
Growth Prevention System for Inhibiting Corrosion Rate in Ship
Operation
Didit Sumardiyanto
1
and Sri Endah Susilowati
2
1
Faculty of Engineering, 17Agustus 1945 Jakarta University, Sunter Permai Raya Street, Jakarta, Indonesia
2
Department of Mechanical Engineering, 17Agustus 1945 Jakarta University, Jakarta, Indonesia
Keywords: MGPS, biofouling, copper electrode RRY17.
Abstract: Marine Growth Prevention System (MGPS) is a system applied on ships to inhibit marine growth or
biofouling, namely colonies of marine animals and plants that grow and cover the surface of structures or
piping systems on ships that can cause corrosion. The reduced flow as a result of the appearance of
impurities on the inner wall of the cooling system pipe causes a failure in cooling the ship's engine, which
results in overheating. Another impact of reducing the inside diameter of the pipe, the pressure received by
the pipe on the discharge side becomes larger, and cavitation occurs in the suction side due to excessive
pressure drop. The purpose of this study was to determine the impact of the use of the Marine Growth
Prevention System which was applied to the MT ship. Savvy in inhibiting the corrosion rate on the JIS
F0507 type seawater pipe by using copper (Cu) electrode type RRY17 based on the ship speed variability
value. From the research, it was found that protection using the Marine Growth Prevention System carbon
anode (copper electrode) is very effective for protecting the pipes in the engine cooling system and ship hull
from marine animals and plants.
1 INTRODUCTION
The operation of a large ship is always equipped
with an engine cooling system and a ship balancing
system with fluid using seawater, so a piping system
is needed for the circulation of the cooling fluid.
This causes the growth of various marine biota
plants such as coral, barnacles, and marine algae that
trigger corrosion. The Motor Tanker (MT) Savvy
ship uses seawater as engine cooling medium and
also as ballast water. The pipe installation used is
JIS-F0507. The use of sea water causes the growth
of marine growth which triggers corrosion which
will affect engine performance and decrease
usability, especially engine effectiveness and cause a
lot of detrimental damage.
To protect against corrosion in the piping system,
MT. Savvy uses a type-SC MGPS mounted on a Sea
Chest (SC) which is equipped with an RRY17 type
copper (Cu) electrode each and an aluminum
electrode placed in strategic locations, as close as
possible to the area to be protected. The anode is
connected to a control panel that regulates the
current flowing to the anode. Ions produced by the
anode will be dispersed by seawater and create an
environment that is not conducive to marine growth
in the area. Another advantage is that the aluminum
hydroxide formed will create a protective layer on
the surface of the channel so as to prevent corrosion.
The use of a stable current on the copper
electrodes and aluminum electrodes on the hull will
produce copper and aluminum hydroxide ions to
protect the pipe from marine biota plants that can be
corrosive and form rust. This method of preventing
the development of corrosion is known as the
Marine Growth Prevention System (MGPS).
MGPS works with an electrolyte method or
principle that works to provide continuous
protection without the use of chemicals. The trick is
to combine two systems, namely the installation of
anti-fouling pipes and corrosion suppression. With
control from a low-voltage power supply panel that
is channeled to an anode which is connected directly
to the liquid in the pipeline to minimize the
influence of acidity of the liquid content on the
corrosion process along the pipeline installation. The
specialty of using this system is that it is
environmentally friendly, does not use chemicals to
neutralize the liquid condition, in accordance with
300
Sumardiyanto, D. and Susilowati, S.
Growth Prevention System for Inhibiting Corrosion Rate in Ship Operation.
DOI: 10.5220/0011980200003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 300-306
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

the rules that apply to the classification of
international rules.
This system consists of a pair of copper and
aluminum called anodes which are attached to the
inlet filter of the liquid to be neutralized. The copper
anode produces ions that flow through a liquid
medium that is in direct contact with it. These ions
have the potential to inhibit the growth of shellfish
and barnacles along the anode current flow range.
Without anti-fouling protection, pipes can be full of
organisms which over time can cause blockages,
reducing system efficiency in pipe installations, with
the help of aluminum hydroxide as flocculation
released by copper. This is the so-called double
protection, where the system can benefit from being
protected from bio-fouling and corrosion processes.
In new installations the anodes are attached to the
sea chests using special retainers or a mounting
flange. Or if the system is installed before the
drydocking vessel, the anode can be installed in the
seawater filter. Another advantage is that it is easy to
replace a new anode.
