
Preparation of Asymmetric Single-Atom Electrocatalysts for
High-Performance Oxygen Reduction Reaction
Qiyue Cui
Denison University, U.S.A.
Keywords: Metal-Organic Frameworks, Single-Atom Catalysts, Oxygen Reduction Reaction, Electrocatalysis, Fuel
Cells.
Abstract: Metal-organic frameworks (MOFs) have been regarded as a kind of supramolecular non-noble metal-organic
hybrids via the strong coordination bonds, which have a highly tunable porous structures, high surface area,
and fully exposed and uniformly dispersed metal centers, facilitating mass transport and highly-efficient
electron transfer. In this study, we explore the synthesis strategy to prepare hierarchical single-atom
electrocatalysts with porous and conductive carbon supports based on a serial of MOFs templates. The various
MOF templates were prepared by room-temperature self-assembly or hydrothermal processes. The as-
synthesized MOFs were well-designed for the construction of hierarchical nanostructures. Subsequently, a
facile and controlled high-temperature pyrolysis treatment was applied for the as-synthesized MOF templates,
which allowed the organic ligands to reduce metal centers by releasing hydrogen by changing themselves to
porous and conductive carbon materials. Finally, the nanostructured morphology and electrical activity of the
as-synthesized single-atom catalysts were investigated by the X-ray diffraction, X-ray photoelectron
spectroscopy, Raman spectrum, Spherical aberration electron microscopy, and synchrotron radiation
characterization, electrochemical impedance, and cyclic voltammetry. Density Functional Theory (DFT)
calculations suggest that the designed asymmetric planar four-ligand structure may be the most favorable
catalytic sites. Previous studies were restricted by using Cu elements as the metal active centers and S
1
N
3
as
the ligands, but broadening the catalyst selection areas, e.g., using monoatomic metal centers such as Fe, Mn,
and introducing P element in the ligand, may improve the electronic structure properties of the catalyst, which
can effectively reduce the energy barrier in the ORR, a key rate-limiting step in fuel cells. Based on this idea,
we investigated six asymmetric monoatomic electrocatalysts, namely, Fe-S
1
N
3
, Cu-S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
,
Fe-P
1
N
3
, and Mn-P
1
N
3
, respectively. The synthesized catalysts have abundant and fully exposed active sites,
which can be applied in the cathode reaction of fuel cells and help human species to cope with the global
energy crisis and the energy transition in fields such as electric vehicles.
1 INTRODUCTION
Metal-organic frameworks (MOFs) combine
inorganic and organic chemistry, which is popular in
the recent nanostructured materials fields. Metal
atoms coordinated by organic ligands can form one-,
two- or three-dimensional structures (crystalline
materials). MOFs can be used in multiple fields such
as adsorption, separation, storage, catalysis, and
energy conversion. Common single-atom catalysts
using the MOFs as templates are Fe, Co, Ni, Cu, Mn,
and W, which have excellent performances in
corresponding applications via a well-designed
preparation. (Sun, 2019)
Metal-organic framework-derived materials
mainly include porous carbon, metal oxides, metal
porous carbon composites, metal oxides, and porous
carbon composites. The main synthesis strategies are
chemical etching, high temperature pyrolysis,
oxidation, etc. Considering some disadvantages of
metal-organic frameworks in practical applications,
such as low crystal stability, poor performance of
electrical conductivity, low catalytic site activity,
limited mass transfer and diffusion in the
microporous structure, etc. There are many synthetic
strategies for high-temperature pyrolysis, such as
direct pyrolysis of templates, pyrolysis of
encapsulated guests. The template of the bulk, the co-
pyrolysis of the template carrier, the first solvent
100
Cui, Q.
Preparation of Asymmetric Single-Atom Electrocatalysts for High-Performance Oxygen Reduction Reaction.
DOI: 10.5220/0012003100003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 100-105
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

etching and then the pyrolysis, etc. During the
pyrolysis process, the heating rate, holding time and
gas atmosphere are the key points of pyrolysis
strategy research, which directly determine the
synthesized metal-organic framework derivatives
properties.
