
Heterogeneous Catalyst of Oxidative Desulfurization for Reducing
Sulfur Content in Indonesia Biosolar
Yandy Yandy, Nurul Fitri Widyasari, Tiffany Berliana and Mohammad Nasikin
Universitas Indonesia, Margonda Raya, Depok, Indonesia
Keywords: Biosolar, Oxidative Desulfurization, Sulfur Content.
Abstract: Sulfur content in Indonesian diesel fuel is still very high, so it needs to be reduced to meet international
regulations and improve the efficiency of diesel engines. This paper aims to reduce sulfur content on the fuel
using Oxidative Desulfurization (ODS) method. Hydrogen peroxide was used as an oxidant with various
heterogeneous catalysts in the ODS process. There are 3 heterogeneous catalysts used in this work, namely
activated carbon-formic acid, Co-Fe/γ-Al
2
O
3
and MoO
3
/γ-Al
2
O
3
. These three catalysts have been used in
other studies and succeeded in significantly reducing sulfur content in various diesel models. The ODS
reaction was carried out using a batch stirring reactor under several reaction conditions and followed by
centrifugation to separate the diesel and the oxidated sulfur compounds. As the results, Co-Fe/γ-Al
2
O
3
catalysts gave the highest percentage of 9.8% desulfurization with reaction conditions of 5 g catalyst, the
molar ratio of H
2
O
2
to sulfur = 120, and 25 mL of Biosolar.
1 INTRODUCTION
Diesel fuel in Indonesia is still far from international
regulatory standards because it has a high sulfur
content. Pertamina DEX has the lowest sulfur content
of 300 ppm, Dexlite has a sulfur content of 1,200
ppm, and Biosolar. Meanwhile, based on the
international standard EURO VI, the sulfur content in
diesel fuel is 0.001% by mass (10 ppm).
Due to this high sulfur content, the sulfur oxide
content can be oxidized to sulfuric/sulfuric acid
which causes corrosion and wear and tear on vehicle
engine parts. In addition, sulfur oxides can affect the
efficiency of the catalyst system in the exhaust gas
pipeline. Therefore, desulfurization technology is
needed to reduce sulfur content in diesel fuel.
One alternative process to reduce sulfur content is
the Oxidative Desulfurization (ODS) method. Many
researchers have reduced the sulfur content by
oxidizing dibenzothiophene to sulfoxide and sulfone,
because dibenzothiophene is the sulfur compound
with the most content (Joskić et al., 2014). Compared
with HDS, ODS has several advantages, such as using
atmospheric pressure operating conditions, relatively
low temperature up to 100℃, low cost, high
selectivity, no use of expensive hydrogen, and
potential for desulfurization of sterically hindered
sulfides such as 4,6-dimethyldibenzothiophene
(DMDBT) (Murata et al., 2004).
Oxidative Desulfurization (ODS) process was
used in this paper, which has been extensively studied
in reducing sulfur content but has not yet been applied
to Indonesia Biosolar fuel (Nikolas et al., 2021).
Thus, further research is needed regarding the use of
ODS technology in Indonesian Biodiesel (B-30) fuel
to reduce its sulfur content.
In the ODS process, hydrogen peroxide (H
2
O
2
) is
the most used oxidant because of its affordable cost,
availability, and producing oxygen and water by-
products that are not harmful to the environment
(Shang et al., 2003). Therefore, this paper used an
oxidant in the form of hydrogen peroxide (H2O2).
For the use of solvents, according to Jia et al., (2011),
the solvent in the ODS process can cause problems in
the separation between the biodiesel oil and the
solvent phase with the loss of some amount of the oil
phase. So, it is recommended that in the ODS process
using a solid catalyst, the use of solvents should be
avoided. And separation can be carried out using
centrifugation.
Based on the phase, catalyst in the desulfurization
process is divided into two types, which are
homogeneous catalysts and heterogeneous catalysts.
However, the homogeneous catalyst is difficult to
separate from the reaction because they have the same
428
Yandy, Y., Widyasari, N., Berliana, T. and Nasikin, M.
Heterogeneous Catalyst of Oxidative Desulfurization for Reducing Sulfur Content in Indonesia Biosolar.
