
Evaluation the Effectiveness of AstraZeneca's COVID-19 Vaccine
After 6 Months of the Second Dose
Windi Marti Ningsih
a
, Liandhajani and Diana Laila Ramatillah
b
Faculty Pharmacy, Universitas 17 Agustus 1945 Jakarta, Indonesia
Keywords: COVID-19, Effectiveness, Astrazeneca.
Abstract: Growing efforts are being made to socialize the vaccination program in order to establish a global herd
immunity. In clinical trials of strategy and development, the astrazeneca vaccine's efficacy is a crucial concern.
The purpose of this study was to evaluated the effectiveness of Astrazeneca’s Covid-19 vaccine after 6 months
of the second dose. This study was conducted to evaluate the effectiveness of the Astrazeneca vaccine after 6
months of the second dose in Indonesian people. This type of research is observational with a cross sectional
design. This method is done by direct observation of the survey data. This research requires ethical approval
No.40/KEPK-UTA45JKT/EC/EXP/07/2022. The results of this study showed that there was a significant
relationship between sociodemography and the incidence of COVID-19 infection after administration of the
AstraZeneca vaccine such as gender, age, and BMI. The most common side effect of receiving AstraZeneca
is pain at the injection site. The effectiveness of the astrazeneca vaccine can be seen from the average not
exposed to covid-19, which is 95% where the Covid-19 vaccine in this study is safe for gender and age > 18
years.
1 INTRODUCTION
The respiratory condition known as Coronavirus
2019 (covid-19) is brought on by the pandemic-
causing severe acute respiratory syndrome
coronavirus 2 (sars-cov-2) (Vesselaldo & Ramatillah,
2022). Chinese health officials alerted the WHO
towards the end of 2019 about unidentified
pneumonic cases in Wuhan, Hubei Province, China.
At early January, throat swab sample from a patient
led to the discovery of a novel coronavirus (ncov-
2019). The pathogen is then given the names
coronavirus disease 2019 (Covid-19) by WHO and
severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) by coronavirus study group.
(Harapan et al., 2020).
The incubation period for COVID-19 is between
1-14 days, ranging from 3 to 7 days. The most
common symptoms in mild to moderate patients are
fever, fatique, and a dry cough accompanied by other
symptoms such as headache, nasal congestion, sore
throat, muscle aches, and joint pain (Yang et al.,
a
https://orcid.org/0000-0001-8144-9907
b
https://orcid.org/0000-0002-3921-3455
2020). Until January 2022 SARS-CoV 2, virus that
started the COVID-19 pandemic, had already killed
more than 5.5 million people. One of the current
vaccine which is one of the most popular treatments
available for the Covid-19 pandemic is an adenoviral
vector based vaccine (Barin et al., 2022).
The astrazeneca vaccine was created by the
University of Oxford and clinical trials were
conducted in the UK, Brazil and South Africa
(Voysey et al., 2021). A vaccine called Astrazeneca
was created using a genetically altered virus (viral
vector). This type of vaccine functions by
encouraging or inducing the body to develop
antibodies that fight infection with the SARS-CoV-2
virus (Pane, 2021). The decline of serum SARS-CoV-
2 antibodies has been brought up questionable long-
term immunity, which have been linked to
breakthrough infections and led to the consideration
of extra booster doses of the vaccine (Padoan et al.,
2022).
Study conducted by scientists at King's College
London showed that protection from a full-dose
Astrazeneca Covid-19 vaccine dropped within 6
Ningsih, W., Liandhajani, . and Ramatillah, D.
Evaluation the Effectiveness of AstraZeneca’s COVID-19 Vaccine After 6 Months of the Second Dose.
DOI: 10.5220/0011978300003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 179-185
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
179

months. Protection 1 month after the second dose of
Astrazeneca vaccine drops from 77% to 67% after 4
to 5 months (Sorongan, 2021).
