
Exposure Evaluation of the Complete Dosage Sinovac and Pfizer
COVID-19 Vaccination into Office Employees
Elvira Potoboda and Diana Laila Ramatillah
Faculty Pharmacy, Universitas 17 Agustus 1945 Jakarta, Indonesia
Keywords: Covid-19, Sinovac, Pfizer, Office Employees.
Abstract: To find out the amount of exposure to covid-19 from Sinovac or Pfizer vaccination participants to office
employees and to find out the relationship between age, gender, type of vaccine, BMI, vaccine side effects,
and exposure to Covid-19 of Sinovac and Pfizer vaccine participants to office employees. This type of research
uses a cross-sectional prospective research design. The data collection technique was carried out using a survey
method using google forms distributed offline and online to office employees vaccinated with complete doses of
Sinovac and Pfizer vaccines with a convenience sampling method. The number of exposures to Covid-19 after
being vaccinated with Sinovac and Pfizer was 127 with Sinovac 111 and Pfizer 16 respectively. Factors that
affect exposure to Covid-19 are age, gender, type of vaccine, BMI, vaccine, and de effects with ap-value of
each variable <0.05. There is a relationship between age, gender, type of vaccine, BMI, and side effects of
exposure to Covid-19.
1 INTRODUCTION
The coronavirus disease 2019 (COVID-19), which
emerged at the end of 2019 has become a public health
threat around the world. The primary destination of
infection for SARS- CoV-2 acute respiratory
syndrome is the lower respiratory tract. Recorded in
the Analysis of Covid-19 Indonesia Data on the
Development of Covid-19 Cases Indonesia, among
the 765,350 confirmed cases of Covid-19 in the
update of October 10, 2021, the percentage of patients
confirmed positive for Covid-19 with an age range of
13-15 y is 1.83% while for the age range of 16-18 y by
2.24%. Very far, patients with an age range over 18 y
with a percentage of 13-29,66%(Sutardi and
Ramatillah 2022). There are 3 categories of severity of
Covid-19: Critical Covid-19 [acute respiratory
distress syndrome (ARDS), sepsis, septic shock, or
patients requiring life support therapy], Severe
Covid-19 [SpO2 <90%, have signs of ARDS and
pneumonia] and Covid-19 is not severe [no sign
criteria] severe or critical(Gee et al. 2020). One way to
prevent diseases caused by the coronavirus is to
increase the immune system or the body's
resistance(Amalia and Hiola 2020).
During the Covid-19 pandemic, several vaccine
platforms have been generated against this virus,
including inactivated whole-virus vaccines, namely
CoronaVac (Sinovac Life Sciences). It is used
particularly in Asia, the Middle East, and South
America. Dermatologic reactions such as erythema,
swelling, and urticaria have been reported. Cutaneous
vascular inflammation, however, has not been
reported from the use of Sinovac (CoronaVac).
Weherein, report 2 cases of CoronaVac-induced
cutaneousvasculitis (Bencharattanaphakhi and
Rerknimitr 2021). The National COVID-19
Vaccination Program details the policies, vaccine
procurement strategies, implementation efforts, and
monitoring necessary tocontain the COVID-19
pandemic at the national level(Chan et al. 2022).
The first vaccine accepts emergency use
Authorization (EUA) from Food and Drug
Administration (FDA) namely an mRNA in lipid
nanoparticles (LNPs), BNT162b2 from Pfizer and
BioNTech, which show 95% vaccine effectiveness.
Vaccine BNT162b2 was developed in less than one
year involving 30,420 volunteers at random to receive
and get an effectiveness vacuole of 94.1%. For
participants, 18 to <65 years old the effectiveness
obtained is 95.6%, and for thoseaged >65 years of
effectiveness was obtained at 86.4%. Pfizer Vaccines-
BioNTech starts to protect the body approx. 10 days
after the first dose, with maximum protection after
dose(Alfatihah, Shafriani, and Irfani 2021). Several
coronaviruses infect humans’ respiratory tracts,
Potoboda, E. and Ramatillah, D.