DOI: 10.5220/0011811800003575
In Proceedings of the 5th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2022), pages 428-433
ISBN: 978-989-758-619-4; ISSN: 2975-8246
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

phase. This gives the heterogeneous catalyst an
advantage in the ODS process because separating the
catalyst from the reaction is easier. In addition,
heterogeneous catalysts have a large surface area
which can increase the interaction of the material with
the catalyst (Haghighi and Gooneh-Farahani, 2020).
In this paper, heterogeneous catalysts are used in
ODS process with three different types of catalysts.
Due to their high ability in oxidation reactions,
various transition metals have been investigated as a
catalyst in the desulfurization process (Rajendran et
al., 2020). Nazmi et al. (2020) conducted a paper to
reduce sulfur content using Co-Fe/γ-Al
2
O
3
catalyst
with the ODS method in n-octane diesel. The research
was conducted with various compositions of catalyst,
oxidant, and oxidation time which succeeded to
reduced 93% sulfur content in 30 min. Therefore, in
this paper, Co-Fe/γ-Al
2
O
3
was used as a catalyst with
hydrogen peroxide as an oxidant to reduce sulfur
content in Biosolar (B-30). Jia et al. (2011) have
investigated transition metals in reducing sulfur
content with ODS using a MoO
3
/γ-Al
2
O
3
catalyst in
n-octane model diesel. The sulfur content of diesel
fuel can be reduced up to 97.2% with an oxidation time
of 10 min. Therefore, in the present investigation,
MoO
3
/γ-Al
2
O
3
is used as a catalyst in the ODS process
to reduce sulfur content in Biosolar. One paper of the
ODS process using activated carbon-formic acid (AC-
HCOOH) catalyst and oxidizing H
2
O
2
resulted in a
desulfurization percentage of 98% in the n-octane
model diesel (Yu et al., 2005). Therefore, in this paper,
the ODS process was carried out to determine the
catalyst with the best desulfurization results, and the
sulfur content was determined by ASTM-FTIR
absorbance correlation.
2 EXPERIMENTAL SECTION
2.1 Materials
Biosolar (B-30) was obtained from PT. Pertamina
with 360.9 ppm. Activated carbon Jacobi AquaSorb®
2000, is granular coal-based, and technical-grade
formic acid were obtained commercially. Ammonium
heptamolybdate tetrahydrate as a precursor for
MoO
3
/γ-Al
2
O
3
catalyst was purchased commercially.
Iron (III) nitrate nanohydrate and cobalt (II)
hexahydrate as precursors for Co-Fe/γ-Al
2
O
3
catalyst
was purchased commercially. γ-Al
2
O
3
as the support
catalyst precursor of MoO
3
/γ-Al
2
O
3
and Co-Fe/γ-
Al
2
O
3
was also purchased commercially. Hydrogen
peroxide (30 wt %, technical-grade reagent) was
purchased commercially.
2.2 Catalyst Preparation
Co-Fe/γ-Al
2
O
3
and MoO
3
/γ-Al
2
O
3
catalysts were
prepared by the incipient wetness impregnation
method. According to the loading of Co-Fe and
MoO
3
, an appropriate amount of cobalt (II) nitrate
hexahydrate, iron (III) nitrate nonahydrate, and
ammonium heptamolybdate tetrahydrate were
dissolved in distilled water and then slowly added to
γ-Al
2
O
3
at ambient temperature. The mixture was
dried in an open vessel with stirring at 373 K for 2 h
to evaporate the excess water. The precursor of Co-
Fe/γ-Al
2
O
3
was calcined at 773 K for 5 h, while the
precursor of MoO
3
/γ-Al
2
O
3
was
calcined for 6 h to
obtain a catalyst.
Activated carbon Jacobi AquaSorb® 2000 was
prepared using 10g of activated carbon soaked and
washed repeatedly in deionized water. This process
aims to neutralize and clean the activated carbon
sample until the water looks clean and not cloudy.
Then, the sample was filtered to separate the solids
from water and dried in an oven at 120℃ for 6 hours
to remove the water content.