According to research by The BMJ Covid-19
booster vaccines: What we know and who's doing
what that immunity is indeed reduced, especially
against the delta variant (Mahase, 2021). Researchers
found that a single dose of the Oxford-Astrazeneca
vaccine was only about 30% effective against delta,
although two doses were more effective: 67% for
Astrazeneca (Mahase, 2021b). In an experimental
study, antibody responses to a single dose of
Astrazeneca (AZ) (ChAdOx1 nCoV-19) vaccine
(Astrazeneca, Lund, Sweden) using five SARS-CoV-
2 antibody tests showed that seroconversion rates
after the first vaccination ranged from 66.2% up to
92.5%, which is consistent with previous studies
(Jeong et al., 2021).
Although data relating to the effectiveness of the
astrazeneca vaccine already exist. Further clinical
research is needed on the Evaluation of the
Effectiveness of the Astrazeneca Covid-19 Vaccine
after 6 months of the second dose, by looking at
various other aspects such as sociodemography that
allows for an association with the efficacy and side
effects of the astrazeneca vaccine.
2 METHOD
2.1 Design
This study used mixed methods and a prospective
cross-sectional study by collecting primary data from
Indonesian people who had received the complete
dose of Astrazeneca vaccine. This research was
conducted over a period of 4 months (April-July). For
inclusion criteria, all Indonesian people aged >18
years who have received the second dose of
AstraZeneca vaccine for more than 6 months and are
willing to be respondents in this study. The exclusion
criteria for all Indonesian people with cancer,
HIV/AIDS patients, TB patients, autoimmune
patients (lupus patients) and less than 6 months of the
second dose will be excluded in this study.
2.2 Participant
Participants in this study were Indonesian people >18
years old who had received the complete dose of
AstraZeneca vaccine with a total of 310 respondents.
2.3 Instrument
This study used questionnaires distributed through
social media (WhatsApp, Facebook, Instagram,
Telegram and Twitter). The total number of
questionnaires in this study were 62 of identity
questions and comorbidities. 62 of these questions
were about side effects received after the first and
second doses of vaccination short-term and long-term
and monitoring of side effects from the vaccine for 1-
6 months after being vaccinated.
2.4 Statistic Analysis
The collected results were analyzed using SPSS
version 25 application. Fisher, Chi-Square, Mann-
Whitney, and Kruskal Wallis tests were used to find
associations between risk factors (gender, age, BMI)
and side effects. A p-value of 0.05 was considered
significant.
2.5
Ethical Approval
This study was reviewed and approved by the
University Ethics Committee 17 August 1945 on 26
July 2022 (No.40/KEPK-
UTA45JKT/EC/EXP/07/2022).
3 RESULT
3.1 Demographic Characteristic
This study was conducted based on demographic
status, namely gender as many as 310 respondents in
this study consisted of 73 men (23.5%), and 237
women (76.5%) with an age range of 18-25 years 225
(72.5 %), 26-40 years 76 (24.3%), 41-50 years 5
(1.5%) and 51-57 years 4 (1.2%). Respondents are
also spread across several parts of Indonesia, around
10.0% from Sumatra, 85.2% from Java, 3.9% from
Kalimantan, 0.3% from Papua, and 0.6% from
Sulawesi. Based on comorbid diseases, most of the
respondents in this study 289 people (93.2%) did not
have comorbid diseases, 5 people (1.6%) with
comorbid diabetes, 2 people (0.6%) hypertension and
the last 14 people ( 4.5%) Asthma. (Table 1).
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
180

Table 1. Demographic characteristic (N=310).
Variables Fre
q
uenc
y
Percenta
g
e (%)
Gende
r
Male 73 23,5%
Female 237 76,5%
Age
18-25 225 72,5%
26-40 76 24,3%
41-50 5 1,5%
51-57 4 1,2%
Domicile
Sumatera 31 10,0%
Jawa 264 85,2%
Kalimantan 12 3,9%
Papua 1 0,3%
Sulawesi 2 0,6%
Comorbi
d
it
y
Diabetes 5 1,6%
Hy
p
ertension 2 0,6%
Asthma 14 4,5%
3.2 Side Effect after Vaccination
Table 2: Side Effect after Vaccination.