Oxygen reduction reaction (ORR) is one of the
common energy conversion reactions, and the
common products of ORR reactions are H
2
O, which
is a four-electron reaction product of oxygen
reduction reaction, and H
2
O
2
, which is a two-electron
product of oxygen reduction reaction. At present, the
most widely used oxygen reduction catalysts in
cathode reaction of fuel cells are precious Pt/C
materials, usually 20% or higher content, which are
expensive and scarce. (Tang, 2016) Therefore, the
three worthwhile goals to design high-performance
ORR catalyst are low cost, high activity, and high
stability. The biggest advantage of single-atom
catalysts is that they can achieve the maximum
utilization of atoms. (Sun, 2019)
Single-atom catalysts has many advantages, such
as high efficiency, maximum atomic utilization,
efficient and unique electronic structures, and unique
geometric construction. To prepare highly active
single-atom catalysts, the homo-dispersedly and
catalytically active sites are necessary. (Sun, 2019)
To improve the transfer of mass and electron as well
as the stability of catalytic active sites, and the tight
interactions of catalytically active site and carrier are
also required and necessary. Many literatures have
reported obvious improvements in the single-atom
catalysts for ORR.
(Shang, 2020; Xie, 2021; Sun,
2019) Typically, Metal-N
x
, such as FeN
4
, is usually
considered as an ideal catalyst, and the design concept
of this configuration has been demonstrated by
density functional theory and electrochemical testing
with excellent results. (Xie, 2021)
Usually, in addition to metal atoms, single-atom
catalysts are N and C elements. In our research, we
want to introduce S and P elements, and the key issue
is how to design an effective synthesis method. At
least one N elements around the metal element needs
to be removed and replaced with a sulfur or
phosphorus elements. In recent days, a few studies
have pointed out that the symmetrical configuration
of FeN
4
is not conducive to stable intermediate
products. Inspired by this clue, we can try to
synthesize single-atom catalysts with asymmetric
coordination. In addition, we would use DFT to
explain the underlying catalytic mechanism for the
enhanced ORR performances. (Xie, 2021) DFT
calculations are a method of quantum chemistry for
studying the electronic structures of multi-electron
systems. The primary research object of DFT focuses
on small molecules or isolated cluster structures, and
can calculate transition state energy, bond and
reaction energy, molecular orbital, thermodynamic
properties, reaction path, etc.
2 EXPERIMENTAL
2.1 Preparation of Materials
2.1.1 Chemicals
The chemicals are usually sourced from Alfa Aesar
and Sigma Aldrich, and the purchased reagents were
not further purified. The synthesis method uses Cu,
Fe, and Mn-ZIF-8 template, the source of S is sulfur
powder, and the source of P is phytic acid.
(Shang,
2020; Benítez, 2020)
2.1.2 Hydrothermal Synthesis of
Metal-Organic Framework Templates
The preparation of MOF templates went through
dissolving and recrystallizing the powder. Heating
and pressurizing in a sealed pressure vessel, using
water as the solvent.
First, mix the solutions of metal ions and organic
ligands. Then, transfer the solution to a hydrothermal
kettle and heat it in a vacuum oven to keep it warm,
allowing the metal ions and organic ligands to
coordinate. After that, cooling the solution to room
temperature, centrifuged and separated from the
MOF, washing several times with methanol solutions,
then dried in a vacuum oven.
The key synthesis step is to first hold the
temperature at 450 ℃ for 2 hours in the environment
of inert Ar. In this process, the sulfur powder is
volatilized into sulfur vapor and embedded in the
MOF framework. After incubation at 950 ℃ for four
hours, the template was completely carbonized to
synthesize single-atom catalysts with porous and
conductive carbon supports. The accurate reaction
Figure 1: Schematic preparation taking Cu, Fe, and Mn-ZIF-8 as the template.
Preparation of Asymmetric Single-Atom Electrocatalysts for High-Performance Oxygen Reduction Reaction
101
temperatures of high-temperature pyrolysis can be
determined by the thermogravimetric analysis
(TGA). (Benítez, 2020) TGA is a method to explore
the physical and chemical properties of a substance
with an increase in temperature over time. TGA can
provide abundant information about phase
transitions, such as evaporation, sublimation,
absorption, and desorption.
