
Preliminary Study of the Characteristics of Nipah Fruit Bioadsorbent
as Greenhouse Gas Adsorption
Dodi Satriawan
1a
, Fadhillah Hazrina
2
and Agus Santoso
3
1
Department of Environmental Pollution Control Engineering, Cilacap State Polytechnic, Cilacap, Indonesia
2
Department of Electrical Engineering, Cilacap State Polytechnic, Cilacap, Indonesia
3
Department of Mechanical Engineering, Cilacap State Polytechnic, Cilacap, Indonesia
Keywords: Nipah Fruit, Bioadsorbent, Adsorption, SNI 06-3730-1995.
Abstract: This research is a preliminary study that aims to determine the bioadsorbent characteristics of nipah fruit
based on SNI 06-3730-1995. Nipah fruit bioadsorbent that is made will later be applied as a medium for
absorbing pollutants from greenhouse gas emissions. Nipah fruit obtained from Kampung Laut, Cilacap
Regency, Central Java, was carbonized at a temperature of 500
o
C for 4 hours. The charcoal obtained is then
pulverized to a size of 50, 100, and 150 mesh. The activation process is carried out using 0% KOH; 2.5%;
5%; and 7.5%. The results obtained in the form of water content and iodine absorption that have met the
quality requirements of SNI 06-3730-1995. However, the ash content and volatile matter content did not meet
the quality requirements of SNI 06-3730-1995. This is because the nipah fruit bioadsorbent has high metal
oxides and organic compounds.
1 INTRODUCTION
Vehicles powered by fossil fuels have altered the side
of the earth over the last few century. The arrival of
these vehicles covered the way for industrialization
though we have been grappling with their ill effects
for a decade. Hydrogen is the neatest fuel known to
people and advances in hydrogen technology are
expected to impact transportation and energy
markets. In addition, greenhouse gases (GHGs)
produced from exhaust fumes from automobiles and
industrial factories are a major cause of global
warming and further harmful environmental impacts
(Mukherjee et al., 2019). The rejection of the global
warming phenomenon by world leaders underscores
the task of scientists in addressing this man-made
catastrophe.
Adsorption of greenhouse gases for instance
carbon dioxide and methane is one of the useful
methods to reduce these effects. In contrast, burning
fossil fuels produces greenhouse gases (GHG) and
dangerous gases such as CO
2
, H
2
S, CH
4
, NO
2
for the
environment, which are improving in line with recent
energy needs for rapid economic improvement
(Mukherjee et al., 2019). Consequently, it is highly
a
https://orcid.org/0000-0001-7064-8484
desired to find a cheap and environmentally
responsive adsorbent to remove pollutants from air.
Many techniques can be used to reduce
greenhouse gas emissions such as filtration,
oxidation, reverse osmosis, flocculation, aerobic,
anaerobic, magnetic separation and adsorption. Of all
the techniques, adsorption is one of common methods
for removing contaminants from wastewater and air
pollution because it is inexpensive, easy to use,
environmentally responsive, harmless to health and
non-harmful (Wickramaratne & Jaroniec, 2013). The
adding of bioadsorbent in the adsorption practice
helps remove various contaminants and carcinogenic
composites such as metallic, drugs and non-metal
pollutants, colorants, and even taste then odor from
aqueous solutions (Idrees et al., 2018).
Compared to other adsorbents such as zeolites,
polymers and clays, activated carbon takes improve
performance and firmness in conditions of adsorption
(Narkiewicz & Morawski, 2016). Recently, the
adsorption of pollutant gases by bioadsorbent has
been established as a capable technology for the
mechanism of attraction among pollutants and
superficial functional groups (Xu et al., 2014).
Bioadsorbent making from biomass can be realized
Satriawan, D., Hazrina, F. and Santoso, A.
Preliminary Study of the Characteristics of Nipah Fruit Bioadsorbent as Greenhouse Gas Adsorption.
DOI: 10.5220/0011723900003575
In Proceedings of the 5th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2022), pages 127-131
ISBN: 978-989-758-619-4; ISSN: 2975-8246
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
127
as an advantage in two aspects, firstly it can avoid
CO
2
production by sequestering carbon and secondly
bioadsorbent can enter the soil naturally (Alabadi et
al., 2015).
Bioadsorbent has more benefits other adsorbents
due to its high thermal constancy and low feedstock
costs. Bioadsorbent can be produced from some of
materials, such as coal, manufacturing by-products,
and wood or more biomass sources (Jia et al., 2020).