2.3 Oxidative Desulfurization of
Biosolar
A typical procedure was as follows. All ODS
reactions were conducted in a 150 mL beaker glass,
equipped with a magnetic stirrer bar and fitted with
the hot plate. For the ODS process using activated
carbon catalyst, a mixture of commercial diesel oil
(100 mL), 30 wt% hydrogen peroxide (3.4 mL), H
2
O
(5 mL), formic acid (1 mL), and AC Jacobi (0.7 g)
was stirred at 750 rpm in a beaker glass under various
oxidation temperatures (30°C, 60°C, and 70°C) for 60
min. For ODS runs of MoO
3
/γ-Al
2
O
3
catalyst, 1 g
catalyst, and 25 mL diesel oil were stirred until the
reaction temperature reached the desired temperature
(40°C, 60°C, and 70°C), and then 1.5 mL hydrogen
peroxide (molar ratio of H
2
O
2
/s = 120/1) were added
to the beaker glass and stirred for 30 min. For ODS
runs of Co-Fe/γ-Al
2
O
3
, catalyst weight variation (1 g,
3 g, and 5 g) and 25 mL diesel oil were stirred until
the reaction reached the desired temperature (30°C,
50°C, and 70°C), and then 1.5 mL hydrogen peroxide
(molar ratio of H
2
O
2
/s = 120/1) were added to the
beaker glass and stirred for 30 min.
The beaker glass was fitted with a condenser, a
mechanical stirrer bar, and a thermometer. The
oxidized oil and adsorbent were separated by
centrifugation.
Heterogeneous Catalyst of Oxidative Desulfurization for Reducing Sulfur Content in Indonesia Biosolar
429

3 METHOD OF ANALYSIS
The separated oil phase was analyzed using Fourier-
Transform Infrared Spectroscopy (FTIR) to
determine the total sulfur content. According to Az-
Zahra et al. (2022), FTIR method can identify and
measure total sulfur content quantitatively and
qualitatively in diesel fuel with 62% accuracy,
towards ASTM D-4294 method. FTIR method as a
sulfur detector does not require sophisticated sample
preparation and expensive costs.
The wavenumber that shows strong absorption of
sulfur is at 1169 cm
-1
. Meanwhile, the wavenumber
that shows the presence of aromatic range is at 1458
cm
-1
, which shows the characteristic of Biosolar since
70% of Biosolar contains diesel oil that formed of the
aromatic ring (Az-Zahra et al., 2022). According to
Coates (2006), in the wavenumber range of 1200-
1100 cm
-1
, it states the presence of sulfone
compounds in the presence of S=O strain. While the
aromatic ring group C=C-C will appear at
wavenumbers 1510-1450 cm
-1
with non-polar
properties and is suitable as a basis for identifying
diesel oil. Bonds with a wave number of about 1458
cm
-1
are C-H bonds with bending vibrations. The
results obtained from comparing the two peaks were
calibrated using a model made from the combined
FTIR data and the sulfur contents from ASTM-D test
results. The absorbance of 1169 cm
-1
and 1458 cm
-1
resulted in IR Spectrum is defined as W
and
W
. So, the equation for determining the sulfur
content in each sample is obtained as follows:
Total Sulfur Content (ppm) =
(
)
,
(1)
For removal rates of sulfur were calculated as follows
Desulfurization
(
%
)
=
× 100% (2)
Where TS
0
is the initial total sulfur content of diesel
fuel and TS
t
is the final total sulfur content of diesel
fuel after ODS reaction.
4 RESULTS AND DISCUSSION
4.1 Catalyst Characterization
4.1.1 Characterization of Co-Fe/γ-Al
2
O
3
and
MoO
3
/γ-Al
2
O
3
Co-Fe/γ-Al
2
O
3
and MoO
3
/γ-Al
2
O
3
catalysts were
characterized by X-Ray fluorescence (XRF) using S2
PUMA Bruker to identify the elemental composition
of the catalyst. In this paper, XRF analysis was
conducted at Pusat Riset Kimia Maju, Puspiptek,
Serpong. Table 1 shows the composition of the XRF
analysis results with a comparison of the theoretical
composition. XRF analysis was only carried out on
one sample of each catalyst to prove the results of the
catalyst preparation.
Table 1: Comparison between theoretical and actual
composition with XRF analysis.
Catalyst Compound
Theoretical
composition
(
wt.%
)
Actual
composition
(
wt.%
)
*
Co-Fe/
γ-Al
2
O
3
CoO 3.32 2.1
Fe
2
O
3
24.63 10.4
Al
2
O
3
72.05 83.1
MoO
3
/
γ-Al
2
O
3
MoO
3
20 20.26
Al
2
O
3
80 75.76
*Based on XRF analysis.