Variables
Frequency /
Persentage
(%)
1st dose
(n=310)
Frequency /
Persentage
(%)
2nd dose
(n=310)
Side effect 1st dose
vaccination
Fever 182 (58,7%) 109 (35,2%)
Pain in the injection
area 233 (75,2%) 188 (60,6%)
Cough 39 (12,6%) 28 (9%)
Flu 60 (19,4%) 37 (11,9%)
Nausea 56 (18,1%) 28 (9%)
Dia
r
rhea 17 (5,5%) 18 (5,8%)
Dizziness 140 (45,2%) 96 (31%)
Sleepiness 156 (50,3%) 130 (41,9%)
Dehyd
r
ate
d
72 (23,2%) 49 (15,8%)
Bleeding 3 (1%) 2 (0,6%)
Pain in the upper ar
m
200 (64,5%) 136 (43,9%)
Cardiovascular
events 10 (3,2%) 7 (2,3%)
Based on table 2, it is known that the common side
effects felt by respondents in this study at the first
dose of AstraZeneca were fever 58.7%, pain at the
injection site 75.2%, cough 12.6%, flu 19.4%, nausea
18,1%, diarrhea 5.5%, dizziness 45.2%, feeling
sleepy 50.3%, thirst or dehydration 23.2%, bleeding
1.0%, upper arm pain 64.5%, and cardiovascular 3.2
%. In the second dose, the side effects were fever
35.2%, pain at the injection site 60.6%, cough 9.0%,
flu 11.9%, nausea 9.0%, diarrhea 5.8%, dizziness
31%, feeling sick. drowsiness 41.9%, thirst or
dehydration 15.8%, bleeding 0.6%, upper arm pain
43.9%, and cardiovascular 2.3%.
3.3 Between Gender and Side Effects
Table 3: Correlation between gender and side effects
Variables
Frequency / Percentage
(%)
p-
value
Male
(n=73)
Female
(n=237)
Side effects 1st
vaccination
Sleepiness
29
(39,7%) 127 (53,5%) 0,045*
Monitoring 1-3
months after
vaccination
Menstrual
problems 0 23 (9,7%) 0,000#
Monitoring 4-6
months after
vaccination
Menstrual
problems 0 16 (6,7%) 0,000#
*Fisher test,
#Chi-square test
In this study, the variable that had a significant
correlation with the side effects that appeared was
gender. From a total of 310 respondents, 23.5% of
respondents were male and 76.5% were female. The
details of the data are presented in table 3. For
questions about side effects of fisher’s test results and
chi-square test results for follow-up 1-3 months and
4-6 months after vaccination.
Evaluation the Effectiveness of AstraZeneca’s COVID-19 Vaccine After 6 Months of the Second Dose
181

3.4 Between Age and Side Effects
Another variable that has a significant relationship is
age and vaccine side effects. In this study, the overall
age of the respondents was 18-57 years. Most of the
side effects are felt by those who are at the age of 23
years. Detailed data are presented in table 4. from the
results of the Mann-Whitney test for questions about
side effects after receiving the vaccine and the results
of the Kruskal Wallis test for questions about
monitoring side effects after 1-3 months and 4-6
months after vaccination. In accordance with research
that has been done, many side effects are felt by those
who are at the age (median: 23 years).
3.5 Between BMI and Side Effects
The last variable that correlates is BMI. In this study,
individuals who experienced side effects of fever (p =
0.035) and loss of smell (p = 0.013) with an average
BMI (Body Mass Index) of 21.
Table 4: Correlation between age and side effects.
Variables Frequency p-value
Age (n:310 median: 23)
Side effects 1st vaccination
Nauseous 56 (18,1%) 0,040*
Covid-19 infection after 1st dose
Cough and Sore Throat 63 (20,3%) 0,001*
Headache 104 (33,5%) 0,029*
Covid-19 infection after 2nd dose
Cough and Sore Throat 44 (14,1%) 0,011*
Monitoring 1-3 months after vaccination
Infected with Covid-19 11 (3,5%) 0,039#
Menstrual problems 24 (7,7%) 0,001#
Heart problems 6 (1,9%) 0,011#
Monitoring 4-6 months after vaccination
Menstrual problems 16 (5,1%) 0,001#
*Mann-whitney test, #Kruskal wallis test
Table 5: Correlation between BMI and side effects.