Bioadsorbent is generally produced by chemical and
physical activation. Physical activation is commonly
undertake with carbon dioxide, air steam, or mixtures
thereof. Chemical activation requires several agents
such as HCl, Ca(CO)
2
, ZnCl
2
, H
2
SO
4
, KOH, HNO
3
,
and K
2
CO
3
(Erawati & Fernando, 2018; Hendrawan
et al., 2017; Hui & Zaini, 2015; Pallarés et al., 2016;
Satriawan et al., 2021). Bioadsorbent adsorption
performance hinge on the structure of pore and
properties of surface. Bioadsorbent achieved by
activation of chemical often have large surface of
areas and well-developed of micropores, making
them desirable materials for carbon dioxide (CO
2
)
adsorption (Heidari et al., 2014).
This study aims to examine the characteristics of
bioadsorbents made from nipah fruit biomass with
variations in size and chemical activation. The
characteristics of the bioadsorbent in the form of
adsorption of iodine content, volatile matter content,
moisture content, and ash content were carried out to
see whether the bioadsorbent of nipah fruit activated
with KOH had complied with SNI 06-3730-95, 1995
regarding technically activated charcoal. This
bioadsorbent will be used for the adsorption of air and
water pollutants in future research.
2 METHODOLOGY
This research was conducted in the Environmental
Pollution Control Engineering study program,
Cilacap State Polytechnic. The raw material for
bioadsorbent comes from the biomass of nipah fruit
obtained in Kampung Laut, Cilacap Regency. Nipah
fruit is split into four parts which are then dried in the
sun. The pyrolysis process was carried out at 500
o
C
for 4 hours to get nipah fruit charcoal. Nipah fruit
charcoal was then mashed with variations of 50 mesh,
100 mesh, and 150 mesh.
The refined charcoal was then activated using
KOH with various concentrations of 0%: 2.5%; 5%;
and 7.5%. Activation of nipah fruit charcoal was
carried out by heating at 70
o
C for 2 hours with 300
rpm stirring. Activation of nipah fruit charcoal is then
left for 24 hours after the heating process.
The filtering process was carried out using filter
paper and the obtained bioadsorbent was then
neutralized with hot distilled water to a neutral pH
(6.5 - 7.5). The neutralized bioadsorbent is then
placed in the oven to remove the water content. The
process of bioadsorbent analysis in the form of iodine
analysis, volatile matter content, ash content, water
content refers to the method of SNI 06-3730-95,
1995.
3 RESULTS AND DISCUSSION
3.1 Moisture Content
Analysis of the water content of the bioadsorbent
refers to SNI 06-3730-95, 1995 regarding technically
activated charcoal. 1 gram of bioadsorbent was put
into the oven at 115
o
C for 3 hours. The bioadsorbent
that has been baked is then put into a desiccator and
weighed with an analytical balance. The difference
between the initial and final weight reduction is the
water content that evaporates in the bioadsorbent. The
results of the analysis of the water content of the
bioadsorbent made from nipah fruit are presented in
figure 1.
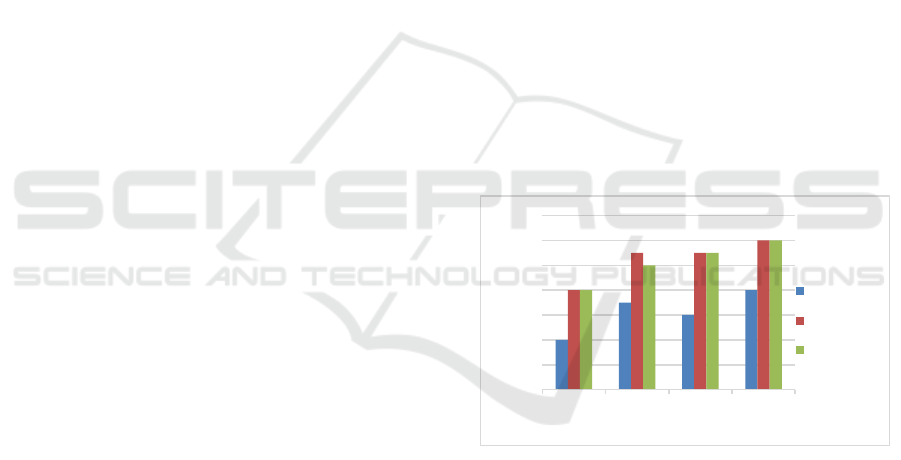
Figure 1: The effect of KOH concentration on the water
content of nipah fruit bioadsorbent.