The actual composition in Table 1 shows that the
catalyst preparation succeeded in obtaining the
desired compound, but the results of the percentage
composition in XRF analysis slightly differed from
the theoretical composition in catalyst preparation.
4.1.2 Characterization of Activated Carbon
The characterization of activated carbon carried out
in this paper aims to determine the surface area and
total pore volume using the Brunauer Emmett-Teller
(BET) Quantachrome Quadrasorb-Evo Surface Area
and Pore Size Analyzer method. This characterization
was conducted at ILRC UI Laboratory. The results of
the characterization are shown in Table 2. In theory,
the higher the surface area of activated carbon, the
greater the ability of activated carbon to adsorb polar
compounds (Jamilatun and Setyawan, 2014). And
with the increased surface area and a decrease in the
average pore radius on activated carbon increased the
total pore volume of activated carbon (Irma,
Wahyuni, and Zahara, 2015).
Table 2: Structural parameters of the activated carbons.
Catalyst
BET surface
area (m
2
/g)
Total pore
volume (cm
3
/g)
Activated carbon
Jacobi
AquaSorb® 2000
775.3 0.52
* Based on BET analysis.
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
430
4.2 Evaluation of Various
Heterogeneous Catalyst Systems
4.2.1 Comparison of Heterogeneous
Catalysts
The results of the ODS process in this paper were
compared to determine the catalyst's performance.
The results can be seen in Table 3 below using the
same solar model and oxidant. The percentage of
desulfurization produced in this paper uses the
Indonesian Biosolar (B-30), which has a total sulfur
content that is too complex and not specific.
Table 3: Desulfurization results in ods process with various
heterogeneous catalysts.
Catalyst T (°C) t (min)
Desulfurization
(
%
)
Co-Fe/γ-Al
2
O
3
50 30 9.8
MoO
3
/γ-Al
2
O
3
60 30 7.7
AC-HCOOH 30 60 7.6
Table 3 shows that using a catalyst can reduce
sulfur content in the ODS process, but there are
differences in the desulfurization percentage of the
heterogeneous catalyst used. The results exhibit that
the highest removal of sulfur is 9.8% using
Co-Fe/γ-
Al
2
O
3
catalyst until 325.6 ppm.
Co-Fe/γ-Al
2
O
3
catalyst
has three components, namely Fe
2
O
3
as an active
core, CoO as the promoter, and γ-Al
2
O
3
as a support.
The combination of Fe
2
O
3
and CoO can increase the
reaction activity, meanwhile γ-Al
2
O
3
has a large
surface area and high pores so that it can increases the
performance of catalytic reactions. A screening of
several transition metal-oxide catalysts showed that
alumina-supported Fe-Co catalyst performed the
highest oxidative desulfurization (Nazmi et al, 2020).
The percentage of sulfur removal using MoO
3
/γ-
Al
2
O
3
catalyst reached 7.7% with sulfur content from
360.9 ppm to 333.3 ppm. The MoO
3
/γ-Al
2
O
3
catalyst
only has two catalyst components, MoO
3
as an active
core and γ-Al
2
O
3
as a support. Molybdenum metal is
used as an active core which can increase the activity
and selectivity of the reaction, while γ-Al
2
O
3
is used
as a support because it has a high surface area and
pore volume so that it can increase catalytic activity
(Argyle and Bartholomew, 2015).
For the AC-HCOOH catalyst, it produces a
desulfurization percentage of 7.6% from 360.9 ppm
to 333.7 ppm. According to previous researchers, the
oxidation of DBT with the AC-HCOOH catalytic
system is better than using only the HCOOH catalyst.
Activated carbon is used as a phase-transfer
adsorption medium, because it is porous and has a
large surface area for the reaction contact area. Large
surface area for the reaction contact area. The
presence of formic acid in the catalyst can increase
the oxidation reaction in activated carbon by
catalyzing the formation of performic acid and results
in high conversion to DBT-Sulfone (Yu et al., 2004).
4.2.2 Effect of Oxidation Temperature
The oxidation of Biosolar was carried out with
hydrogen peroxide catalyzed by various
heterogeneous catalysts under various oxidation
temperatures.