Variables
Frequency / Persentage (%)
p-value
BMI (n:310 median 21)
Side effects 1st vaccination
Fever 21 (6,7%) 0,035*
Side effects 2nd vaccination
Loss of smell 13 (4,1%) 0,013*
*Mann-whitney test
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
182
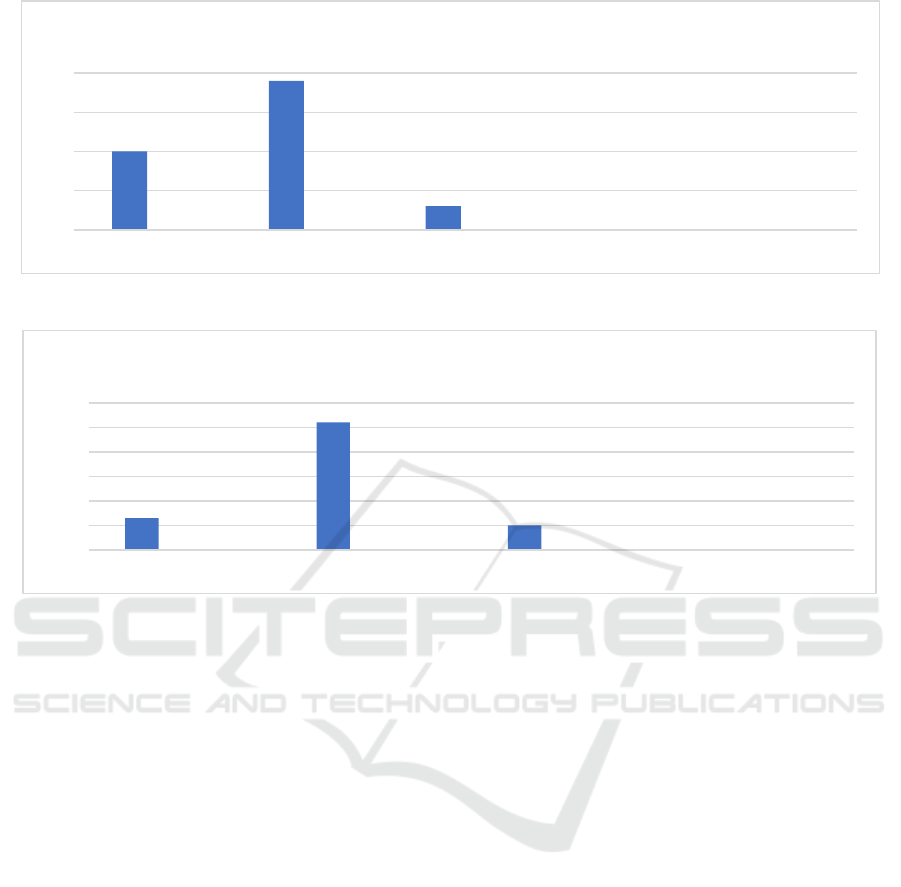
Figure 1: Symptoms of Exposure to Covid-19 1st Dose.
Figure 2: Symptoms of Exposure to Covid-19 2nd Dose.
3.6 The Wffectiveness of the
AstraZeneca Vaccine Based on the
Symptoms of Exposure to Covid-19
Based on Fig 1 and 2. above, it can be seen that most
of the respondents in this study were not exposed to
COVID-19 after the first dose of AstraZeneca
vaccination as much as 97%, asymptomatic 1%, mild
symptoms 1.9%, moderate symptoms 0.3%, and 0%
respondents with severe symptoms. 93% were not
exposed to covid-19 after the second dose of
AstraZeneca vaccination, 1.3% asymptomatic, 5.2%
mild symptoms, 1% moderate symptoms, and 0% of
respondents with severe symptom.
4 DISCUSSION
4.1 Vaccine Side Effects
This study involved 310 people who were fully
vaccinated, participants received a questionnaire that
they experienced the side effects of the vaccine. As
shown in Table 2 of this study found a slight
difference with the study in Vietnam, it was found
that the most common side effects were fever
(69.4%), muscle aches (68.6%), fatigue/drowsiness
(62, 5%), body aches (59.4%), headache (58.3%) and
chills (45.7%) (Tran et al., 2020). Whereas in another
study from Saudi Arabia reported some side effects
such as fatigue (90%), injection site pain (85%), fever
(66%), and headache (62%) (Alhazmi et al., 2021).
