
Effect of Hydroxyl Aluminum Ratio on Preparation of PAC from
Aluminum Ash
Yikun Zhao
1
, Hui Yuan
1
, Yongdong He
1
and Changke Cheng
2
1
College of Physical Science and Technology, Xinjiang University, Urumqi, Xinjiang, China
2
Xinjiang Zhonghe Co., Ltd., Urumqi, Xinjiang, China
Keywords: PAC; CaO; Al
13
Molecule; Hydroxy Aluminum Ratio; Phase Transformation.
Abstract: The oligomeric polymeric aluminum chloride prepared from secondary aluminum ash was used as raw
material, and hydrolytic polymerization was carried out with different ratios of calcium oxide, and the
structure and phase transformation process of the polymeric aluminum chloride produced by hydrolytic
polymerization were characterized by XRD and infrared spectroscopy. The results showed that at the
hydroxyl-aluminum ratio of 0.25, the phases of hydrolysis polymerization products were Al(OH)
3
, AlOCl,
AlCl
3
-6H
2
O, Ca
3
Al
2
(OH)
12
; at the hydroxyl-aluminum ratio of 0.5, the main phases were Al(OH)
3
, AlO(OH),
AlCl
3
-6H
2
O, Ca
3
Al(OH)
7
-3H
2
O, Ca
3
Al
2
(O
4
H
4
), the main phase is AlCl
3
-6H
2
O,
((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
when the hydroxyl-aluminum ratio is 0.75, and the polymeric
((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
molecules account for 70.4%, When the hydroxy-aluminum ratio is 1, the
main phase is indeterminate; A large amount of OH- appears in the prepared high polymer polyaluminum
chloride molecule, and the peak height of the absorption peak of Al-OH-Al can also indicate that there are a
large number of Al-OH-Al bonds in the sample, which proves that the polyaluminum chloride contains a large
number of Al-OH-Al bonds. There are ((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
molecules.
1 INTRODUCTION
By the end of 2021, China produced 60.9 million tons
of aluminum, depending on the raw material, each ton
of aluminum produces raw material feeding amount
ranging from 0.3% to 10% aluminum ash, and the
disposal fee of 2000-4000 RMB/ton is required to be
paid for the disposal of aluminum ash. The new
environmental protection law in April 2014 requires
that enterprises must dispose of hazardous waste
generated from electrolytic aluminum according to
the hazardous waste disposal requirements. China
included aluminum ash in the National Hazardous
Waste List in 2016. Nitrides and carbides in
aluminum ash hydrolyze when exposed to moisture,
emitting strong irritating gases, and fluoride pollutes
soil and groundwater resources and causes fluorosis
to human and animal bones.
China relies on imported bauxite resources, while
the electrolytic aluminum industry consumes more
than 100 million tons of bauxite resources annually.
Secondary aluminum ash is a mixture of metallic
aluminum, alumina, aluminum nitride and salt
solvent with 50%-80% aluminum content, which is a
high-quality secondary aluminum resource, so it is
significant to realize the effective recycling of
secondary resources of aluminum ash.
Polymeric aluminum chloride is a kind of water-
soluble inorganic polymer with a wide range of
applications. The preparation of polymeric aluminum
chloride using
aluminides in aluminum ash leached
from hydrochloric acid is an effective way to
realize the resource
utilization of secondary
aluminum ash from hazardous waste. Kefeng Du et al.
experimentally studied the preparation of
polymerized aluminum chloride from aluminum ash
residue and waste hydrochloric acid. The degree of
polymerization of aluminum chloride affects its
physicochemical properties, application areas and use
effects, and according to the degree of polymerization,
aluminum chloride is divided into monomer (Al
3+
,
Al(OH)
2+
), dimer (Al
2
(OH)
2
4+
), trimer
(Al
3
(OH)
3
(H
2
O)
9
6+
), and hyperpolymer (Al
13
, Al
30
),
etc. Al
13
is considered the best among PACs because
of its large charge and molecular weight, it is easy to
bond and bridge in water and form flocs, thus Al
13
is
considered the best component in PAC, and the more
Al
13
content proves the higher quality of its PAC.