Exposure Evaluation of the Complete Dosage Sinovac and Pfizer COVID-19 Vaccination into Office Employees.
DOI: 10.5220/0011977900003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 165-172
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
165

causing everything from coughs and colds to more
serious diseases. The symptoms are normally minor
and come gradually, and some infected people may
not display any symptoms at all and yet feel
fine(Ramatillah et al. 2021).
At this time, it is very important to understand the
scientific evidence regarding the extent to which a
previoushistory of COVID-19 infection or a history of
vaccination can prevent further transmission from an
infected individual to others However, currently
available scientificevidence is lacking. Therefore,
researchers want to conduct research on the
Evaluationof Exposure to Covid 19 after
beingvaccinated with the Sinovac and Pfizer vaccine
types to Office Employees.
2
MATERIAL AND
METHODS
2.1 Design
This research was conducted with a quantitative
approach using a prospective cross-sectional design
study. The data collection technique was carried out
using a survey method using google forms distributed
offline and online to office employees vaccinated
with complete doses of Sinovac and Pfizer vaccines
with a convenience sampling method.
2.2 Participants
Participants in this study were all office workers in
Indonesia aged 18-60 years who had been vaccinated
against Sinovac or Pfizer with a complete dose of 600
respondents.
2.3 Instrument
This study uses a questionnaire distributed through
social media (WhatsApp, Twitter, Facebook,
Instagram, and Telegram). The number of
questionnaires in this study was 67 questions about
waste and comorbidities. The 67 questions were
about the side effects received after the first and
second doses of vaccination in the short and long
term, as well as monitoring the vaccine’s side effects
for 1-6 months after being vaccinated.
2.4 Statistical Analysis
The collected results were analyzed using the
SPSS version 25 application.
Fisher, Chi-square,
Mann-Whitney tests we Chi-square fand and the
relationship between risk factors (sex, age, BMI,
vaccine type) and side effects. A p-value of 0.05
was considered.
2.5 Ethical Approval
As stated in fig 1, ethical approval was obtained before
conducting the study. Ethical approval was sourced from the
health research ethics committee 17 Augustus 1945
JaethicsUcommitty, with an approval letter, No.48/KEPK-
UTA45JKT/EC/EXP/07/2022.
Figure 1: Research Framework
3 RESULT
The number of respondents from this study was 600
respondents who had received two doses of the
Sinovac vaccine and Pfizer vaccine and were included
in the inclusion criteria. Included dentists in this study
received questionnaires through social media such as
Facebook, WhatsApp, and Instagram.
Figure 2: Participants Based on Gender
Based on pictures 2, out of 600 respondents, 49%
Respondents are female respondents and 51% (305
respondents) were male respondents.
