplating processing. This material is a good
electric and heat conductor. Copper is used as a base
coat because it can cover the surface of the material
being coated and has good leveling ability. A copper
base plating is required for further plating with
nickel which can then, be followed by a chrome,
brass, silver or gold plating. Its characteristic gives
the demanded final products properties. Copper
plating used on plastics, rotogravure rolls, printed
circuit board, semiconductor manufacturing and
also widely used as coating on steel wire to increase
conductivity (12%). Copper can be utilized as
leveling material in electrorefining and
electroforming. It has a decorative value if applied
on the surface and buffed. Coat of copper also can
act as an heat inhibitor in metal selective heat
treatment (Purwanto and Huda, 2005; Dini and
Snyder, 2020; Lowenheim, 1978).
To achieve the purposed uses, the electroplating
results need to have a certain level of quantity and
quality. To be used as an effective conductor
material the plated layer has to be at a certain
thickness. The surface glossiness of the end result is
highly demanded for decorative purposes and also
the layer adhesion strength to its substrate is
important when used as inhibitor in selected parts of
heat treated components. To get a good coating
quality, there are several influencing factors, such as
surface condition of coated base material, coating
anode quality, electrolyte quality, process
temperature, electrical current and process time.
These parameters must be studied to achieve
Cipta, D. and Ramli, I.
The Influence of Variation of Electrical Current and Electroplating Process Time on Coating Thickness, Glossiness and Adhesion of Copper on Low Carbon Steel.
DOI: 10.5220/0010941000003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 117-124
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
117

optimum results (Fotovvati et al., 2019; Aygar,
2009).
The modelling and optimization pf copper
electroplating adhesion strength was conducted by
Suryanto et al. (2017). The base material
investigated was stainless steel which coated with
copper through elctroplating with variation in the
copper content in the electrolyte and also variation
of the current density. The adhesion strength was
tested using Teer ST-30 tester. This study finds the
highest current density gives the highest adhesion
strength.
Margen et al. (2018) studied the effect of process
time of 60 seconds, 120 seconds, and 180 seconds
on electroplating nickel layer upon AISI 304
stainless steel and copper with an enhancement from
an ultrasonic batch. The findings of this work tell
that the longest process time gave the highest
thickness number and the conclusion is that the
electroplating process time has a direct relationship
to level of thickness formed on the research test
subject.
The effect of current density on hardness and
thickness properties of copper coating on low carbon
steel has been studied (Sudarsono et al., 2020) This
study varies the current density of 6, 9, and 12
Ampere. The result shows the highest current
density variables gave the thickestcoating layer and
also resulting the hardest copper layer on the test
specimen.
This study aims to understand the effect of some
variables against different aspects and needs from
copper coating quality at the same time. The layer
thickness relates to mechanical properties such as
wear-resistance and hardness. The glossiness value
is directly related to the function of copper coating
as a decorative value enhancer. The adhesion test is
needed to understand the effect of heat and sudden
temperature changes to the durability of coated
copper layer for instance in the use of copper coating
as a barrier in selective heat treatment. Process time
and electrical current are two of the main basic
controllable variables in electroplating process, thus
having a deep understanding about this parameters
would have a great impact on producing quality
coating layer.
2 RESEARCH METHODOLOGY
2.1 Research Method Flowchart
The research flowchart is as shown in Figure 1.
Figure 1: Research method flowchart.
2.2 Preparations
In this stage, activities planning is arranged and the
materials are prepared for the testing and research
activities stage.
2.2.1 Test Design
In this test, there are two variables, the current
strength and processing time. Where for the current
strength variation used is 5 Ampere, 10 Ampere and
15 Ampere. As for the variation of processing time,
the selected time is 20 seconds, 40 seconds and 60
seconds.The material to be coated is low carbon steel
with nickel ,and copper as plating materials.
2.2.2 Equipment
In this study, several equipment and tools were used
for the implementation of activities.
a) Electroplating Process Equipment
The equipment used in this electroplating process
are plate cutting tool, polishing machine, coating tub,
compressor, Ampere meter, rectifier
b) Testing Methods and Equipment
• Layer Thickness Test
The layer thickness test aims to determine the
thickness of the copper deposit layer on the surface of
the workpiece after the electroplating process. The
thickness test here uses the Inverted Metallurgical
Microscope.