The purpose of analyzing the water content of this
nipah fruit bioadsorbent is to determine and analyze
the hygrascopic properties of rice husk charcoal.
Satriawan et al., (2021) stated that the hydroscopic
nature of the bioadsorbent or activated carbon shows
the ability of the bioadsorbent to absorb water vapor
in the air when it is in the cooling process. In addition,
this hydroscopic nature shows the percentage of water
vapor that is still absorbed in the carbonization
process of the bioadsorbent (Husin & Hasibuan,
2020). Based on SNI 06-3730-95, 1995, the water
content that meets the quality requirements of
activated charcoal is a maximum of 15%. This
2
3,5
3
4
4
5,5
5,5
6
4
5
5,5
6
0
1
2
3
4
5
6
7
0,0% 2,5% 5,0% 7,5%
Water content (%)
KOH concentration (%)
50 mesh
100 mesh
150 mesh
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
128
maximum level indicates the ability of dry activated
charcoal to absorb moisture in the air. Figure 3.1
shows that all nipah fruit bioadsorbents have met the
standard of SNI 06-3730-95, 1995 with a maximum
moisture content of 15%.
3.2 Ash Content
Analysis of ash content of bioadsorbent made from
nipah fruit refers to SNI 06-3730-95, 1995 regarding
technically activated charcoal. 2.5 grams of
bioadsorbent was weighed and put into porcelain. The
bioadsorbent was then put into the furnace for 1 hour
at a temperature of 600
o
C. This temperature was used
to ash the test sample for the nipah bioadsorbent.
After one hour, the temperature of the furnace was
raised to 900
o
C for 2 hours. The bioadsorbent that
has been in the furnace is then put into a sedikator and
weighed. The results of the analysis of the ash content
of the bioadsorbent made from nipah fruit are
presented in figure 2.
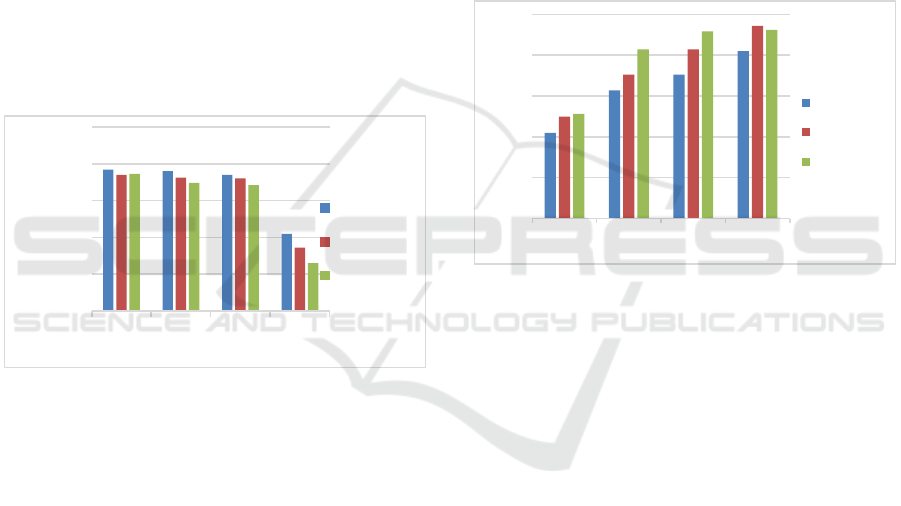
Figure 2: The effect of KOH concentration on ash content
of nipah fruit bioadsorbent.
The ash content indicates the weight of metal
oxides contained in the nipah fruit bioadsorbent. This
metal oxide is a mineral that has an influence on the
quality of the bioadsorbent adsorption made
(Satriawan et al., 2021). High ash content indicates
the amount of metal oxides contained in the
bioadsorbent. This metal oxide will cause blockage of
the pores of the nipah bioadsorbent so that the
adsorption process that occurs cannot be optimal
(Suherman et al., 2021). Based on SNI 06-3730-95,
1995, the requirement for ash content that meets the
standard of activated carbon or bioadsorbent is a
maximum of 10%. Figure 3.2 shows that the nipah
fruit bioadsorbent does not meet the ash content
standard based on SNI 06-3730-95, 1995. The ash
content of unactivated nipah bioadsorbent was 81.5 -
85.5%; while the ash content of activated nipah fruit
reaches >90%. From these data it can be shown that
the addition of KOH activation can increase the ash
content of the nipah fruit bioadsorbent.