Yuan Huizhou
[11]
et al. prepared polyaluminum
solutions with different degrees of alkalinity by
106
Zhao, Y., Yuan, H., He, Y. and Cheng, C.
Effect of Hydroxyl Aluminum Ratio on Preparation of PAC from Aluminum Ash.
DOI: 10.5220/0011918300003536
In Proceedings of the 3rd International Symposium on Water, Ecology and Environment (ISWEE 2022), pages 106-110
ISBN: 978-989-758-639-2; ISSN: 2975-9439
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
controlling the amount of alkali addition to explore
the effect of pH on polyaluminum morphology. Lv
Jianxiao et al. explored the effect of total aluminum
concentration on the distribution of Al morphology in
polymerized aluminum chloride, and high aluminum
concentration is more likely to form products with
high polymerization, but too high aluminum
concentration will produce aluminum hydroxide
precipitation, which affects the coalescence effect.
Liu, Liang et al. explored the effects of total
aluminum concentration, alkalinity, reaction
temperature and alkalinity rate on the content of the
generated Al
13
.Yaneth Cardona
et al. studied the
formation pattern of Al
13
molecules and Al
30
molecules。
Regarding the structure and phase transformation
process of polymeric aluminum chloride generated by
the hydrolysis and polymerization of calcium oxide
and oligomeric PAC, there are few relevant studies in
China. This paper adopts the method of CaO
regulation of oligomeric PAC hydroxyaluminum ratio
(-OH to Al ratio) to study the structure and phase
transition law of polymerized aluminum chloride
generated by hydrolysis polymerization. It provides
technical support to explore the comprehensive
utilization of high value of high purity aluminum ash
residue.
2 EXPERIMENTS AND
MATERIALS
Xinjiang Zhonghe Co., Ltd. with particle size less
than 425 μm, deionized water, 35% hydrochloric
acid (analytically pure) and CaO (analytically pure)
were used as raw materials, and the aluminum ash
was washed and dried in acid solution. Take 150 g of
the dried acid solution powder, add 875 ml of
deionized water to a constant temperature water bath
at 80℃, and under the effect of mechanical stirring,
add CaO according to the hydroxy-aluminum ratio of
0, 0.25, 0.5, 0.75, 1.0, respectively, and wait for the
reaction for 4h, then cool to room temperature and
leave for 24h, followed by drying the polymeric
aluminum chloride solution using an electric
thermostatic drying oven at 75℃, and wait for the