The side effect that is most commonly felt is pain in
the injection area, this is in accordance with previous
research conducted in Ethiopia which described that
the most common local effect was pain at the
injection site (65.48%) (Solomon et al., 2021).
4.2 Correlation Between Gender and
Vaccine Side Effects
There was a significant difference between the side
effects felt by the patients and gender. As it was found
that female vaccine recipients had more side effects
than male vaccine recipients. 53.3% of female
respondents experienced side effects of feeling
drowsy after receiving the first dose of vaccine, while
39.7% for males. For monitoring 1-3 months after
vaccination it was found that 9.7% of women who
received the vaccine experienced a change in their
menstrual cycle, while for monitoring 4-6 months
1% (3)
2% (6)
0,3% (1)
0,% (0)
0%
1%
1%
2%
2%
No symptoms Mild Moderate Severe
Symptoms of Covid-19 After Vaccination First Dose
1,3% (4)
5,2% (16)
1% (2)
0% (0)
0,0%
1,0%
2,0%
3,0%
4,0%
5,0%
6,0%
No symptoms Mild Moderate Severe
Symptoms of Covid-19 After Vaccination Second Dose
Evaluation the Effectiveness of AstraZeneca’s COVID-19 Vaccine After 6 Months of the Second Dose
183

after vaccination it was found that 6.7%. See table 3.
Females experience side effects more than males. Out
of 310 respondents reported that women experienced
significant side effects of the first dose such as feeling
drowsy (53.5% ; p-value 0.045) this proves that
women generally have a greater level of antibody
response to viruses, infections and vaccinations
(Scully et al., 2020).
4.3 Correlation Between Age and
Vaccine Side Effects
As the data in table 4 shows, age has an impact on
vaccine side effects. The findings of this study are in
accordance with research that has been done, many
side effects are felt by those who are at the age
(median: 23 years). These results are in agreement
with a study conducted on Bangladeshi population
that reported side effects were much greater in young
adults than in older adults (p = 0.02) (Jahan et al.,
2021). In Hamed Zare's study on health workers in
Iran the frequency of side effects such as injection site
pain, fatigue, headache and fever was higher in those
under 40 years of age (Zare et al., 2021). This
phenomenon is interpreted using the concept of
immunosenescence which refers to the decline in the
function of the immune system with increasing age
(Ramasamy et al., 2020).
4.4 Correlation Between BMI and
Vaccine Side Effects
The last variable that correlates with side effects and
vaccine efficacy is BMI. In this study, individuals
who experienced side effects of fever (p= 0.035) and
loss of smell (p=0.013) with an average BMI (Body
Mass Index) of 21. Vaccine recipients with a BMI
<25 had a higher risk of experiencing side effects
from the vaccine Covid-19 (Sutardi & Ramatillah,
2022).
4.5 The Effectiveness of the
AstraZeneca Vaccine based on the
Symptoms of Exposure to Covid-19
In Figures 1 and 2 of this study, the average 310
respondents who were exposed to COVID-19 after
the first dose of vaccination were 3.3%, and 7.5%
exposed to Covid-19 after the second dose of
vaccination. The results of this study prove that the
effectiveness of the complete dose of AstraZeneca
vaccine can reduce the risk and prevent COVID-19
by up to 94%. A study conducted in the United States
in a phase III trial found that the vaccine produced by
the University of Oxford (AstraZeneca) was 79%
effective in preventing symptoms of COVID-19 and
100% effective in preventing serious illness and
hospitalization (Boytchev, 2021).
5 CONCLUSIONS
The most common side effects is pain in the injection
after the first and second doses. The variables that
affect the side effects and effectiveness of the vaccine
are gender, age and BMI. For gender, female vaccine
recipients experienced significantly more side effects
than males. The age of the vaccine recipients was
dominated by the age of 23 years who experienced
side effects after vaccination. As for BMI, it was
found that vaccine recipients who have BMI < 25 had
a higher risk of experiencing side effects. The
effectiveness of the astrazeneca vaccine can be seen
from the average not exposed to COVID-19, which is
95%. Overall, based on this study the Covid-19
vaccine was safe for gender and age >18 years, in this
study no severe side effects or fatal allergic reactions
were found in AstraZeneca vaccine recipients.