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
166
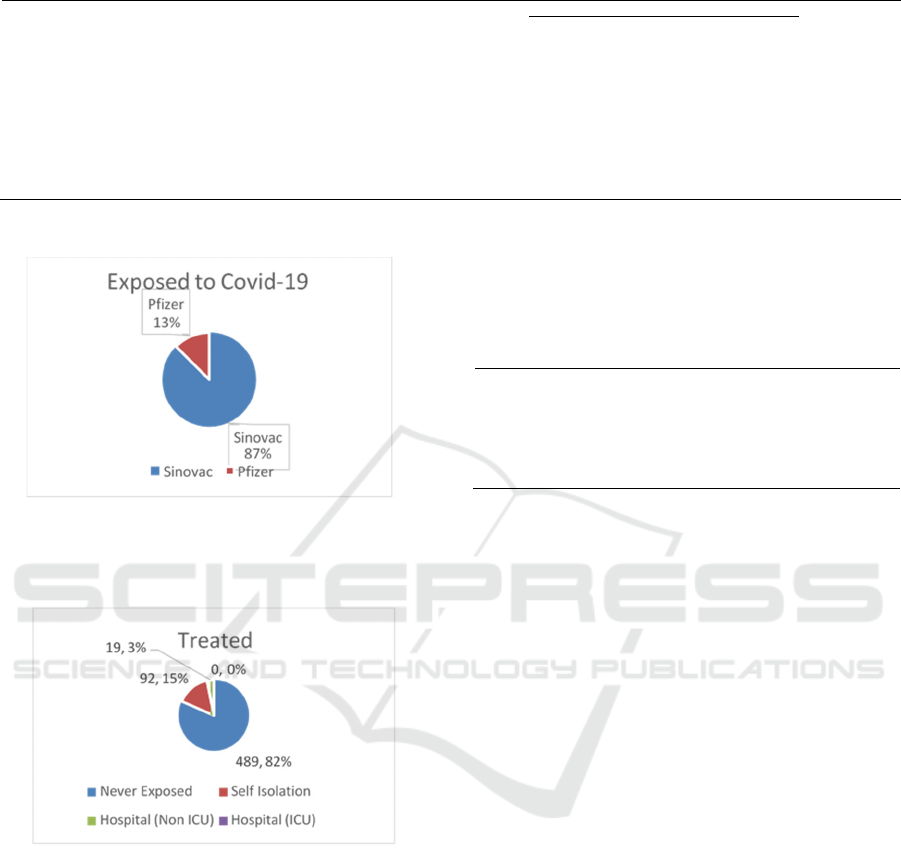
Table 1. The complicated disease of Sinovac and Pfizer vaccine participants since the 1st dose.
Frequency/Percentage (%)
Variable Sinovac (n=300) Pfize
r
(n=300)
p
-value
History of Diabetes 0/0 0/0 0.000
History of Gou
t
0/0 0/0 0.000
History of Asthma 0/0 0/0 0.000
History of Rheumatic Disease 0/0 0/0 0.000
History of Hear
t
Disease 0/0 0/0 0.000
*Fisher test, #Chi-square test
Figure 3: Participants exposed to Covid-19.
Based on pictures 3 out of 600 respondents, 21% (127
respondents) have been exposed to Covid-19.
Figure 4: Percentage treated of Covid-19.
Based on picture 4, which is never exposed
489, self- isolation 92, hospital (non-ICU) 19 and
hospital (ICU) 0.
Of the total 600 participants with 300 Sinovac
participants and 300 Pfizer participants each, none
had comorbid diseases as listed in table 1, namely
diabetes, gout, asthma, rheumatism, and heart disease.
The results of the statistical test can be seen from the
P-Value = 0.005, meaning P-Value <0.05 which
indicates that there is a significant relationship
between comorbidity and the condition of the
occurrence of Covid-19 (Sutardi and Ramatillah
2022).
Table 2. Correlation between type of vaccine and exposure
to Covid-19.
Kind of vaccine Exposed to Covid-
19/percenta
g
e (%)
P value
Sinovac 111/37
Pfizer 16/5.3
Total p-value 0.000
Fisher test, #Chi-square test
As shown in Table 2, those who were most
exposed to Covid-19 were participants who received
the Sinovac vaccine with apercentage of 37% and
Pfizer with 5.3%.
There is a significant difference between age,
BMI, and vaccine type with the results obtained at a
median overall age of 4.3%, anda median BMI of
3.8%. At median age Sinovac 8.7%, median BMI
7.8%, median age Pfizer 8.3%, median BMI 7.2%.
There was signed between the type of vaccine and
the side effects felt by patients after the receipt of dose
1 where the results showed that Pfizer’s vaccine
provided more side effects than Sinovac. As found
that out of 300 respondents who received the Pfizer
vaccine felt the side effects of fever as much as
46.6%, 69% felt pain at the injection site, 14.6% felt
coughing, 8% felt experienced diarrhea, 5% felt
dizziness, and 40.3% felt sleepy after the first dose of
vaccination, while for 300 other respondents who
received the Sinovac vaccine 32.6% felt fever, 60%
felt pain at the injection site, 5.6% felt coughing, 3.3%
felt experienced diarrhea, 1.3% felt dizziness, and
56.6% felt sleepy after the first dose of vaccinations.