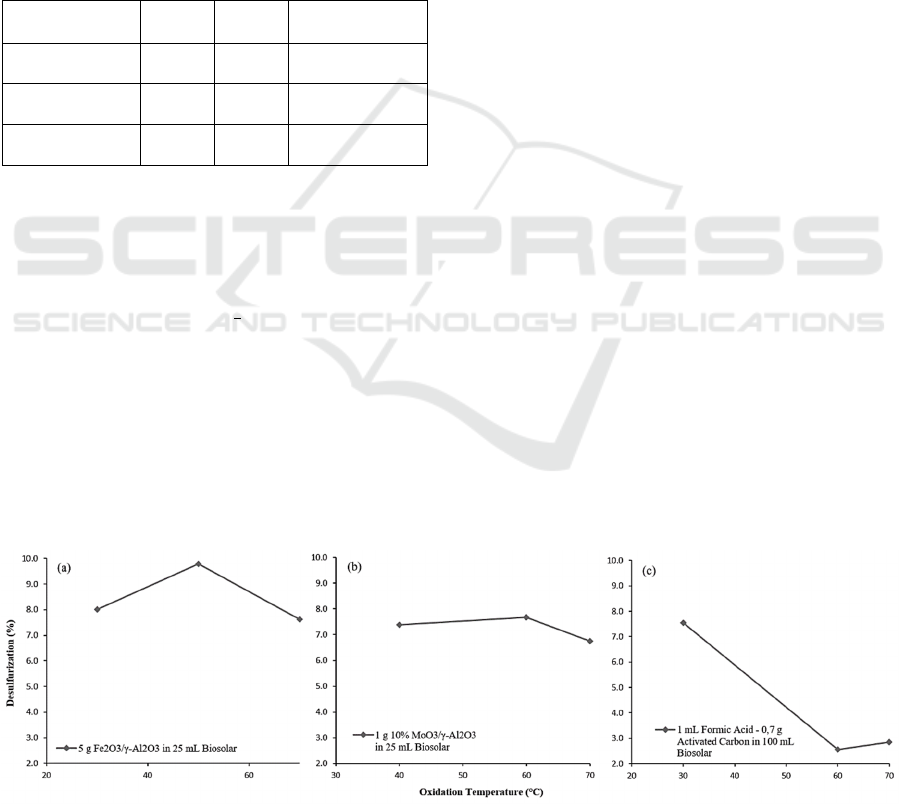
Figure 1 shows the sulfur removal at 30°C, 50°C,
and 70°C using 5 g Co-Fe/γ-Al
2
O
3
in 25 mL Biosolar
with an oxidation time of 30 min. The removal of
sulfur content increased from 30°C until it reached
the highest condition at 50°C. Then when the
temperature was increased to 70°C, the percentage of
sulfur removal was decrease. This can happen
because each catalyst has an optimum and
equilibrium point in working (Pahlevi et al, 2015). In
this operation, 50°C is the optimum condition.
Figure 1: Effect of temperature on the ODS catalyzed by (a) Co-Fe/γ-Al
2
O
3,
(b) MoO
3
/γ-Al
2
O
3,
(c) AC-HCOOH.
Heterogeneous Catalyst of Oxidative Desulfurization for Reducing Sulfur Content in Indonesia Biosolar
431

The effect of reaction temperature on sulfur
removal was also carried out on MoO
3
/γ-Al
2
O
3
catalyst. Figure 1 shows the sulfur removal at 40°C,
60°C, and 70°C using 1 g 10% MoO
3
/γ-Al
2
O
3
in 25
mL Biosolar with an oxidation time of 30 min. The
sulfur removal increased from 40°C until it reached
the highest condition at 60°C. After that, the sulfur
removal decreased at 70°C.
For the ODS process catalyzed by AC-HCOOH,
as shown in Figure 1 that the percentage of
desulfurization decreases as the reaction temperature
increases. With an oxidation time of 60 min, the
composition of the AC-HCOOH catalyst of 0.7 g-1
mL in 100 mL Biosolar will experience a decrease in
the percentage of desulfurization as the oxidation
temperature increases from 30℃ to 60℃. However,
the desulfurization percent increased again at an
oxidation temperature of 60℃ to 70℃. This shows
that the use of high temperatures in the ODS process
in this paper can reduce the performance of the
oxidation results.
This is in accordance with the research conducted
by Tugrul Albayrak & Ali Gurkaynak (2012), where
the ODS process with hydrogen peroxide has been
carried out using a formic acid catalyst, and the
desulfurization is greater at 30℃ compared to 40℃.