• Glossiness Test
This test aims to determine the level of surface
glossiness of the test speciments, which is related to
the decorative value of the results of the
electroplating process. The glossiness test is carried
out using a Lux meter.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
118
• Adhesion Test
This test aims to perceive the adhesiveness
property of the copper electroplated layer on the
surface of substrate material. The test, named heat
quench test [lowenheim] where the testing factor is the
high temperature obtained by heating objects in an
electric furnace and then the speciment subsequently
quenched in water at room temperature. The heat and
sudden temperature changes will affect the strength of
adhesion of the electroplated copper layer to the base
material. The layer’s adhesion strength is measured by
differentiating the percentage of the test specimens
surface area left covered with the copper layer after
the heat quench process. The surface area is calculated
using a transparent millimeter block [3].
2.2.3 Materials
The coating material (anode) is copper while the
material to be coated (cathode) is a low carbon steel
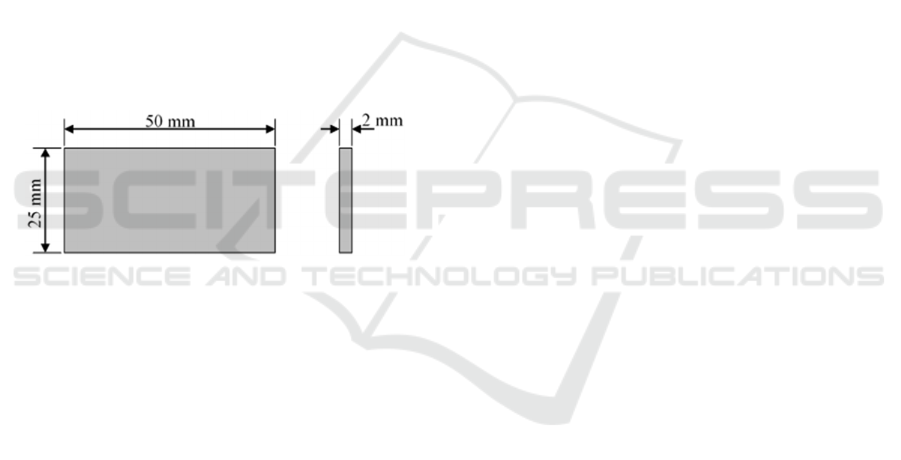
plate with a size: 50 mm long, 25 mm wide and 2 mm
thick. The dimensions of the test object are shown in
Figure 2.
Figure 2: Test object dimension.
2.2.4 Electrolyte
a) Composition of Nickel Solution
The composition of the solution in 1 litre of
distilled water:
• Nickel sulfate, NiSO4.6H2O = 240 - 300
gram/litre
• Nickel chloride, NiCl2.6H2O = 40 - 60
gram/litre
• Boric acid, H3BO3 = 25 - 40 gram/litre
• Brigthener Magnum SS =2.5 cm3/litre
• Brigthener Magnum AM = 2.5 cm3/litre
• pH 1.5− 4.5
b) Copper Solution Material
The composition of the solution in 1 litre of
distilled water:
• Acid copper, CuSO4 = 195 - 250 gram/litre
• Sulfuric acid, H2SO4 = 45 - 90 gram/litre
• Hydrochloric acid, HCl = 40 – 80 mL/litre
2.3 Method of Data Collection
The data collection for this research was carried out
by conducting experiments on the copper plating
process with different parameters of electrical current
strength and processing time, with the purpose of
understanding the effect of changing these parameters
on the thickness, gloss and adhesion of the copper
layer resulting from the electroplating process. The
results of the data tests are then compiled and
documented. Then an analysis of the interconnections
between the test results and the process parameters is
performed, to obtain conclusions about the research
conducted.
2.4 Electroplating Process
The electroplating process is carried out in several
stages, namely the preparation of electroplating
equpment, the preparation of electrolyte for the nickel
and copper coating, the preparation of the test object,
the nickel and copper electroplating process and the
final finishing process. The coating process is carried
out in 2 steps. The first step is nickel plating, to form
a base layer on the surface of the test object because
acidic copper cannot applied directly over ow carbon.