Table 1, it is explained office employees who were
exposed to Covid-19 and those who received the
Sinovac vaccine (37%).
Exposure Evaluation of the Complete Dosage Sinovac and Pfizer COVID-19 Vaccination into Office Employees
167

Table 3. Correlation between age and BMI with the type of vaccine.
Var
i
a
bl
e
Frequency
/
Percentage
(
%
)
p
-va
l
ue
Overall
(
n=600
)
Sinovac (n=300) Pfize
r
(
n=300
)
Median Age 25.57/4.3 26.14/8.7 25.00/8.3 0.005
Median BMI 22.64/3.8 23.51/7.8 21.77/7.2 0.003
*Man-Whitney test, #Kruskal Wallis test
Table 4. Correlation between Type of Vaccine and Side Effect Dose 1.
Frequency/Percentage (%)
Sinovac
(n=300)
Pfizer
(n=300)
p
-value
Variables
Side Effects of Fever After The 1st Vaccination
98/32.6 140/46.6 0.001
Pain In The 1st Vaccination Injection Area
180/60 207/69 0.026
Side Effects of Coughing After The 1st Vaccination
17/5.6 44/14.6 0.028
Experienced Diarrhea After The 1st Vaccination
10/3.3 24/8 0.020
Feeling Dizzy After The 1st Vaccination
4/1.3 15/5 0.017
Feel Sleepy After The 1st Vaccination
170/56.6 121/40.3 0.000
Fisher test, #Chi-square test
Table 5. Correlation between type of vaccine.
Fisher test, #Chi-square test
Side Effect Dose 2
For side effects after vaccination dose 2, 35.3% of
respondents who received Pfizer type vaccination
experienced side effects of fever while for Sinovac
vaccine recipients were 19.3%. another significant
side effect between the two vaccines was 7.6% felt
coughing for Pfizer vaccine recipients and 3% for
Sinovac vaccine recipients, 4.3% experienced
diarrhea for Pfizer vaccine and 0.3% for Sinovac
vaccine and 30% felt sleepy for Pfizer vaccine
recipients, 38% for Sinovac vaccine recipients.
For side effects after vaccination dose 2, 35.3% of
respondents who received Pfizer type vaccination
experienced side effects of fever while for Sinovac
vaccine recipients were 19.3%. another significant
side effect between the two vaccines was 7.6% felt
coughing for Pfizer vaccine recipients and 3% for
Sinovac vaccine recipients, 4.3% experienced
diarrhea for Pfizer vaccine and 0.3% for Sinovac
vaccine and 30% felt sleepy for Pfizer vaccine
repellents, 38% for Sinovac vaccine recipients.
Frequency/Percentage (%)
Variables Sinovac (n=300) Pfizer (n=300) p-value
Side Effects of Fever After The 2nd Vaccination 58/19.3 106/35.3 0.000
Side Effects of Coughing After The 2nd Vaccination 9/3 23/7.6 0.024
Experienced Diarrhea After The 1st Vaccination 1/0.3 13/4.3 0.002
Feel Sleepy After The 2nd Vaccination 114/38 90/30 0.047
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
168

Table 6. Correlation between Type of Vaccine and Efficacy of the Vaccine Dose 1.
Variables Frequency/Percentage (%) p-value
Sinovac (n=300) Pfizer (n=300)
Loss of Loss and Taste after the 1st Vaccination
15/5 7/2.3 0.012
Experience
d
Cough an
d
Sore Throa
t
Afte
r
the 1s
t
Vaccination
28/9.3 37/12.3 0.002
Experienced Diarrhea After The 1st Vaccination
7/2.3 11/3.6 0.004
Having Head Pain After The 1st Vaccination
55/18.3 62/20.6 0.000
*Fisher test, #Chi-square test
Table 7. Correlation between type of vaccine and efficacy of the vaccine dose 2.