appearance of a large number of The solid-liquid
separation was carried out when a large number of
crystals appeared; the physical phase changes and
molecular structure of the prepared polymeric
aluminum chloride were analyzed by X-ray
diffraction (XRD) and infrared spectroscopy.
3 EXPERIMENTAL RESULTS
AND ANALYSIS
3.1 Preparation of Low Hydroxyl Ratio
Oligomeric Aluminum Chloride by
Acid Leaching Method
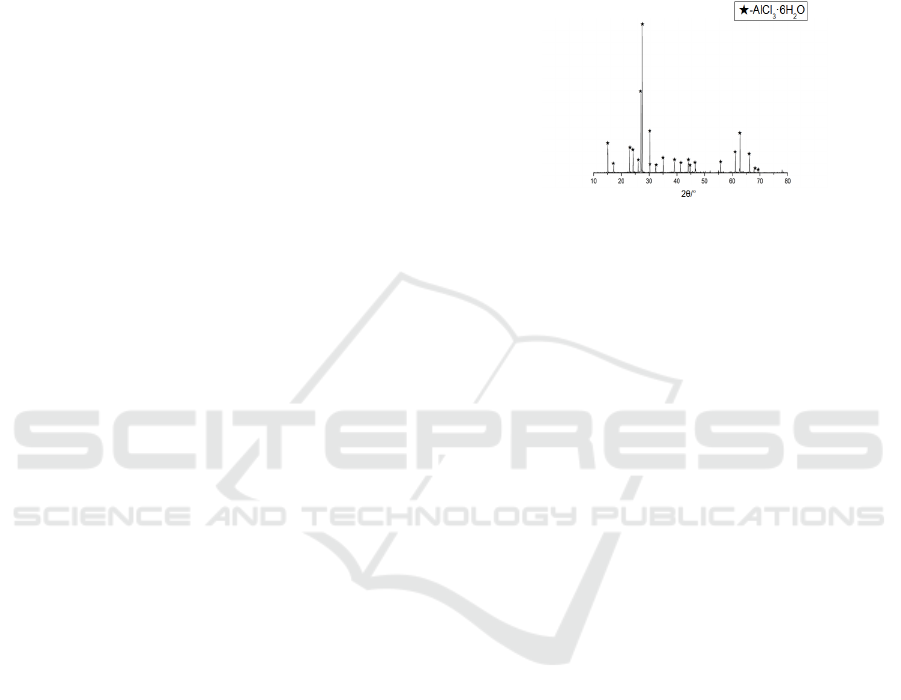
Figure 1: XRD analysis of polymeric aluminum chloride
prepared by acid leaching method
As shown in Figure 1, the XRD pattern of
polymeric aluminum chloride prepared by acid
leaching method. From Fig. 1, it can be seen that the
material phase obtained after acid dissolution of high
purity aluminum ash is relatively single with less
impurities. The aluminum ash can be obtained as
relatively pure AlCl
3
-6H
2
O after acid washing. the
polymerization degree of AlCl
3
-6H
2
O prepared
directly by acid leaching method is low, and the water
purification effect of this low hydroxyl ratio low
polymerization degree aluminum chloride is poor.
High polymerization contains a large number of
hydroxyl groups, and the hydroxyl groups, because
they can form hydrogen bonds in water, prompt the
polymerization of aluminum chloride molecules and
adsorption of impurities in water, so improving the
polymerization degree of polymerized aluminum
chloride can improve the water purification effect of
polymerized aluminum chloride.
In order to improve the polymerization degree of
polymerized aluminum chloride and strengthen the
water purification effect, the polymerized aluminum
chloride prepared directly by acid leaching method is
adjusted by adding CaO.
3.2 Analysis of the Effect of Different
Hydroxy-Aluminum Ratio
Adjustment on the Physical Phase
of PAC
From Fig. 2(a), the XRD spectrum of
hydroxyaluminum ratio of 0 shows that there is no
significant change in the structure of the PAC
Effect of Hydroxyl Aluminum Ratio on Preparation of PAC from Aluminum Ash
107
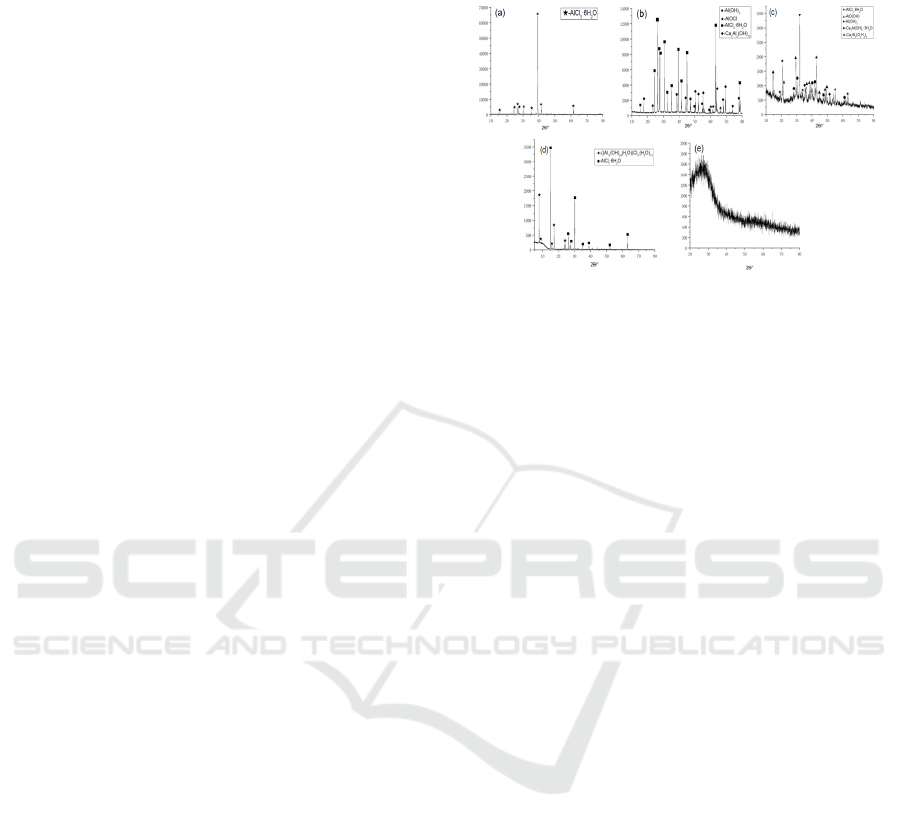
prepared without the addition of CaO hydrolysis
polymerization product (PAC) and acid leaching,
indicating that the aluminum chloride polymerization
reaction is not obvious. From Fig. 2(b), the XRD
spectrum of hydroxyaluminum ratio of 0.25 shows
that the composition of the physical phase is Al(OH)
3
,
AlOCl, AlCl
3
-6H
2
O, Ca
3
Al
2
(OH)
12
, and the
appearance of Ca
3
Al
2
(OH)
12
indicates that the PAC