Another study related to this problem was carried
out by Seung Jun Lee and Jun Lee Seong (2011)
which was about the effect of flow velocity on
marine growth using electrochemical alloy Al 5083-
H116 on the suction pipe. The results showed that
the corrosion rate increased linearly with flow
velocity compared to static conditions. Meanwhile,
other studies have shown that the installation of
aluminum alloy carbon anodes on the hull plate of
KM ADRI XLIV technically has good performance
and is very influential in slowing the corrosion rate
of ship steel plates (Nur Aziz, 2012).
This study aims to analyze the use of MGPS on
MT. Savvy at inhibiting the corrosion rate on the
ship engine cooling pipe due to Marine growth. The
method used is to calculate the corrosion rate in the
JISF0507 seawater pipe and weigh the decrease in
weight of the RRY17 type copper electrode based on
the value of ship speed variability.Be advised that
papers in a technically unsuitable form will be
returned for retyping. After returned the manuscript
must be appropriately modified.
2 RESEARCH METHODS
The research was conducted on the MT Savvy Ship,
which uses the MGPS system using a combination
of Aluminum (Al) and Copper (Cu) electrodes.
2.1 Equipment Used Tup
Megger insulation tester, 1 set
Multimeter, 1 set
Toll set, 1 box
Vernier calipers, 1pc
2.2 Mgps Materials
Prevent corrosion resistance electrode, type
RRY17, 1 pc
Pipe, JIS F 0507 standard pipe (the level of
UNS G10200 Carbon steel) with a diameter of
20 cm, a length of 150 cm as the sample metal
(seawater pipe).
2.3 Data Collection Techniques
Data collection through observation techniques and
field note documentation. This research instrument
emphasizes the observation technique because it is
considered in accordance with the characteristics of
action research.
2.4 Implementation of Corrosion Test
In the corrosion test, the normal concentration level
or the average pH of the seawater environment is 8.0
and the electrode sample (Cu) is immersed in a sea
chest box for 3 days or 72 hours non-stop with
several treatments for variations in seawater speed
based on the speed of ship movement, including:
Ship totally stop, moving water, 0.0m/s
Full speed ship, 12.9knots, or moving water
6.6363m/s.
Medium speed ship, 9.7knots, or water
moving 4.9901m/s.
Slow speed ship, 6.5knots, or water moving
3.3438m/s.
Dead speed ship, 3.3knots, or water moving at
1.6976m/s.
The condition in the case of sea chest intake is
not protected with MGPS as shown in Figure 1.
Growth Prevention System for Inhibiting Corrosion Rate in Ship Operation
301

Figure 1. Marine growth and its effects, (a) intake hole, (b)
pipe interior, (c) filter, (d) pole base plate
Ship particulars of MT. Savvy:
Specifications:
Tonnage, gross (6,694tons), nett (3,785tons),
displacement (13,263tons), dead weight (10,327
tons), lightship (2,935tons), length over all (120m),
length between perpendicular (114m), breadth
molded (20.50m), depth molded (10m), summer
draft (7.61 m), height from keel (29.16m).
Machineries:
Main engine, Hanshin, LH46LG, power:
3,960hp, speed: 220rpm (1 unit), Auxiliary
engine,Yanmar, 6LAAL-DTN, power: 250kW,
speed: 1,200 rpm (3 units), Harbor generator,
Yanmar, 6CHL-TN 80 kAV, power: 75kW, speed:
1,800rpm (1unit), Emergency generator, Mitsui
Deutz, F4910, power: 40 kW, speed: 1,800 rpm (1
unit)), Bow thruster engine, Yanmar, 6KY-ET1
4CyC, power: 370kW, speed: 2,040rpm (2 units),
Cargo pump, screw type, CSL- 1000P, speed: 600
rpm, pressure: 0.98MPa, capacity 1,000m3/h (2
units), Stripping pump, screw type, CSL-300, speed:
600rpm, pressure: 0.98MPa, capacity: 300 m3/h (1
unit), air conditioner (Daikin Ind US20H, power:
75kW (2 units), Fan, power: 2.5kW (1 unit).
Tanks:
Cargo tank, capacity: 13,307m3, slop tank,
capacity: 1,476m3, MFO tank, capacity: 8,300liter +
343.22m3, MDO tank, capacity: 6,000 liter + 76m3,
ballast tank, capacity: 4,264m3.
Application of MGPS on MT. Savvy
Application of the MGPS pipe guard system on
MT. Savvy uses Hikari production Sangyo Co., Ltd.