REFERENCES
Alhazmi, A., Alamer, E., Daws, D., Hakami, M., Darraj,
M., Abdelwahab, S., Maghfuri, A., & Algaissi, A.
(2021). Evaluation of side effects associated with
covid‐19 vaccines in Saudi Arabia. Vaccines, 9(6).
https://doi.org/10.3390/vaccines9060674
Barin, B., Kasap, U., Selçuk, F., Volkan, E., & Uluçkan, Ö.
(2022). Comparison of SARS-CoV-2 anti-spike
receptor binding domain IgG antibody responses after
CoronaVac, BNT162b2, ChAdOx1 COVID-19
vaccines, and a single booster dose: a prospective,
longitudinal population-based study. The Lancet
Microbe, 274–283. https://doi.org/10.1016/s2666-
5247(21)00305-0
Boytchev, H. (2021). Covid-19: Germany struggles with
slow uptake of Oxford AstraZeneca vaccine. BMJ
(Clinical Research Ed.), 372(March), n619.
https://doi.org/10.1136/bmj.n619
dr. Merry Dame Cristy Pane. (2021). Vaksin AstraZeneca
atau AZD1222 adalah vaksin untuk mencegah penyakit
COVID-19. Vaksin ini merupakan hasil kerja sama
antara Universitas Oxford dan AstraZeneca yang
dikembangkan sejak Februari 2020. Alodokter.Com.
https://www.alodokter.com/vaksin-astrazeneca
Harapan, H., Itoh, N., Yufika, A., Winardi, W., Keam, S.,
Te, H., Megawati, D., Hayati, Z., Wagner, A. L., &
Mudatsir, M. (2020). Coronavirus disease 2019
(COVID-19): A literature review. Journal of Infection
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
184

and Public Health, 13(5), 667–673.
https://doi.org/10.1016/j.jiph.2020.03.019
Jahan, N., Rahman, F. I., Saha, P., Ether, S. A.,
Roknuzzaman, A. S. M., Sarker, R., Kalam, K. T., Haq,
K., Nyeen, J., Himi, H. Z., Nazmul Hossain, M.,
Chowdhury, M. H., Uddin, M. M., & Alam, N. H.
(2021). Side effects following administration of the first
dose of Oxford-AstraZeneca’s covishield vaccine in
Bangladesh: A cross-sectional study. Infectious
Disease Reports, 13(4), 888–901.
https://doi.org/10.3390/IDR13040080
Jeong, S., Lee, N., Lee, S. K., Cho, E. J., Hyun, J., Park, M.
J., Song, W., Jung, E. J., Woo, H., Seo, Y. Bin, Park, J.
J., & Kim, H. S. (2021). Comparison of the results of
five sars-cov-2 antibody assays before and after the first
and second chadox1 ncov-19 vaccinations among
health care workers: A prospective multicenter study.
Journal of Clinical Microbiology, 59(12).
https://doi.org/10.1128/JCM.01788-21
Mahase, E. (2021a). Covid-19: Two vaccine doses are
crucial for protection against delta, study finds. BMJ
(Clinical Research Ed.), 374(August), n2029.
https://doi.org/10.1136/bmj.n2029
Mahase, E. (2021b). Covid-19 booster vaccines: What we
know and who’s doing what. BMJ (Clinical Research
Ed.), 374(August), n2082.
https://doi.org/10.1136/bmj.n2082
Padoan, A., Cosma, C., Bonfante, F., Della Rocca, F.,
Barbaro, F., Santarossa, C., Dall’olmo, L., Pagliari, M.,
Bortolami, A., Cattelan, A., Cianci, V., Basso, D., &
Plebani, M. (2022). Neutralizing antibody titers six
months after Comirnaty vaccination: Kinetics and
comparison with SARS-CoV-2 immunoassays.