polymerization increases when the hydroxyaluminum
ratio is 0.25. From Fig. 2(c), which shows the XRD
spectrum of the hydroxyaluminum ratio of 0.5, it can
be seen that the composition of the phases are
Al(OH)
3
, AlO(OH), AlCl
3
-6H
2
O, Ca
3
Al(OH)
7
-3H
2
O,
Ca
3
Al
2
(O
4
H
4
), from which it can be seen that the
diffraction peak of AlCl
3
-6H
2
O is weakened and the
appearance of the dimeric phase with higher
polymerization Ca
3
Al
2
(O
4
H
4
), indicating that the
degree of PAC polymerization continued to increase
when the hydroxyl-aluminum ratio was 0.5, but the pH
remained low due to the addition of less CaO, less
Ca(OH)
2
was generated, and thus no polymers
appeared in the physical phase. Fig. 2(d) shows the
spectrum of hydroxyl-aluminum ratio of 0.75, and the
XRD results show that the composition of the physical
phase is AlCl
3
-6H
2
O,
((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
, and it can be seen
from the figure that the polymeric phase
((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
appears in the
physical phase, and the PAC polymerization further
increased. Fig. 2(e) shows the XRD spectrum of
hydroxyaluminum ratio of 1. The XRD results
indicate an indefinite phase. In summary, the
polymerization degree of PAC increased with the
increase of the hydroxyaluminum ratio from 0 to 0.75,
and the indefinite phase was formed when the
hydroxyaluminum ratio was 1. This indicates that the
increase of CaO is too large, which will make the
polymeric phase ((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
hydrolyze and polymerize to form the indefinite
aluminum phase, but the phase will directly
polymerize to form the indefinite phase in the later
The reaction will directly polymerize to form
indefinite Al(OH)
3
, which will affect the water
purification effect of PAC.
Due to the high degree of polymerization of the
generated PAC in Fig. 2(d), cell refinement and
quantitative analysis were performed using JADE
software, and the results showed that
((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
in the prepared
polymeric aluminum chloride accounted for 70.4% of
the total amount and the structure was highly
polymeric aluminum chloride cluster structure, and
the cell parameters of the generated Al13 molecules
were a = 13.9859, b = 23.4673, c = 22.3724, α = 90°,
β = 91.05°, γ = 90°, and the molecular radius is 1.08
nm.
Figure 2: Analysis of the influence of CaO content on
phase.