Japan with two combination types of installation,
namely:
a. Typical seachest anode arrangement
Copper anodes and aluminium anodes are
installed in the sea chest if the tanker is planned for
drydocking for less than 5 years, assuming there is
sufficient space available.
Figure 2. Type of anode installation on sea chest
Typical strainer anode arrangement
The advantage of the filter mount system is that
the anode can be changed at any time without the
need for drydocking. In this case, the sea water
coming from the sea chest must still be ensured free
from dirt and contamination.
Figure 3. Type of anode installation on filter strainer
Figure 4. MGPS copper electrode and aluminum electrode
Measurement/identification
The dimensions and mass of the electrodes are
measured before carrying out the test. Dimensions
are measured using a vernier caliper and steel ruler.
Figure 5. Dimensional measurement of copper electrode
RRY17
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
302
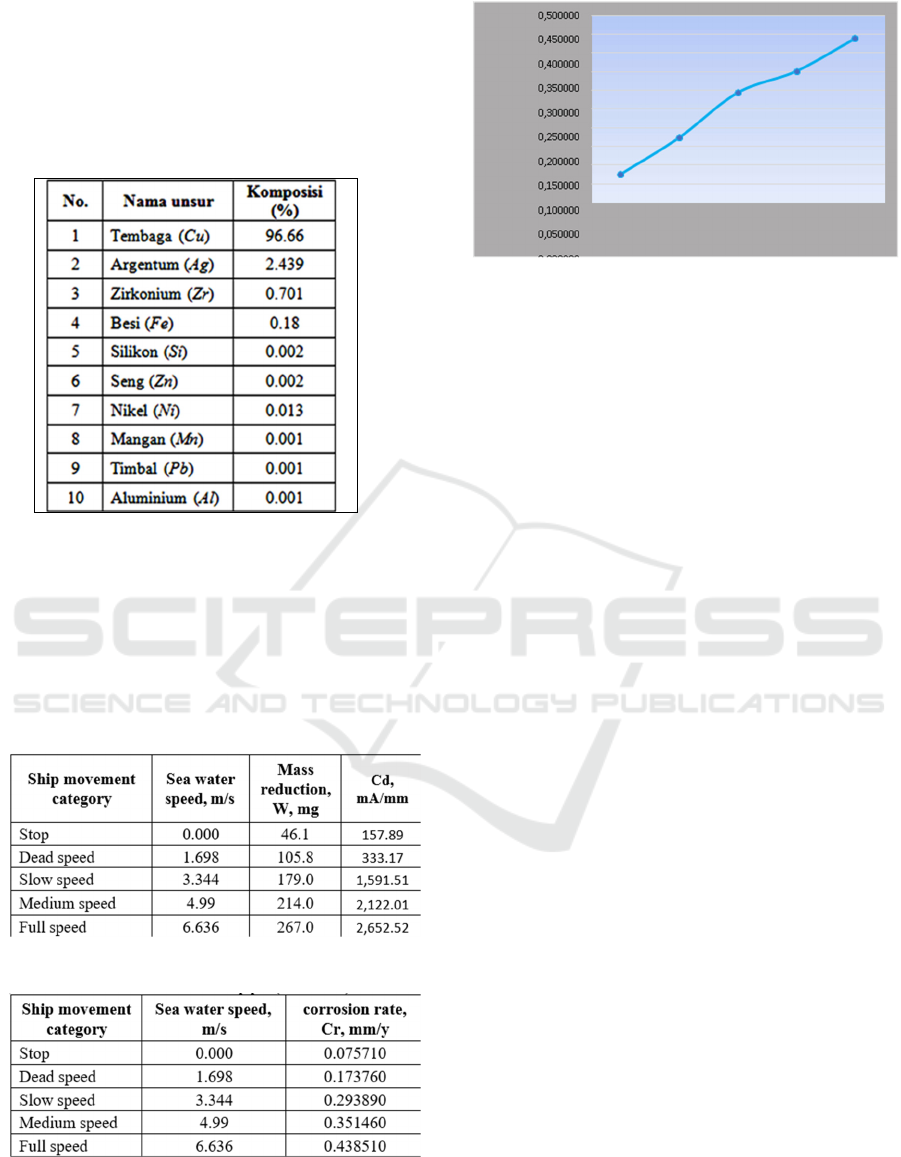
3 RESULT AND DISCUSSION
The chemical composition of the electrodes used is
obtained from the delivery order sheet, as follows:
Table 1. Chemical composition of copper electrode
RRY17.