In the second step, the coating process with an acidic
copper solution. Nickel plating is carried out with the
same process parameters for all test objects, process
time for 10 minutes and 10 Ampere current strength.
The copper plating is performed with variation of
current strength are 5, 10 and 15 Ampere. Variation
in processing time are 20, 40 and 60 seconds.
2.5 Testing
2.5.1 Layer Thickness Test
This thickness test is carried out to determine the
thickness of coating layer after the nickel and chrome
plating process. This test uses a Metallurgical
Microscope.
2.5.2 Glossiness Test
The glossiness test was performed using a Lux meter.
This equipment works by measuring the strength of
light reflection from a source (electric lamp) by the
surface of the test object. As a benchmark of
comparison a mirror was used, assuming that it
reflects the light received by 100 (one hundred)
percent. The power of light is measured in Lumens.
The Influence of Variation of Electrical Current and Electroplating Process Time on Coating Thickness, Glossiness and Adhesion of Copper
on Low Carbon Steel
119
2.5.3 Adhesion Testing
This adhesion test uses a qualitative test with the heat-
quench test method. Where the test object reaches a
certain temperature and then cooled with water
immersion at room temperature. The heating
temperature based on the type of base material and
coating material [3]. In this study the test object is
heated in an electric furnace to a temperature of
250°C.
3 TEST RESULTS AND
DISCUSSION
The data presented in this section are the results
obtained from the research. Consists of the results of
calculations for the electroplating process, data from
the results of thickness test, glossiness test and coating
adhesion test.
3.1 Electroplating Result Data
This research was carried out with variations in the
large current and time of the coating process. In
addition, the weight of the test object is also carried
out before and before the coating process, which
determines the weight of the object at the time it
occurs. The results of the weighing are shown in
Table 1.
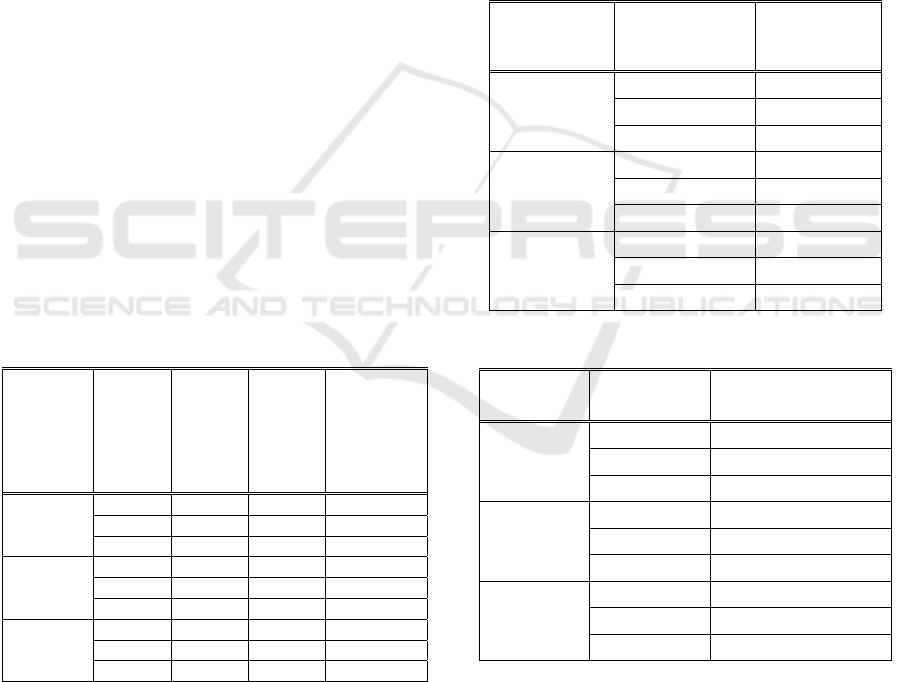
Table 1: Actual deposition weight.