3.3 High Polymeric Aluminum
Chloride Molecular Infrared
Spectrum Analysis
Fig. 3 shows the results of infrared spectrum analysis
of the prepared PAC when the hydroxyl-aluminum
ratio is 0.75. From Fig. 3, it can be seen that the
prepared PAC has a strong and wide absorption band
at 3058.427 cm
-1
, and the absorption band is located at
3600-2800 cm
-1
, where the absorption peak is
generated by the stretching vibration of the -OH group
in the PAC connected with the aluminum ion and the
-OH group in the adsorbed water molecule, indicating
the presence of a large number of -OH groups in
polymeric aluminum chloride. A sharp peak at
1632.877 cm
-1
, which is an absorption peak generated
by the bending vibration of H-O-H of bound water in
the Al
13
molecule, indicating that the prepared
polymeric aluminum chloride contains a large amount
of bound water. The absorption peaks appearing at
1138.840 cm
-1
, 837.896 cm
-1
are in-plane bending
vibration absorption peaks produced by Al-OH-Al,
the intensity of which can indicate the number of
bonds, and the reaction aluminum chloride
polymerizes between Al atoms during hydrolysis by
Al-OH-Al bond bridging to form polymeric aluminum
chloride. The two sharper absorption peaks at 596.463
cm
-1
and 538.643 cm
-1
are the bending vibration peaks
of Al-OH, and there are components of the
polymerization state in the reaction PAC; the peak at
2413.293 cm
-1
is the peak caused by atmospheric CO
2
.
From the infrared spectrogram, it can be seen that
a large number of -OH groups appear in the PAC
prepared with a hydroxyl aluminum ratio of 0.75, and
the peak height of the absorption peak of Al-OH-Al
can also indicate the presence of a large number of Al-
OH-Al bonds in the prepared PAC, which can indicate
ISWEE 2022 - International Symposium on Water, Ecology and Environment
108
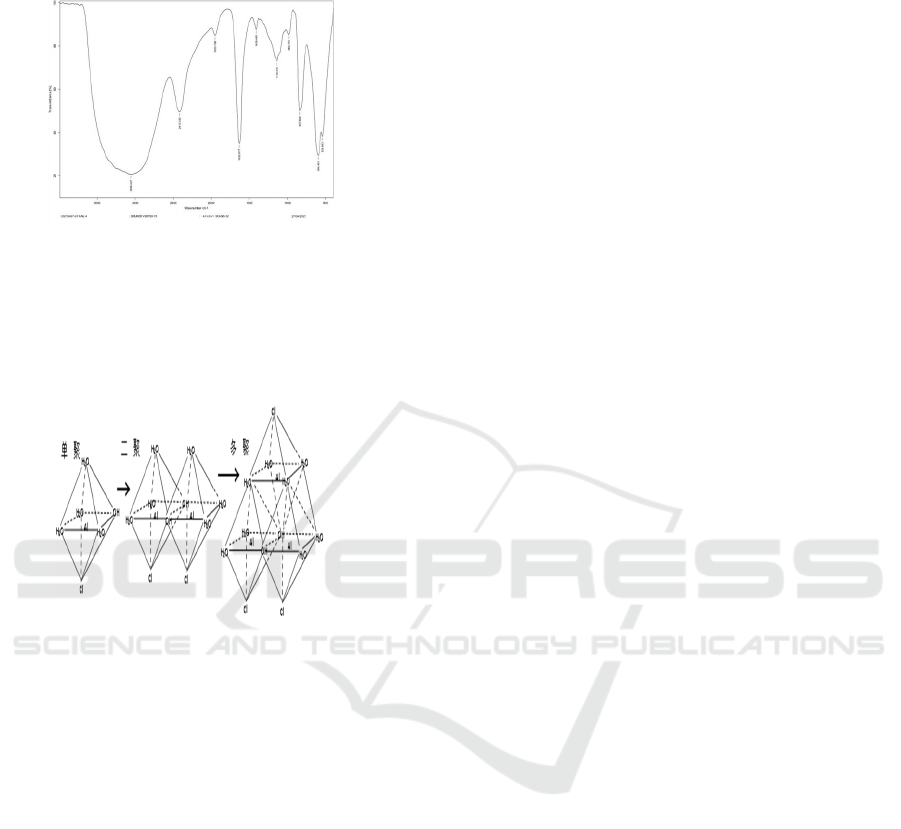
that ((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
is indeed
present and the content very high.