The form should be completed and signed by one
author on behalf of all the other authors.
The electrodes (samples) were immersed in a sea
chest box with variations in seawater discharge
based on the speed of the ship for 72 hours. After
observing the data obtained in the form of reduction
of the anode mass as shown in table 2 below:
Table 2. Mass reduction in seawater pipes (JIS F-0507)
Table 3. Corrosion rate in seawater pipes (JIS F 0507)
Figure 6. Effect of ship speed on corrosion rate in
seawater pipes
Steel pipe in seawater (NaCl) environment will
corrode due to the presence of Cl ions. These ions
will break down the passive layer on the seawater
pipe steel (JIS F 0507). When in contact with metal
surfaces, Cl- ions will dissolve metal ions and make
it easier to enter the solution. From Figure 6, it can
be seen that the corrosion rate of the seawater pipe
(JIS F 0507) will increase along with the increase in
the value of the seawater speed, as a modeling of
ship movement
Other research conducted by Seung-Jun Lee et al
also showed that the corrosion rate that occurred in
the sea chest of the ship increased with increasing
flow velocity compared to the static state. When the
ship is traveling at full speed away, the propulsion
engine burns more fuel, thus requiring a cooling
fluid, which in this case is seawater, with a larger
flow capacity, and causes the velocity of the water
flow in the pipe to increase.
2. Weight Reduction on RRY1 Copper
Electrode
The total weight of the anode, Wtot required for
protection can be calculated by the equation:
Wtot = I p Y C
Explanation:
dimana Ip = A Cd
1000
Ip, the current strength needed for protection Y, test
time = 3days or 0.008year
C, anode reduction number = 3.6kg/Amp.y
Cd, electric current density (console panel), mA/m2
A, seawater pipe area = 0.09425m2
µ, utilization factor = 90%
Growth Prevention System for Inhibiting Corrosion Rate in Ship Operation
303
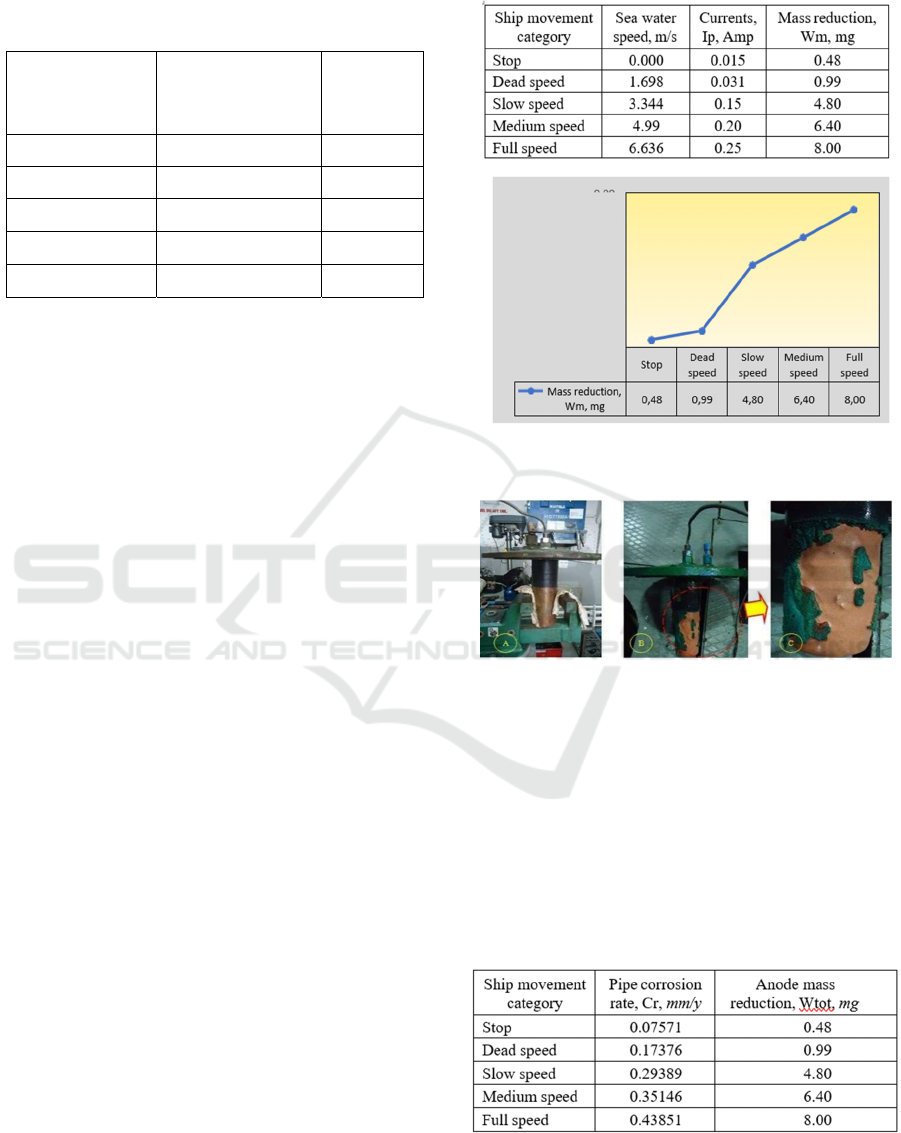
The following is the current density data (Cd) from
various ship speeds as shown in Table 4 below.