Clinical Chemistry and Laboratory Medicine, 60(3),
456–463. https://doi.org/10.1515/cclm-2021-1247
Ramasamy, M. N., Minassian, A. M., Ewer, K. J., Flaxman,
A. L., Folegatti, P. M., Owens, D. R., Voysey, M., Aley,
P. K., Angus, B., Babbage, G., Belij-Rammerstorfer, S.,
Berry, L., Bibi, S., Bittaye, M., Cathie, K., Chappell,
H., Charlton, S., Cicconi, P., Clutterbuck, E. A., … Zizi,
D. (2020). Safety and immunogenicity of ChAdOx1
nCoV-19 vaccine administered in a prime-boost
regimen in young and old adults (COV002): a single-
blind, randomised, controlled, phase 2/3 trial. The
Lancet, 396(10267), 1979–1993.
https://doi.org/10.1016/S0140-6736(20)32466-1
Scully, E. P., Haverfield, J., Ursin, R. L., Tannenbaum, C.,
& Klein, S. L. (2020). Considering how biological sex
impacts immune responses and COVID-19 outcomes.
Nature Reviews Immunology, 20(7), 442–447.
https://doi.org/10.1038/s41577-020-0348-8
Solomon, Y., Eshete, T., Mekasha, B., & Assefa, W.
(2021). Covid-19 vaccine: Side effects after the first
dose of the oxford astrazeneca vaccine among health
professionals in low-income country: Ethiopia. Journal
of Multidisciplinary Healthcare, 14, 2577–2585.
https://doi.org/10.2147/JMDH.S331140
Sutardi, A. Q. I., & Ramatillah, D. L. (2022). Evaluation
Comparison Between Sinovac and Pfizer Vaccine
Among Indonesian Children and Teenager Under 18
Years Old. International Journal of Applied
Pharmaceutics, 14(Special issue 2), 22–30.
https://doi.org/10.22159/ijap.2022.v14s2.44745
Tommy Sorongan. (2021). Riset Ungkap Perlindungan
Vaksin AstraZeneca Cuma 6 Bulan. CNBC Indonesia.
https://www.cnbcindonesia.com/news/2021082520541
4-4-271294/riset-ungkap-perlindungan-vaksin-
astrazeneca-cuma-6-bulan
Tran, V. N., An, H., Thanh, T., Le, A., Tung, T., & Thao,
P. (2020). Since January 2020 Elsevier has created a
COVID-19 resource centre with free information in
English and Mandarin on the novel coronavirus
COVID- 19 . The COVID-19 resource centre is hosted
on Elsevier Connect , the company ’ s public news and
information . January.
Vesselaldo, M., & Ramatillah, D. L. (2022). Evaluation of
Bmi Relationship With Increased D-Dimer in Covid-19
Patients At a Jakarta Private Hospital. International
Journal of Applied Pharmaceutics, 14(Special Issue 2),
49–53. https://doi.org/10.22159/ijap.2022.v14s2.44749
Voysey, M., Clemens, S. A. C., Madhi, S. A., Weckx, L.
Y., Folegatti, P. M., Aley, P. K., Angus, B., Baillie, V.
L., Barnabas, S. L., Bhorat, Q. E., Bibi, S., Briner, C.,
Cicconi, P., Collins, A. M., Colin-Jones, R., Cutland, C.
L., Darton, T. C., Dheda, K., Duncan, C. J. A., …
Zuidewind, P. (2021). Safety and efficacy of the
ChAdOx1 nCoV-19 vaccine (AZD1222) against
SARS-CoV-2: an interim analysis of four randomised
controlled trials in Brazil, South Africa, and the UK.
The Lancet, 397(10269), 99–111.
https://doi.org/10.1016/S0140-6736(20)32661-1
Yang, C. L., Qiu, X., Zeng, Y. K., Jiang, M., Fan, H. R., &
Zhang, Z. M. (2020). Coronavirus disease 2019: A
clinical review. European Review for Medical and
Pharmacological Sciences, 24(8), 4585–4596.
https://doi.org/10.26355/eurrev_202004_21045
Zare, H., Rezapour, H., Mahmoodzadeh, S., & Fereidouni,
M. (2021). Prevalence of COVID-19 vaccines (Sputnik
V, AZD-1222, and Covaxin) side effects among
healthcare workers in Birjand city, Iran. International
Immunopharmacology, 101(PB), 108351.
https://doi.org/10.1016/j.intimp.2021.108351
Evaluation the Effectiveness of AstraZeneca’s COVID-19 Vaccine After 6 Months of the Second Dose
185