Current
(Ampere)
Time
(second)
Initial
weight,
W
0
(gram)
Final
weight,
W
1
(gram)
Actual
deposition
weight,
W
a
= W
1
-
W
0
(gram)
5
20 23 23.02 0.02
40 23 23.02 0.02
60 23 23.05 0.05
10
20 23 23.04 0.04
40 23 23.04 0.04
60 23 23.06 0.06
15
20 23 23.04 0.04
40 23 23.06 0.06
60 23 23.05 0.05
3.2 Calculation of Electroplating
Results
In this section, theoretical calculations are carried out
for the copper electroplating process according to the
parameters of the research carried out.
3.2.1 Calculation of Theoretical Deposit
Weight
From Faraday's Law, the weight calculation at the
beginning of the coating process variable is carried
out. The results obtained are as in Table 2.
3.2.2 Current Efficiency Calculation
By obtaining data on weight at the time of storage and
theoretical storage, it is possible to calculate the
current efficiency of each electroplating process
carried out. Then the current efficiency data obtained
for the electroplating process in this study, which is
shown in Table 3.
Table 2: Theoretical weight.
Current
(Ampere)
Time
(second)
Theoretical
weight, W
t
(gram)
5
20 0,03
40 0,07
60 0,09
10
20 0,07
40 0,13
60 0,19
15
20 0,09
40 0,19
60 0,29
Table 3: Current efficiency.
Current
(Ampere)
Time
(second)
Eficiency
(%)
5
20 66, 67
40 28,57
60 71, 43
10
20 57, 14
40 30, 77
60 31, 16
15
20 44, 44
40 31, 15
60 17, 24
3.2.3 Current Density Calculation
By using Faraday's Law, it is possible to find the large
density that occurs in each coating process variable.
Where the amount of current used is divided by the
surface area of the workpiece. So that separate results
are obtained which are shown in Table 4.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
120

Table 4: Electroplating process current density.
Current
(Ampere)
Surface area
(dm
2
)
Current density
(A/dm
2
)
5 0,28 17,81
10 0,28 35,71
15 0,28 53,57
3.3 Thickness Test Results
Observation of the layer is done visually with the aid
of a microscope. Measurements are made by
comparing the thickness with the scale lines already
on the ocular lens. Measurements are made at 3 points
on each test variables combination, then the
calculated average is taken as the thickness value of
the coresponding test object. The images from the
observations on the microscope is then photographed,
which is shown in Figure 3.
a.) 5 A and 20 seconds, (b.) 5 A and 40 seconds, (c.) 5 A
and 60 seconds, (d.) 10 A and 20 seconds, (e.) 10 A and 40
seconds, (f.) 10 A and 60, (g.) 15 A and 20 seconds, (h.) 15
A and 40 seconds, (i.) 15 A and 60 seconds
Figure 3: Thickness test result images.
Figure 4: Process time vs layer thickness graph.
The thickness value of each test object as shown
in Table 5. From the results of the tests carried out, it
can be seen from the strong influence of the current
coating process. Figure 4 showing the relationship
between current and process, where it can be seen that
the highest thickness is achieved in the 10 Ampere
process with a time of 60 seconds, although at the
same current, 10 Ampere with a time of 20 seconds
and 40 seconds the layer formed is thinner. While in
the process with a current of 5 Ampere there is no
significant difference in layer thickness. This is
because the current density that occurs is not
sufficient to move the copper electrons to move and
increase the pairing effectively.
Table 5: Layer thickness.
Current
(Ampere)
Time
(second)
Thickness
(μm)
Average
thickness
(μm)
point 1 point 2 point 3
5
20 0.63 0.31 0.31 0.42
40 0.31 0.47 0.47 0.42
60 0.63 0.16 0.47 0.42
10
20 0.16 0.63 0.16 0.32
40 0.31 0.31 0.31 0.31
60 0.31 0.31 0.16 0.26
15
20 0.31 0.63 0.63 0.52
40 0.31 0.63 0.31 0.42
60 0.31 0.31 0.31 0.31
3.4 Glossiness Test Results
The degree of glossiness relates to the decorative
function of a coating. The higher the gloss of the
coating, the better the quality of the coating will be.