Variables Frequency/Percentage (%) p-value
Sinovac (n=300) Pfizer (n=300)
Experienced Cough and Sore Throa
t
Afte
r
the 2nd
Vaccination
11/3.6 30/10 0.003
Experienced Diarrhea After The 2nd Vaccination 4/1.3 12/4 0.000
Having Head Pain After The 2nd Vaccination 31/10.3 49/16.3 0.041
Experienced Fever After The 2nd Vaccination 31/10.3 75/25 0.000
*Fisher test, #Chi-square test
Based on table 7 above, the efficacy of the second
dose of the vaccine found significant results >0.05.
For coughs and sore throats, the p-value is 0.003, for
those who have diarrhea the p-value is 0.000, for
those who feel headache, the p-value is 0.041, and for
those who feel fever, the p-value is 0.000.
Table 8. Monitoring of side effects and efficacy after several months of vaccination.
Frequency/Percentage (%)
Variables Sinovac (n=300) Pfizer (n=300) p-value
Have been exposed to Covid-19 1-3 months after
Vaccination
5/1.7 22/7.3 0.001
Have been exposed to Covid-19 4-6 months after
Vaccination
29/9.7 13/4.3 0.015
Feel easy fatigue 1-3 months after vaccination 23/7.7 41/13.7 0.024
Feel easy fatigue 4-6 months after vaccination 3/1 8/2.7 0.222
Feel pain in arm 1-3 months after vaccination 7/2.3 0/0 0.015
Feel pain in arm 4-6 months after vaccination 2/0.7 0/0 0.000
Bleeding 1-3 months after vaccination 2/0.7 1/0.3 0.001
Experienced heart disorder 1-3 months after vaccination 6/2 0/0 0.030
Experienced heart disorder 4-6 months after vaccination 16/5.3 0/0 0.000
Cholesterol levels increase 4-6 months after vaccination 13/4.3 4/1.3 0.046
*Fisher test, #Chi-square test
Exposure Evaluation of the Complete Dosage Sinovac and Pfizer COVID-19 Vaccination into Office Employees
169

Based on table 8, on monitoring side effects and
vaccine efficacy after a complete dose of vaccine, 1
insignificant result was found with a p-value of 0.222.
The rest obtained significant results with a p-value>
0.05.
4 DISCUSSION
In this study, more than half of the respondents were
male (51%) compared to female (49%). Gender has an
impact on acceptance status, attitudes, and overall
vaccination outcomes. Women are less likely to
receive vaccines, but after vaccination women tend to
develop a more durable protective antibody response
when compared to men. However, women are also
more likely to experience side effects from
vaccines(Arumsari, Desty, and Kusumo 2021).
An alternative explanation is that vaccination
causes an increased risk of COVID-19, as well as in
other clinical and real-world trials. Another
explanation that some aspects of the vaccination event
increase the risk of infection may be, for example,
through exposure to others during the vaccination
event or while traveling from the vaccination site.
However, the increase occurred three days,before
the typical incubation period of Covid-19(Bernal
et al. 2021). Most of the currently published papers
evaluate COVID-19 patients based on their severity
and complications(Ramatillah et al. 2022).
Age has an impact on vaccine side effects. The
study's findings also revealed that 17-year-olds were
the most affected by the side effects of the vaccine.
However, the findings of this study differ from a
study in Malaysia of vaccine recipients aged 18-60
years, which found that the younger age group (18–
30) was 7.4 times more likely to experience vaccine-
related side effects(Elnaem et al. 2021). This is most
likely due to the immune system of younger people
being stronger and more efficient than older
people(Sutardi and Ramatillah 2022). Vaccine
recipients with a BMI of less than 25 have a higher risk
of experiencing side effects from the covid vaccine.