Table 4. Test results for copper (Cu) RRY17 electrodes
Ship
movement
category
Sea water
speed, m/s
C
d
,
mA/m
2
Stop 0.000 157.89
Dead speed 1.698 333.16
Slow speed 3.344 1,591.51
Medium speed 4.990 2,122.01
Full spee
d
6.636 2,652.52
Then the value of Wtot for various ship speeds:
a. Water is still or when the ship stops (0m/s)
Cd = 157.89mA/mm
So:
Ip = 0.09425 157,89 = 0.015 Amp
1000
Wtot = 0.015 0.008 3.6 = 0.48 mg
0.90
b. Dead slow ship, 3.3knots (1.698m/s)
Cd = 333.156mA/m2,
so :
Ip = 0.031Amp
Wtot = 0.99mg
c. Ship slow ahead, 6.5 knots (3,344 m/s)
Cd = 1591.51mA/m2,
so :
Ip = = = 0.15 Amp
Wtot = = 4.8mg
d. Half ahead, 9.7 knots (4.99 m/s),
Cd = 2122.01mA/m2,
so:
Ip = = 0.20Amp
Wtot = 6.4mg
e. Full away ship, 12.9knots (6,636m/s),
Cd = 2652.52mA/m2,
so:
Ip = 0.25Amp Wtot = 8.0mg
Table 5. mass reduction of RRY17 copper (Cu) anode at
various vessel speeds
Figure 7. The effect of ship speed on the reduction of the
anode mass of copper (Cu) RRY17
Figure 8. Weight reduction on the copper electrode
RRY17, (A). Initial conditions, (B). Condition after 72
hours, (C). Image magnification B
From the calculation results of the relationship
between the corrosion rate of seawater pipes (JIS F-
0507) compared to the reduction in mass of the
copper anode (Cu) RRY17 as shown in Table 6 and
Figure 9 below.
Table 6. Reduction of mass of copper anode (Cu) RRY17
and corrosion rate of seawater pipe (JIS F-0507) at various
ship speeds
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
304

Figure 9. Reduction of mass of RRY17 copper anode (Cu)
and corrosion rate of seawater pipe (JIS F-0507)
If you look at the mass reduction value at the JIS F-
0507 seawater pipe cathode in Table 6, it is clear
that the reduction that occurs is very large when
compared to the mass reduction that occurs at the
copper (Cu) RRY17 anode. Steel pipe for seawater
pipe JIS F 0507 is at the top of the table and has the
most negative electrode potential value when
compared to copper (Cu) RRY17. Thus, (Fe) is the
most reactive when compared to (Cu). This causes
the cathode weight reduction (JIS F-0507) to be
greater when compared to the RRY17 copper anode
weight reduction. Likewise with (Al), aluminum has
a more negative electrode potential than copper
which is more positive when compared to (Fe).
When comparing the anode weight reduction value
obtained in the field and the anode weight reduction
value obtained from the formula calculation, it is
seen that there is a difference in the anode weight
requirement value. This happens because the
formula does not take into account the speed of the
sea water (ship speed), while the data in the field
shows that the speed of the sea water greatly affects
the weight requirements of the anode. The higher the
speed of the water (vessel speed), the higher the
anode weight requirement which is used to prevent
the growth of marine growth that causes corrosion.
4 CONCLUSIONS
Corrosion rate of seawater pipe (JIS F 0507) on MT.