3.3 Volatile Matter Content
Analysis of volatile substances or missing parts in
bioadsorbents made from nipah fruit refers to SNI 06-
3730-95, 1995 regarding technically activated
charcoal. Nipah fruit bioadsorbent was weighed as
much as 1.5 g and put into porcelain. The
bioadsorbent is then put into the furnace at a
temperature of 950
o
C. After the temperature reaches
950
o
C, the furnace is then cooled. The bioadsorbent
is then put into a desiccator and weighed. The results
of the analysis of volatile substances are presented in
figure 3.
Figure 3: The effect of KOH concentration on the level of
volatile substances of nipah fruit bioadsorbent.
The analysis of volatile substances or the missing
part of the bioadsorbent aims to find out how many
organic compounds are bound to the nipah fruit
bioadsorbent made (Erawati & Fernando, 2018).
Based on SNI 06-3730-95, 1995, the content of the
volatile substance or part lost in the bioadsorbent is a
maximum of 25%. Figure 3.3 shows the value of the
volatile content of the nipah fruit bioadsorbent has
exceeded the SNI limit for the volatile substance
content that has been set up to >70%. This indicates
that the nipah fruit bioadsorbent has high organic
compounds in the form of methanol, acetic acid
vapor, tar, and hydrocarbons (Rezvani et al., 2019).
3.4 Iodine Absorption Analysis
Analysis of iodine absorption in bioadsorbent made
from nipah fruit also refers to SNI 06-3730-95, 1995
regarding technically activated charcoal. The nipah
fruit bioadsorbent sample was first oven-dried at 115
o
C for 1 hour. The bioadsorbent that has been baked
in the oven is then cooled in a desiccator. Weigh 0.5
g of nipah bioadsorbent and add 50 ml of 0.1 N iodine
94,2
94
93,5
85,5
93,5
93,1
93
83,6
93,6
92,4
92,1
81,5
75
80
85
90
95
100
0,0% 2,5% 5,0% 7,5%
KadarAbu(%)
KOHconcentration(%)
50mesh
100mesh
150mesh
70,5
75,7
77,6
80,5
72,5
77,6
80,7
83,6
72,8
80,7
82,9
83,1
60
65
70
75
80
85
0,0% 2,5% 5,0% 7,5%
Volatile Matter Content (%)
KOH concentration (%)
50 mesh
100 mesh
150 mesh
Preliminary Study of the Characteristics of Nipah Fruit Bioadsorbent as Greenhouse Gas Adsorption
129
solution. Stirring was carried out for 15 minutes and
then filtered. Take 10 ml of the obtained filtrate and
titrate with 0.1 N sodium thio-sulphate. Titrate until a
faint yellow color. Added 1% starch solution and
titrate again with 0.1 N sodium thio-sulfate until the
blue color disappears. The results of the analysis of
iodine absorption are presented in figure 4.
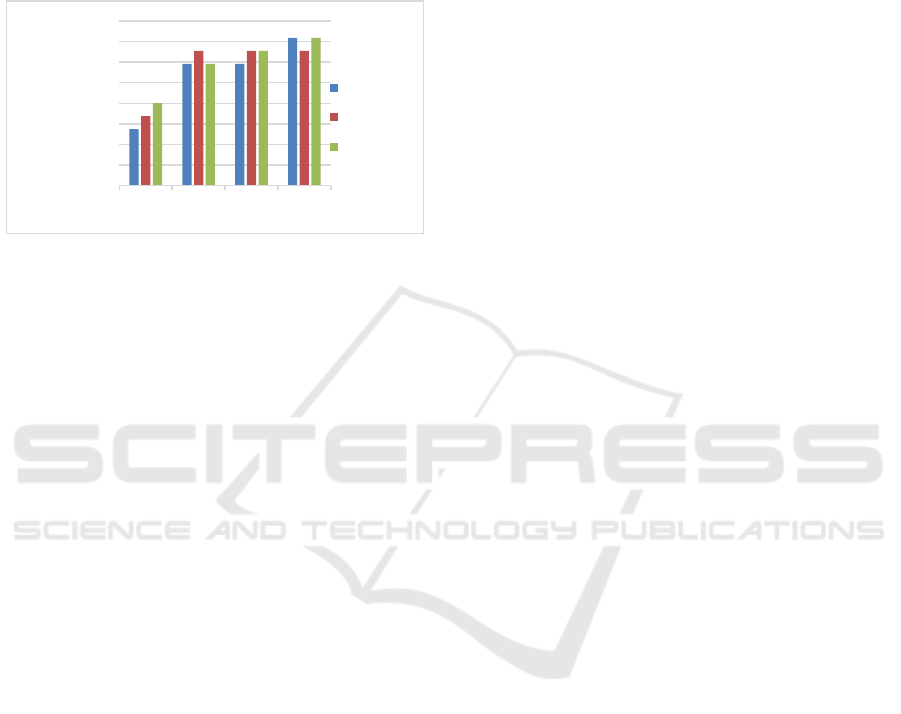
Figure 4: Effect of KOH concentration on iodine absorption
in nipah fruit bioadsorbent.