Figure 3: Infrared spectrum analysis of polyaluminum
chloride molecule with high ratio
4 DISCUSSION
Figure 4: The phase transformation law of polyaluminum
chloride molecules
CaO adjusts the PAC hydroxy-aluminum ratio, and
its phase change pattern is shown in Fig. 4.
Aluminum chloride molecules ionize Al
3+
in water,
Al
3+
will spontaneously carry out hydrolysis reaction
with H
2
O molecules to form octahedral Al(OH)
2
+
with Al
3+
as the core, octahedral Al(OH)2+ will
hydrolyze with OH- in solution again to Al(OH)
4
-
,
Al(OH)
4
-
is the precursor for the formation of
polymer molecules.
Al
H
O→Al
OH
1
AlOH
OH
→AlOH
2
When CaO is added to the PAC solution, CaO will
first react with H
2
O to form Ca(OH)
2
. The presence
of Ca(OH)
2
will cause the pH of the solution to rise,
which will further accelerate the hydrolysis reaction
of Al
3+
and increase the amount of octahedral
Al(OH)
2
+
, and the octahedral Al(OH)
2
+
with Al
3+
as
the core will polymerize through the Al-OH-Al bond
to form dimer Al
2
(OH)
2
4+
, but due to the strong
acidity of the solution, the amount of OH- is low,
forming monomeric Al(OH)
4
-
, Al(OH)
2
+ and dimeric
Al
2
(OH)
2
4+
in smaller amounts, preventing the
formation of polymeric molecules.
CaO H
O→Ca
OH
3
AlOH
H
O→Al
OH
4
As CaO increases and the pH continues to rise,
forced hydrolysis of Al
3+
occurs, resulting in an
increase in Al(OH)
4
-
molecules. Al(OH)
4
-
will use ion
bridging to aggregate octahedral Al(OH)
2
+
monomers
and dimeric Al
2
(OH)
2
4+
to form Al
13
molecules with
Al(OH)
4
-
as the core, and Al
13
can decompose to Al
3+
,
again or form [Al
13
]n by physical aggregation and ion
bridging.
AlOH
Al
OH
AlOH
OH
→
Al
OH
5
With further increase of CaO, the amount of
Ca(OH)
2
formed by reaction with H
2
O is also
increasing, and the pH of the sample is increasing, the
content of Al
13
will also increase, and subsequently,
Al
13
will be transformed into [Al
13
]n, and then Al
13
will be bonded with the transformed [Al
13
]n under the
effect of ion bridging. The strong electrostatic
adsorption ability of [Al
13
]n allows a large amount of
Al
13
to be deposited on the [Al
13
]n molecule, forming
a gel-like indefinite solid phase. When an excessive
amount of CaO is added, these indefinite solid phases
are transformed into amorphous Al(OH)
3
, which
makes the purification effect of PAC poor.
Al
OH
OH
H
O→Al
OH
6
5 CONCLUSION
(1) The degree of polymerization of aluminum
chloride can be adjusted by adding a certain amount
of CaO, but the addition of excessive CaO will form
an indefinite form of Al(OH)
3.
(2) When the hydroxyl-aluminum ratio is 0.25, the
phases of hydrolysis polymerization products are
Al(OH)
3
, AlOCl, AlCl
3
-6H
2
O, Ca
3
Al
2
(OH)
12
; when
the hydroxyl-aluminum ratio is 0.5, the main phases
are Al(OH
)3
, AlO(OH), AlCl
3-
6H
2
O, Ca
3
Al(OH)
7
-
3H
2
O, Ca
3
Al
2
(O
4
H
4
). At the hydroxyl aluminum ratio
of 0.75, the main phases were AlCl
3
-6H
2
O,
((Al
13
(OH)
24
(H
2
O)
24)
)Cl
15
(H
2
O)
13
, and the polymeric
((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
molecules
accounted for 70.4%, and at the hydroxyl aluminum
ratio of 1, the main phases were indefinite phases
(3) The presence of a large amount of OH- in the
prepared polymerized aluminum chloride molecules
in the polymerized state and the peak height of the
absorption peak of Al-OH-Al can also indicate the
presence of a large amount of Al-OH-Al bonds in the
Effect of Hydroxyl Aluminum Ratio on Preparation of PAC from Aluminum Ash
109

sample, which proves the presence of
((Al
13
(OH)
24
(H
2
O)
24
))Cl
15
(H
2
O)
13
molecules in the
polymerized aluminum chloride.