This test aims to determine the effect of the current
and time of the coating process on the resulting
copper layer. So it is expected to know the best
parameters for maximum coating quality. Lux meter
scale with mirror (for comparison) is 56 lumens,
The Influence of Variation of Electrical Current and Electroplating Process Time on Coating Thickness, Glossiness and Adhesion of Copper
on Low Carbon Steel
121

which the higher the lumens number means the
surface is more bright. The comparison graph of the
glossiness test data for each coating process variable
is shown in Figure 5.
Figure 5: Process time vs glossiness graph.
From Figure 5 it can be seen that the maximum
gloss level is obtained in the coating process with a
current of 5 Ampere and a time of 40 seconds. This is
sufficient because the copper layer formed is still thin,
so the level of gloss is affected by the nickel layer
used as the base layer. Where visually the nickel layer
has a higher gloss than the copper layer. In the coating
process with a strong current of 15 Ampere, the
current density is too high which causes heat that
burns the workpiece so that the layer ages or gets
darker. This causes the level of gloss to decrease. In
addition, gloss is also influenced by several factors,
such as: the level of cleanliness and accuracy of the
initial processing, stirring of the electrolyte during the
process, the content of contaminants in the
electrolyte, the purity of the anode and the influence
of additives.
3.5 Adhesion Test Results
The adhesion strength data was obtained from the
calculation of the surface area of the copper layer that
was still left after the heat-quench test process. Where
the surface area of the object being observed is one of
the widest sides. The dimensions of the test object
are; length = 42 mm, width = 25 mm which give the
surface area = 1.050 square mm.
From the tests carried out, the area of the copper
layer that is still left on the surface of the workpiece
and the percentage of copper layer left is shown in
Table 6.
The graph of the comparison of the data from the
adhesion test results to the coating process time is
shown in Figure 6. From the test results, it is known
that the best adhesion is obtained under coating
conditions with a strong current of 10 Ampere for 40
seconds. One of the reasons for this is because the
Table 6: Remaining coating layer area.
Current
(Ampere)
Time
(second)
Remaining layer
area
(mm
2
)
Adhesion
percentage
(%)
5
20 41 3,9
40 97 9,2
60 36 3,4
10
20 120 11,4
40 750 71,6
60 49 46,7
15
20 525 50
40 90 8,6
60 40 3,9
current density at these conditions is good, resulting
in good adhesion. In the current condition of 5
Ampere and 15 Ampere there is a drastic difference
in adhesive power, both of which are related to the
process current density. It's just that at the condition
of 5 Ampere the current density meets the needs of an
ideal coating process, while at the condition of 15
Ampere the excessive current density results in
saturation of the layer, so that even though it has a
good thickness, the adhesion is low. In addition,
related to the level of glossiness, the pre-treatment
process also affects the adhesion of the coating.
Figure 6: Process time vs adhesion percentage graph.
3.6 Discussion of the Relationship
between Test Results
For the coating process with a current density of 5
Ampere, the layer formed is thin, the adhesion is low
as well as the gloss value. The test results obtained for
each process time also do not show significant
differences. From this it is known that the
electroplating process of copper with a current of 5
Ampere is less effective. Due to the low current, the
covering power of the coating anode is not good
which results in a thin layer and poor adhesion.
Layers that have not been formed properly also affect
the glossiness, so that in this test the gloss value is
lower than the process with other conditions.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
122

In the coating with a current of 10 Ampere, at the
processing time of 20 seconds and 40 seconds, the
layer formed is thinner, although then a layer of
processing time of 60 seconds occurs. If with the
value of gloss and adhesion, the coating process
carried out under these conditions gives the maximum
value, compared to the coating results under other
conditions. It can be concluded that at a current of 10
Ampere it takes longer time to increase the thickness,
the time required for the coating metal to cover the
surface of the workpiece and then develop to form a
layer of coating. With current density in this
condition, will give coating results with good
glossiness and adhesion strength.
In the coating process with a current of 15
Ampere, it can be seen the longer the time the thinner
the layer, as well as the bonding of the layer. This is
due to the large current density which forces the
electrons to move faster than needed, resulting in a
layer on the workpiece, while the growth of the layer
is not perfect which affects the adhesion of the
coating. For a time of 20 seconds, it gets bigger
because the large current affects the formation of the
layer. But as previously explained, the longer the
processing time, the less effective it will be and the
less the coating layer. Large currents also cause
excess heat which causes a decrease in aged colors
and the level of glossiness of the coating. It is very
important to consider the surface area of the
workpiece with the large current being used.