The p-value, which is less than 0.05, indicates this.
According to a study conducted in Iran, the occurrence
of side effects was higher in people with a BMI
over 25.
Headaches and flu-like symptoms, on the other
hand, were more common in people with a low
BMI(Zare et al. 2021).
From this study is known that there are 489
participants who have never been exposed to Covid-
19, 92 participantswho have mild symptoms (Self-
isolation), and 19 participants who have moderate
symptoms and no severe symptoms. For patients with
mild severity, the government is recommended to do
self-isolation at home, for the severity level being
treated in hospital (non-ICU) and for severe severity
treatment in the hospital (ICU)(Patel 2019).
The results of this study indicate that the Pfizer
vaccine provides more side effects than the Sinovac
vaccine. This is supported by the p-value of several
variables showing the number> 0.005, some of the
side effects reported to be significant are: fever,
dizziness, pain in the upper arm, cough, feeling
drowsy, and diarrhea. This is because the Sinovac
vaccine is an inactivated vaccine, while the Pfizer
vaccine is a nucleic acid and viral vector vaccine. As
a result, variations in the severity and pattern of
adverse events may be ascribed to the type of
vaccine(Cava, Bertoli, and Castiglioni 2020). The
side effects of vaccines are something that must be
taken into account. Common effects experienced by
some people after getting the vaccine include pain,
redness or swelling at the injection site, fatigue,
headache, muscle aches, chills, fever, and nausea. In
fact, these are normal signs that the body is building
up protection against COVID-19(Patel et al. 2021).
The results of this study stated that in the second
dose of vaccination, Pfizer vaccine gave more side
effects than Sinovac vaccine. This is supported by the
p-value of several variables showing the number>
0.005, BNT162b2 shows the percentage of protection
95% Cl, 90.3-97.6) with safety problems indicated by
temporary pain on injection, fever, headache which is
assessed as a reaction. normal locale. Less than 1%
experience severe pain at the injection site. This
vaccine is considered safe for the prevention of Covid-
19 infection and the antibodies last for 2 months.
The reported side effects were mainly fever and
headache (59% and 52% respectively)(Halim 2021).
Side effects tend to be more pronounced with the
second dose, especially those who receive the Pfizer-
BioNTech vaccine. These findings can be explored
further in the context of pre-treatment of the vaccine
to reduce the severity of side effects and those who
received the Sinovac vaccine were less likely to
experience side effects(Elnaem et al. 2021).
From the results of research on Indonesian office
employees aged 18-60 years who received Pfizer and
Sinovac vaccines, it was found that Pfizer vaccine
efficacy was higher than Sinovac vaccine. This is
because the method for Pfizer has utilized lipid
nanoparticles (LNPs) with formulated mRNA
vaccines(Mascellino et al. 2021). The mRNA-based
BNT162b2 COVID-19 vaccine (Pfizer vaccine) has
demonstrated 95% efficacy in preventing COVID-19
in a phase III randomized placebo-controlled trial,
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
170

with early protection from disease proven already 12
days after the first dose(Cc-by-nc 2021).
The efficacy of the second dose of vaccination
was found, Pfizer vaccine was higher than the
Sinovac vaccine with cough, diarrhea, head pain, and
fever obtained. This is because the method for Pfizer
has utilized lipid nanoparticles (LNPs) with
formulated mRNA vaccines(Mascellino et al. 2021).
Monitoring of side effects and vaccine efficacy
has been carried out within a period of 1-3 months and
4-6 months after being vaccinated. monitoring results
show a p-value >
0.005 which means significant. The Sinovac and
Pfizer vaccines are among the most notable
achievements in the development of next-generation
vaccines that demonstrate a significant role in meeting
the growing demand for global vaccines. Both
vaccines consist of lipid nanoparticles showing 95%
efficacy(Simnani, Singh, and Kaur 2022).