Savvy increases with increasing sea speed (ship
speed). At the stop speed (0.0m/s) corrosion rate is
0.07571mm/y, at full speed (6636m/s) corrosion rate
is 0.43851mm/y.
The reduction in the mass of the copper (Cu)
RRY17 electrode is higher or faster in proportion to
the speed of sea water (ship speed). At the stop
position (0.0 m/s) the reduction in the mass of the
electrode (Cu) RRY17 is 0.48 mg and the current at
the electrode is 0.015 Ampere. Meanwhile, at full
speed (6.636 m/s) the reduction in the mass of the
electrode (Cu) RRY17 is 8.0 mg and the current at
the electrode is 0.25 Ampere.
The amount of current density (Cd) depends on
the effect of the environment, maintenance and
coating, in this case the speed of the ship is very
influential because when the ship stops the Cd value
is 157.89 mA/m2 while at full speed Cd is 2652.52
mA/m2.
REFERENCES
Borys, B., Jared. C, S. Subramanian, Therese van der
Hoorn, Waheed Zaman., 2015, Corrosion and
Prevention Methods In Desalination Plants,
http://group16chem 409.wikispaces.com
Cheremisinoff., Nicholas, et all, 1983. Cooling Towers-
Selection, Design and Practice. Michigan: Ann Arbor
Science.
Dalimunthe, I., 2004, Kimia dari Inhibitor Korosi, Medan:
USU Digital Library.
Deviyani, Larisa dan Isdiriayani Nurdin., 2006, Inhibisi
Korosi Baja Dalam Air Laut Mengandung Sulfida
Menggunakan Glutaraldehida. Jurnal Teknik Kimia
Indonesia 5 (1) 341-349. Bandung: ITB.
Djatmiko, Eddy dan Budiarto., 2009. Analisis Laju Korosi
dengan Metode Polarisasi dan Potensiodi- namik
Bahan Baja SS 304L, Prosiding Seminar Nasional ke-
15 Teknologi dan Keselamatan PLTN Serta Fasilitas
Nuklir ISSN: 0854 - 2910 . Jakarta: UP dan BATAN.
Fontana, G. Mars and Norbert. D. Greene., 1988.
Corrosion Engineering 2nd , International Student
Edition. Jurong: McGraw-Hill Int. Book. Co.
Geiger, Gary and Mel. J. Esmacher, P.E., 2012,
Controlling Corrosion in Cooling Water Systems-
Part2: Inhibiting and Monitoring Corrosion. New
York: American Institute of Chemical Engineers.
Herro, Harvey. M and Robert D. Port., 1993, The Nalco
Guide to Cooling Water System Failure Analysis,
New York, McGraw-Hill Inc.
Heusler, K.E and L. Fischer. 1976. Kinetics of Pit
Initiation at Passive Iron. Journal Materials and
Corrosion 27 (8) 551–556. Weinheim: Verlag GmbH
& Co. KGaA.
NALCO., 1987, Standard Manual Procedure for Corrosion
Coupon Rate, Illinois, Nalco. Co.
Nur Aziz O., 2012, Studi Pengaruh Laju Alir Fluida
Terhadap Laju Korosi Baja Api SL X-52
Menggunakan Metode Polarisasi Pada Lingkungan
NaCl 3,5% Yang Mengandung Gas CO2. Universitas
Indonesia
Olmsted, John and Greg Williams., 2007, Handbook of
Chemistry and Physics, 5th edition, New York: CRC
Press-Taylor & Francis Group.
Growth Prevention System for Inhibiting Corrosion Rate in Ship Operation
305

Priyotomo, Gadang., 2015, Korosi, Serpong: Pusat
Penelitian Metalurgi & Material LIPI. Rozenfeld, I. L.,
1988, Corrosion Inhibitors, New York: McGraw-Hill
Co, Inc.
Seung Jun Lee and Jun Lee Seong, 2011, Effects of flow
velocity on electrochemical behavior of sea chest
5083-H116 Al alloy for ship, Transactions of
Nonferrous Metals Society of China, Vol. 21 (8) p.
1703-1709.
Uhlig, H.H and Robert W. Revie., 2008, Corrrosion and
Corrosion Control, An Introduction to Corrosion
Science and Engineering, 4th Edition, New Jersey:
John Willey and Sons. Inc.
Yari, Mehdi., 2015, An Intro to Pipeline Corrosion in
Seawater, Ontario: University Of Western Ontario.
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
306