Low temperatures and low formic acid-H
2
O
2
amounts
are more efficient because peroxyformic acid,
produced in situ by H
2
O
2
and formic acid,
decomposes slowly at 30 °C, thus increasing the
reaction conversion. Oxidation temperatures that are
too high can reduce oxidation yields due to the
oxidation degradation of H
2
O
2
(Houda et al., 2018).
According to W. Mohammed and R. K. Almilly in
2015, the temperature is the most significant factor
because it shows the interaction between temperature
and the H
2
O
2
/diesel fuel ratio. The excess oxidant is
required at high temperatures due to the loss of H
2
O
2
due to thermal decomposition.
4.3 Analysis of IR Spectrum
FTIR Spectroscopy produces an infrared spectrum
from the absorption of a sample for further use in
identifying compounds and functional groups. The
infrared spectrum produces peaks that indicate the
absorbance value of the sample at various
wavenumbers.
The absorbance of sulfur compounds is
represented by a wavenumber of 1169 cm
-1
,
indicating the presence of sulfur compounds. Figure
2 shows the differences in absorbance levels for
wavenumber 1169 cm
-1
of Biosolar and Biosolar after
ODS. The Biosolar spectrum has a peak with a higher
Figure 2: IR spectrum from Biosolar without ODS process
and Biosolar after treated by ODS process.
absorbance value, which is 0.045 compared to 0.044
for Biosolar after ODS. The difference in absorbance
levels proves that Biosolar has higher sulfur
compounds. The spectrum from Biosolar also has a
wavenumber of 579 cm
-1
, which is not present in the
Biosolar after ODS. The wavenumber is a disulfide
shown in the wavenumber range of 705-570 cm
-1
(Coates, 2000). This proves that the ODS process can
remove disulfide compounds which are sulfur-
derived compounds.
5 CONCLUSIONS
From the three heterogeneous catalysts, which are
Co-Fe/γ-Al
2
O
3
, MoO
3
/γ-Al
2
O
3
, and AC-HCOOH,
catalyst Co-Fe/γ-Al
2
O
3
gave the highest percentage
of desulfurization in the ODS process with 9.8% with
5 g of catalyst, 1.5 mL of hydrogen peroxide (molar
ratio of H2O2/s = 120/1), and 25 mL of Biosolar. The
best-operating conditions for this mixture are at a
temperature of 50℃ with an oxidation time of 30
minutes. The results showed that many factors could
affect the performance of the catalysts, including
temperature.
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
432

ACKNOWLEDGEMENTS
HIBAH PTUPT partially funded this research.
REFERENCES
Argyle, M. and Bartholomew, C. (2015). Heterogeneous
Catalyst Deactivation and Regeneration: A Review.
Catalysts, 5(1), pp.145-269.
Az-Zahra, D. J., Gibran, A. A., & Dondo, T. (2022).
Reduction of Total Sulfur Content in Biosolar TM with
Catalytic Oxidative Desulfurization Method Using
Hydrogen Peroxide as Oxidant and Acetic Acid as a
Catalyst. EVERGREEN Joint Journal of Novel Carbon
Resource Sciences & Green Asia Strategy, 09(01), 126–
132.
Coates, J. (2000). Interpretation of infrared spectra, a
practical approach. Encyclopedia of analytical
chemistry, 12, 10815-10837.
Direktur Jendral Minyak dan Gas Bumi. (2020). Standar
dan Mutu (Spesifikasi) Bahan Bakar Minyak Jenis
Solar yang Dipasarkan di Dalam Negri. Keputusan
Direktur Jendral Minyak dan Gas Bumi Nomor
146.K/10/DJM/2020
Haghighi, M., & Gooneh-Farahani, S. (2020). Insights to
the oxidative desulfurization process of fossil fuels over
organic and inorganic heterogeneous catalysts:
advantages and issues. Environmental Science and
Pollution Research, 27(32), 39923–39945.
https://doi.org/10.1007/s11356-020-10310-4
Houda, S., Lancelot, C., Blanchard, P., Poinel, L., &
Lamonier, C. (2018). Oxidative desulfurization of
heavy oils with high sulfur content: A review.