5 CONCLUSION
This study found that side effects were significantly
correlated, indicating that the Pfizer vaccine had more
side effects than the Sinovac vaccine. Side effects are
fever, dizziness, pain at the injection site, feeling
drowsy, coughing and diarrhea. For exposure to
Covid-19, it was found that there were 127 exposed,
with Sinovac 111 and Pfizer 16 respectively.
Variables affecting exposure to Covid-19 were age,
gender, type of vaccine, BMI and side effects.
REFERENCES
Alfatihah, B, N R Shafriani, and F N Irfani. 2021.
“Literature Review: Respon Imun Adaptif Terhadap
Vaksin Pfizer Pada Penderita Covid-19.”
http://digilib.unisayogya.ac.id/6042/%0Ahttp://digilib.
unisayogya.ac.id/6042/1/Beska
Alfatihah_1711304158_Naskah Publikasi - Beska
Alfatihah.pdf.
Amalia, Lia, and Febriani Hiola. 2020. “Analysis of
Clinical Symptoms and Immune Enhancement to
Prevent COVID-19 Disease.” Jambura Jurnal 2 (2):
71–76.
Arumsari, Wahyuni, Rani Desty, and Wahyu Kusumo.
2021. “Indonesian Journal of Health Community
Gambaran Penerimaan Vaksin COVID-19 Di Kota
Semarang Info Articles.” Indonesian Journal of Health
Community 2 2 (1): 35-45-undefined. http://e-
journal.ivet.ac.id/index.php/ijheco.
Bencharattanaphakhi, Rungrot, and Pawinee Rerknimitr.
2021. “Sinovac COVID-19 Vaccine–Induced
Cutaneous Leukocytoclastic Vasculitis.” JAAD Case
Reports 18: 1–3.
https://doi.org/10.1016/j.jdcr.2021.10.002.
Bernal, J. L., Nick Andrews, Charlotte Gower, Chris
Robertson, Julia Stowe, Elise Tessier, Ruth Simmons,
et al. 2021. “Effectiveness of the Pfizer-BioNTech and
Oxford-AstraZeneca Vaccines on Covid-19 Related
Symptoms, Hospital Admissions, and Mortality in
Older Adults in England: Test Negative Case-Control
Study.” The BMJ 373.
https://doi.org/10.1136/bmj.n1088.
Cava, Claudia, Gloria Bertoli, and Isabella Castiglioni.
2020. “In Silico Discovery of Candidate Drugs against
Covid-19.” Viruses 12 (4): 1–14.
https://doi.org/10.3390/v12040404.
Cc-by-nc, Lisensi Internasional. 2021. “MedRxiv Versi Ini
Diposting 24 Mei 2021. Pemegang Hak Cipta Untuk
Pracetak Ini.”
Chan, Nee Nee, Khang Wei Ong, Ching Sin Siau, Kai Wei
Lee, Suat Cheng Peh, Shakila Yacob, Yook Chin Chia,
Vei Ken Seow, and Pei Boon Ooi. 2022. “The Lived
Experiences of a COVID-19 Immunization
Programme: Vaccine Hesitancy and Vaccine Refusal.”
BMC Public Health 22 (1): 1–13.
https://doi.org/10.1186/s12889-022-12632-z.
Elnaem, Mohamed Hassan, Nor Hidayah Mohd Taufek,
Norny Syafinaz Ab Rahman, Nor Ilyani Mohd Nazar,
Che Suraya Zin, Wesley Nuffer, and Christopher John
Turner. 2021. “Covid-19 Vaccination Attitudes,
Perceptions, and Side Effect Experiences in Malaysia:
Do Age, Gender, and Vaccine Type Matter?” Vaccines
9 (10): 1–15. https://doi.org/10.3390/vaccines9101156.