Iodine absorption analysis aims to determine the
ability of nipah fruit bioadsorbent in absorbing
pollutants. Based on SNI 06-3730-95, 1995, the
bioadsorbent quality requirement based on iodine
absorption is at least 750 mg/g. The higher the value
of iodine absorption, the better the ability of the
bioadsorbent to absorb pollutants. Figure 3.4 shows
the results of the analysis of iodine absorption in the
nipah fruit bioadsorbent. Iodine absorption in nipah
fruit bioadsorbent has met the quality requirements of
SNI 06-3730-95, 1995, namely >750 mg/g. Iodine
absorption in nipah fruit bioadsorbent without
activation also has a good value for iodine absorption,
namely 1154.79 - 1180.17 mg/g. This is because the
nipa fruit bioadsorbent has been physically activated
by using heating during the pyrolysis process
(carbonization) so that the nipa fruit bioadsorbent is
physically activated (Ogungbenro et al., 2018).
4 CONCLUSIONS
Nipah fruit bioadsorbent has met the quality
requirements of activated charcoal based on SNI 06-
3730-1995 for water content and iodine absorption.
However, nipah fruit bioadsorbent did not meet the
quality requirements for activated charcoal based on
SNI 06-3730-95, 1995 based on analysis of ash and
volatile matter content. This is because the nipah fruit
bioadsorbent has high metal oxides and organic
compounds. These high metal oxides and organic
compounds can reduce the effectiveness of nipah
bioadsorbent adsorption.
ACKNOWLEDGEMENTS
Thank you to the Directorate General of Vocational,
Ministry of Education, Culture, Research and
Technology for providing research grants to
researchers so that this research can be carried out
smoothly.
REFERENCES
Alabadi, A., Razzaque, S., Yang, Y., Chen, S., & Tan, B.
(2015). Highly porous activated carbon materials from
carbonized biomass with high CO2 capturing capacity.
Chemical Engineering Journal, 281, 606–612.
https://doi.org/10.1016/j.cej.2015.06.032
Erawati, E., & Fernando, A. (2018). Pengaruh Jenis
Aktivator Dan Ukuran Karbon Aktif Terhadap
Pembuatan Adsorbent Dari Serbik Gergaji Kayu
Sengon (Paraserianthes Falcataria). Jurnal Integrasi
Proses, 7(2), 58–66. https://doi.org/10.36055/
jip.v7i2.3808
Heidari, A., Younesi, H., Rashidi, A., & Ghoreyshi, A. A.
(2014). Evaluation of CO2 adsorption with eucalyptus
wood based activated carbon modified by ammonia
solution through heat treatment. Chemical Engineering
Journal, 254, 503–513. https://doi.org/10.1016/
j.cej.2014.06.004
Hendrawan, Y., Sutan, S. M., & Kreative, R. Y. R. (2017).
Pengaruh Variasi Suhu Karbonisasi dan Konsentrasi
Aktivator terhadap Karakteristik Karbon Aktif dari
Ampas Tebu (Bagasse) Menggunakan Activating
Agent NaCl. Jurnal Keteknikan Pertanian Tropis Dan
Biosistem, 5(3), 200–207. https://jkptb.ub.ac.id/index.
php/jkptb/article/view/420
Hui, T. S., & Zaini, M. A. A. (2015). Potassium hydroxide
activation of activated carbon: A commentary. Journal
Carbon Letters, 16(4), 275–280. https://doi.org/10.