Catalysts, 8(9). https://doi.org/10.3390/catal8090344
Irma, K. N., Wahyuni, N., & Zahara, T. A. (2015). Adsorpsi
Fenol Menggunakan Adsorben Karbon Aktif Dengan
Metode Kolom. JKK, 4(1), 24–28.
Ismagilov, Z., Yashnik, S., Kerzhentsev, M., Parmon, V.,
Bourane, A., Al-Shahrani, F., Hajji, A. and Koseoglu,
O. (2011). Oxidative Desulfurization of Hydrocarbon
Fuels. Catalysis Reviews, 53(3), pp.199-255.
Jamilatun, S., & Setyawan, M. (2014). Pembuatan Arang
Aktif dari Tempurung Kelapa dan Aplikasinya untuk
Penjernihan Asap Cair. Spektrum Industri, 12(1), 1–
112.
Jia, Y., Li, G., & Ning, G. (2011). Efficient oxidative
desulfurization (ODS) of model fuel with H 2O2
catalyzed by MoO3/γ-Al 2O3 under mild and solvent
free conditions. Fuel Processing Technology, 92(1),
106–111. https://doi.org/10.1016/j.fuproc.2010.09.011
Joskić, R., Margeta, D., & Sertić-Bionda, K. (2014).
Oxidative desulfurization of model diesel fuel with
hydrogen peroxide. Goriva i Maziva, 53(1), 11–18.
Mohd Nazmi, N. A. S., Wan Abdullah, W. N., Adam, F.,
Wan Mokhtar, W. N. A., Yahaya, N. F., and Mohd
Shukri, N. (2020) ‘Iron Oxide Catalyst for Oxidative
Desulfurization of Model Diesel Fuel’, Materials
Science Forum, 1010, pp. 418–423. DOI:10.4028/
www.scientific.net/msf.1010.418
Murata, S., Murata, K., Kidena, K., & Nomura, M. (2004).
A novel oxidative desulfurization system for diesel
fuels with molecular oxygen in the presence of cobalt
catalysts and aldehydes. Energy and Fuels, 18(1), 116–
121. https://doi.org/10.1021/ef034001z
Nikolas, M., Nugraha, R. A., Sejati, R. I., & Nasikin, M.
(2021). Investigation of Influential Parameters in
Oxidative Desulfurization of Indonesia ’ s Diesel Fuel
with Methanol Solvent and Formic Acid Catalyst.
Pahlevi, M.R., Nurhayati and Anita, S. (2015). Variasi
Berat Katalis dan Suhu Reaksi Transesterifikasi Crude
Palm Oil Menggunakan Katalis Cangkang Kerang
Darah Kalsinasi 800°c. JOM FMIPA, 2(1), pp.186–
191.
Rajendran, A., Cui, T., Fan, H., Yang, Z., Feng, J. and Li,
W. (2020). A comprehensive review on oxidative
desulfurization catalysts targeting clean energy and
environment. Journal of Materials Chemistry A, 8(5),
pp.2246-2285.
Shang, H., Zhang, H., Du, W., & Liu, Z. (2013).
Development of microwave assisted oxidative
desulfurization of petroleum oils: A review. Journal Of
Industrial And Engineering Chemistry, 19(5), 1426-
1432. https://doi.org/10.1016/j.jiec.2013.01.015
Tugrul Albayrak, A., & Ali Gurkaynak, M. (2012).
Sonocatalytic oxidative desulfurization of thiophene
and its derivatives. Procedia Engineering, 42(August),
1711–1719.
https://doi.org/10.1016/j.proeng.2012.07.563
W. Mohammed, R. K. Almilly, S. B. A. A. (2015).
Desulfurization of Diesel Fuel by Oxidation and
Solvent Extraction. Journal of Engineering, 21(2), 87–
102.
Yu, G., Lu, S., Chen, H., & Zhu, Z. (2005). Oxidative
desulfurization of diesel fuels with hydrogen peroxide
in the presence of activated carbon and formic acid.
Energy and Fuels, 19(2), 447–452. https://doi.org/
10.1021/ef049760b
Zhao, D., Wang, J., & Zhou, E. (2007). Oxidative
desulfurization of diesel fuel using a Brønsted acid
room temperature ionic liquid in the presence of H2O2.
Green Chemistry, 9(11), 1219. https://doi.org/10.1039/
b706574d
Heterogeneous Catalyst of Oxidative Desulfurization for Reducing Sulfur Content in Indonesia Biosolar
433