From the discussion carried out, to obtain copper
electroplating results with maximum quality under
existing constraints, 10 Ampere is used with a plating
time of 40 to 60 seconds.
4 CONCLUSIONS AND
SUGGESTIONS
In this the work the study of effect from electrical
current and process time variations in copper
electroplating were carried out. The wide application
of copper electroplating with different quality
characteristic needs, demand a deep understanding
about the process variables. In the limitation of this
study, it can be concluded that the greater current
gave the largest layer deposited. For the level of
glossiness and adhesiveness of the coating, the best
results were obtained under coating conditions with a
current of 10 Ampere for 40 seconds. This shows that
large currents and longer times does not always
provide the best coating quality. Current strength is
related to the surface area of the workpiece which
gives the value of the process current density. Where
in this study, the surface area of the test object is
relatively small so that large currents actually give a
poor final result. Therefore, it is necessary to pay
attention to the surface area of the workpiece to
determine the current strength used. To better
understand the electroplating process, especially with
copper coatings, other studies can be carried out.
Research on other process variables is encouraged,
such as about current density which relates to the
dimension of the object to plated, uses of additives in
the electrolyte, agitation and different electrolyte
bath.
ACKNOWLEDGEMENTS
There are many obstacles in completing this research,
and this work would not have been possible without
the support of several parties. Authors would like to
thank Director of the Nunukan State Polytechnic who
has provided support for completing this research.
Authors also want to thank family and colleagues
who have always supported in completing this
research.
REFERENCES
Purwanto, Huda, S. (2005). Electroplating industry
technology, Badan Penerbit Universitas Diponegoro,
Semarang. pp.7-10.
Kumar, S., Pande, S., Verma, P. (2015). Factor effecting
electro-deposition process. In International Journal of
Current Engineering and Technology, Vol.5, No.2
(April 2015), pp. 700-703. Inpressco.
Dini, J.W., Snyder D.D. Electrodeposition of copper. In
Modern Electroplating, 5
th
Edition, M. Schlesinger and
M. Paunovic, Eds. New Jersey: John Wiley & Sons, Inc,
2010, pp. 33-78.
Lowenheim, F.A. (1978) Electroplating. New York:
McGraw-Hill Book Company, pp.496.
Ivshin Y.V., Shaikhutdinova F.N., Sysoev,V.A (2018).
Electrodeposition of Copper on Mild Steel: Peculiarities
of the Process. Surface Engineering and Applied
Electrochemistry, 2018, Vol. 54, No. 5, pp. 452–458.
Allerton Press, Inc.
Fotovvati, B., Namdari, N., Dehghanghadikolaei, A. (2019)
On coating techniques for surface protection: a review.
In Journal of Manufacturing and Material Processing 3,
2019, No.1:28.
Aygar, A. M. (2009). Investigation on the factors that affect
the amount of metal coated in an electroplating process.
Retrieved from core.ac.uk: https://core.ac.uk/download/
pdf/11725439.pdf
The Influence of Variation of Electrical Current and Electroplating Process Time on Coating Thickness, Glossiness and Adhesion of Copper
on Low Carbon Steel
123

Suryanto, Haider, F. I., Ani, M. H., & Mahmood, M. (2017).
Modelling and Optimization of Copper Electroplating
Adhesion Strength. In IOP Conference Series:
Materials Science and Engineering 204 012017. IOP
Publisihing Ltd.
Margen, S. Y., Sulistyo, S., Nugroho, S., & Nugroho, Y. S.
(2018). Enhancement Surface Coating Stainless Steel
And Copper Using Ultrasonic Batch. MATEC Web of
Conferences Volume 159. EDP Sciences.
Sudarsono, Aminur, Nurjannah, I., Hidayat, & Othman, R.
(2020). Effect of Current Density on Hardness of Low
Carbon Steel Electroplated by Copper, Nickel and
Copper-Nickel. IOP Conference Series: Materials
Science and Engineering, Volume 797. IOP Publishing
Ltd.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
124