5714/CL.2015.16.4.275
Husin, A., & Hasibuan, A. (2020). Studi Pengaruh Variasi
Konsentrasi Asam Posfat (H3PO4) dan Waktu
Perendaman Karbon terhadap Karakteristik Karbon
Aktif dari Kulit Durian. Jurnal Teknik Kimia USU,
9(2), 80–86. https://doi.org/10.32734/jtk.v9i2.3728
Idrees, M., Rangari, V., & Jeelani, S. (2018). Sustainable
packaging waste-derived activated carbon for carbon
dioxide capture. Journal of CO2 Utilization Journal,
26(May), 380–387. https://doi.org/10.1016/j.jcou.
2018.05.016
Jia, L., Shi, J., Long, C., Lian, F., & Xing, B. (2020).
Science of the Total Environment VOCs adsorption on
activated carbon with initial water vapor contents :
Adsorption mechanism and modi fi ed characteristic
curves. Science of the Total Environment, 731, 139184.
https://doi.org/10.1016/j.scitotenv.2020.139184
Mukherjee, A., Okolie, J. A., Abdelrasoul, A., Niu, C., &
Dalai, A. K. (2019). ScienceDirect Review of post-
combustion carbon dioxide capture technologies using
1154,79
1218,24
1218,24
1243,62
1167,48
1230,93
1230,93
1230,93
1180,17
1218,24
1230,93
1243,62
1100,00
1120,00
1140,00
1160,00
1180,00
1200,00
1220,00
1240,00
1260,00
0,0% 2,5% 5,0% 7,5%
Iodine absorption analysis
(mg/g)
KOH concentration (%)
50 mesh
100 mesh
150 mesh
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
130

activated carbon. Journal of Environmental Sciences,
83, 46–63. https://doi.org/10.1016/j.jes.2019.03.014
Narkiewicz, U., & Morawski, A. W. (2016). Modification
of Commercial Activated Carbons for CO2 Adsorption.
Vol. 129 (2016) ACTA PHYSICA POLONICA A,
129(3), 394–401. https://doi.org/10.12693/APhysPolA.
129.394
Ogungbenro, A. E., Quang, D. V., Al-Ali, K. A., Vega, L.
F., & Abu-Zahra, M. R. M. (2018). Physical synthesis
and characterization of activated carbon from date
seeds for CO2 capture. Journal of Environmental
Chemical Engineering, 6(4), 4245–4252. https://
doi.org/10.1016/j.jece.2018.06.030
Pallarés, J., González-Cencerrado, A., & Arauzo, I. (2016).
Production and characterization of activated carbon
from barley straw by physical activation with carbon
dioxide and steam (Issue July).
Rezvani, H., Fatemi, S., & Tamnanloo, J. (2019). Activated
carbon surface modification by catalytic chemical
vapor deposition of natural gas for enhancing
adsorption of greenhouse gases. Journal of
Environmental Chemical Engineering, 7(3), 1–10.
https://doi.org/10.1016/j.jece.2019.103085
Satriawan, D., Santoso, A., & Widianingsih, B. (2021).
Analisis Kuantitatif Pengaruh Waktu Karbonisasi Dan
Kosentrasi Koh Pada Pembuatan Karbon Aktif Sekam
Padi. Seminar Masional Terapan Riset Inovatif
(SENTRINOVE), 6(2), 139–146.
SNI 06-3730-95. (1995). Arang Aktif Teknis. In Badan
Standardisasi Nasional - BSN.
Suherman, Hasanah, M., Ariandi, R., & Ilmi. (2021).
PENGARUH SUHU AKTIVASI TERHADAP
KARAKTERISTIK DAN MIKROSTRUKTUR
KARBON AKTIF PELEPAH KELAPA SAWIT (Elaeis
guinensis) The Effect of Activation Temperature on The
Characteristics and Microstructure of Active Carbon
From Palm Oil Fronds (Elaeis guinensis). Jurnal Industri
Hasil Perkebunan, 16(1), 1–9.
Wickramaratne, N. P., & Jaroniec, M. (2013). Activated
carbon spheres for CO2 adsorption. ACS Applied
Materials and Interfaces, 5(5), 1849–1855.
https://doi.org/10.1021/am400112m
Xu, J., Chen, L., Qu, H., Jiao, Y., Xie, J., & Xing, G. (2014).
Preparation and characterization of activated carbon
from reedy grass leaves by chemical activation with H
3 PO 4. Applied Surface Science, 320, 674–680.
https://doi.org/10.1016/j.apsusc.2014.08.178.
Preliminary Study of the Characteristics of Nipah Fruit Bioadsorbent as Greenhouse Gas Adsorption
131