ACKNOWLEDGMENTS
This work is supported by Major R & D Projects
Xinjiang by the Office of Science and technology
(2020B02007) ;Supported by the National Natural
Science Foundation of China (51861033).
REFERENCES
Yongdong He, Chunrong Jin , Zhichen Sun, et al. Study on
the dissolution behavior and phase transformation
pattern of high purity aluminum ash[J]. Special Casting
and Nonferrous Alloys, 2021,41(10):1204-1209.
Chao He, Yongdong He, Yikun Zhao et al. Study on the
synthesis of calcium aluminate from secondary
aluminum ash and its physical phase change[J]. Special
Casting and Nonferrous Alloys,2021,41(11):1436-1440.
Yujing OU , Xiaolong LI, Pengguo ZHI et al. Recovery
process of Al2O3 from aluminum ash[J]. Chemical
Technology,2018,26(06):31-36.
Yong ZHANG,Zhaohui GUO,Ziyu HAN et al.Effects of Al
N hydrolysis on fractal geometry characteristics of
residue from secondary aluminium dross using
response surface methodology[J] The Chinese Journal
of Nonferrous Metals,28(2018) 2574-2581.
Tripathy A K,MahalikS,Sarangi C K,etal.A pyro-
hydrometal-lurgical process for the recovery of alumina
from waste aluminium dross[J].Minerals
Engineering,2019,137:181-186.
Cao Y.Multi-stage electrostatic separation for recovering of
aluminum from fine granules of black dross[J].Journal
of Wuhan University of Technology-
Mater.Sci.Ed.,2019,34(4):925-931.
Yanling Li, Yongdong He, Xiaohan Sun et al. Study on the
effect of wet process on the harmless denitrification of
secondary aluminum ash [J] Special Casting and
Nonferrous Alloys,2020,6.
Yangmin Zhou, Gang Xie et al. Preparation of aluminum
hydroxide by alkali sintering of aluminum ash[J] Light
Metals, 2015,No.9,12-14
Lingling Li, Ming Song, Qiang Jin. Research progress on
recycling of aluminum ash [J] Inorganic Salt Industry,
2018,Vol.50,No.8,6-10.
Kaifeng Du, Xingxing Wang, Hongjun Ni, et al. Progress in
the preparation of polymeric aluminum chloride from
aluminum-containing resources and its process
research[J] Modern Chemical Industry,
2018,Vol.38,No.8,48-51.
Huizhou Yuan, Shuizhou Ke, Jiayong Tu et al. Effect of pH
on the distribution of polymeric aluminum morphology
and coagulation effect[J]. Industrial Water
Treatment,2016,36(04):50-53.
Jianxiao Lv, Ying Cui. The effect of total aluminum
concentration on the distribution of Al morphology in
polymeric aluminum chloride[J]. Journal of Henan
Institute of Science and Technology (Natural Science
Edition),2015,43(01):34-36.
Liang Liu, Hailin Yao , Zheng Lu et al. Experimental study
on multi-factor optimization of Al137+ content of
thirteen polyalumina based on orthogonal experimental
design[J]. Science Technology and
Engineering,2017,17(29):174-179.
Shota Suzuki, ReikoMurao, RaykoSimuraet al. Crystal
structure of tridecaaluminium
tetra(nickel,ruthenium),Al13Ni1.26Ru2.74 [J].
Zeitschrift fur Kristallographie. New crystal
structures,2012,227(1)
ISWEE 2022 - International Symposium on Water, Ecology and Environment
110