Gee, Siobhan, Fiona Gaughran, James MacCabe, Sukhi
Shergill, Eromona Whiskey, and David Taylor. 2020.
“Management of Clozapine Treatment during the
COVID-19 Pandemic.” Therapeutic Advances in
Psychopharmacology 10 (2): 204512532092816.
https://doi.org/10.1177/2045125320928167.
Halim, Michael. 2021. “COVID-19 Vaccination Efficacy
and Safety Literature Review.” Journal of Immunology
and Allergy 3 (February).
https://doi.org/10.37191/mapsci-2582-4333-3(1)-058.
Mascellino, Maria Teresa, Federica Di Timoteo,
Massimiliano De Angelis, and Alessandra Oliva. 2021.
“‘Overview of the Main Anti-SARS-CoV-2 Vaccines:
Mechanism of Action, Efficacy and Safety’ [Response
To Letter].” Infection and Drug Resistance 14: 4501–2.
https://doi.org/10.2147/IDR.S344230.
Patel. 2019.
済無
No Title No Title No Title.
Patel, Yash R., David W. Louis, Michael Atalay, Saurabh
Agarwal, and Nishant R. Shah. 2021. “Cardiovascular
Magnetic Resonance Findings in Young Adult Patients
with Acute Myocarditis Following MRNA COVID-19
Vaccination: A Case Series.” Journal of
Cardiovascular Magnetic Resonance 23 (1): 1–8.
https://doi.org/10.1186/s12968-021-00795-4.
Ramatillah, Diana Laila, Siew Hua Gan, Ika Pratiwy, Syed
Azhar Syed Sulaiman, Ammar Ali Saleh Jaber, Nina
Jusnita, Stefanus Lukas, and Usman Abu Bakar. 2022.
“Impact of Cytokine Storm on Severity of COVID-19
Exposure Evaluation of the Complete Dosage Sinovac and Pfizer COVID-19 Vaccination into Office Employees
171

Disease in a Private Hospital in West Jakarta Prior to
Vaccination.” PLoS ONE 17 (1 1): 1–14.
https://doi.org/10.1371/journal.pone.0262438.
Ramatillah, Diana Laila, Siew Hua Gan, Syed Azhar Syed
Sulaiman, Dama Puja, Usman Abubakar, Ammar Ali
Saleh Jaber, Stefanus Lukas, and Nina Jusnita. 2021.
“Evaluation of Treatment Outcome for Pneumonia
among Pre-Vaccinated Covid-19 Patients with/without
Comorbidity in a Public Hospital in Bengkulu,
Indonesia.” Vaccines 9 (12): 1–9.
https://doi.org/10.3390/vaccines9121411.
Simnani, Faizan Zarreen, Dibyangshee Singh, and Ramneet
Kaur. 2022. “COVID-19 Phase 4 Vaccine Candidates,
Effectiveness on SARS-CoV-2 Variants, Neutralizing
Antibody, Rare Side Effects, Traditional and Nano-
Based Vaccine Platforms: A Review.” 3 Biotech 12 (1):
1–30. https://doi.org/10.1007/s13205-021-03076-0.
Sutardi, Azzahrotul Qona’Ah Ibnatus, and Diana Laila
Ramatillah. 2022. “Evaluation Comparison Between
Sinovac and Pfizer Vaccine Among Indonesian
Children and Teenager Under 18 Years Old.”
International Journal of Applied Pharmaceutics 14
(Special issue 2): 22–30.
https://doi.org/10.22159/ijap.2022.v14s2.44745.
Zare, Hamed, Hadis Rezapour, Sara Mahmoodzadeh, and
Mohammad Fereidouni. 2021. “Prevalence of COVID-
19 Vaccines (Sputnik V, AZD-1222, and Covaxin) Side
Effects among Healthcare Workers in Birjand City,
Iran.” International Immunopharmacology 101 (PB):
108351. https://doi.org/10.1016/j.intimp.2021.108351.
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
172