2.2 Electrochemical Measurements
Cyclic voltammetry is the commonly used
experimental method in electrochemistry. A three-
electrode system was used for electrochemical
measurements, with Ag/AgCl in saturated potassium
chloride solution as the reference electrode, graphite
rod as the counter electrode, glassy carbon as the
working electrode, and 0.1 M KOH solution as the
electrolyte solution. The catalyst was dispersed in a 5
wt% Nafion solution and dried by a heat lamp before
the testing. (Zhou, 2020)
2.2.1 Grind and Disperse Nanomaterials
Grind the nanomaterials into fine particles, and then
disperse the nanomaterials in the Nafion solution.
Usually, ultrasonic treatments are required for around
0.5 hours to 2 hours. The dispersed solution is usually
water and ethanol, the volume ratio is 1:1. (Zhou,
2020)
2.2.2 Preparation of Nanomaterial Working
Electrode by the Drop Coating Method
Take 5 microliters of nanomaterial dispersion liquid
and drop it on the 3 mm glassy carbon working
electrode, and then usually need to volatilize the
solvent through an electric heating lamp.
2.2.3 Assembling the Three-Electrode
System
The nanomaterial-modified electrode is the working
electrode and usually uses the Ag/AgCl electrode or
saturated calomel electrode (SCE) as the reference
electrode. (Shang, 2020)
2.3
Materials Characterizations
Material characterizations were divided into three
parts: (1) Crystal Structure Analysis; (2) Morphology
analysis by electron microscope; and (3) Material
surface element and valence analysis. The
performance characterization of ORR materials is
mainly performed by voltammetry.
3 RESULTS AND DISCUSSION
3.1
Novelties
Our study provides a general approach for
synthesizing and tuning the activity of single-atom
catalysts and applying in the fields of energy
conversion. The synthesis of Cu and S
1
N
3
-
coordinated single-atom catalysts based on metal-
organic framework templates has been reported,
which is a Cu-S
1
N
3
coordination form, namely,
asymmetrically Cu-S
1
N
3
. (Shang, 2020) Inspired by
this report, we explored high-performance ORR
catalysts using Fe, Cu, and Mn as single-atom metal
centers coordinated with S
1
N
3
, and P
1
N
3
, namely, Fe-
S
1
N
3
, Cu-S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-
P
1
N
3
, respectively. We hope the oxygen reduction
activity is higher than that of Cu-S
1
N
3
while
introducing multiple mental atoms and phosphorus.
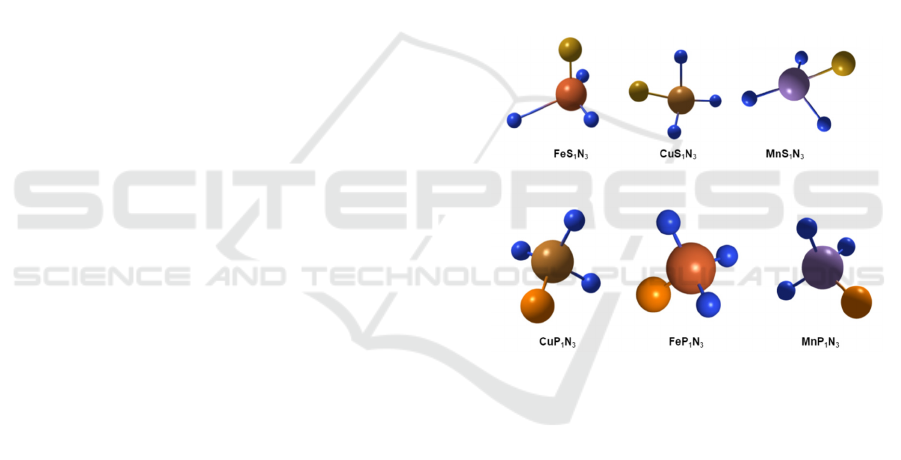
Figure 2: Schematic atomic interface model of Fe-S
1
N
3
,
Cu-S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-P
1
N
3
.
3.2
Materials Characterizations
Tuning atomic interfaces is an important method to
tune the activity of single-atom catalysts. Two
strategies to improve catalyst activity are to improve
the number of active sites and improve the activity of
a single catalytic active site, while the metal single-
atom is the catalytic center.
Mesoporous metal-organic framework materials
are good templates to increase the number of exposed
active sites and improve catalytic performance.
Pyrolysis of metal-organic framework materials is an
effective way to increase the number of catalysts, the
key lies in the formation of mesoporous structure,
which is beneficial to improve electron transfer and
material structure.
FSB 2022 - The International Conference on Food Science and Biotechnology
102
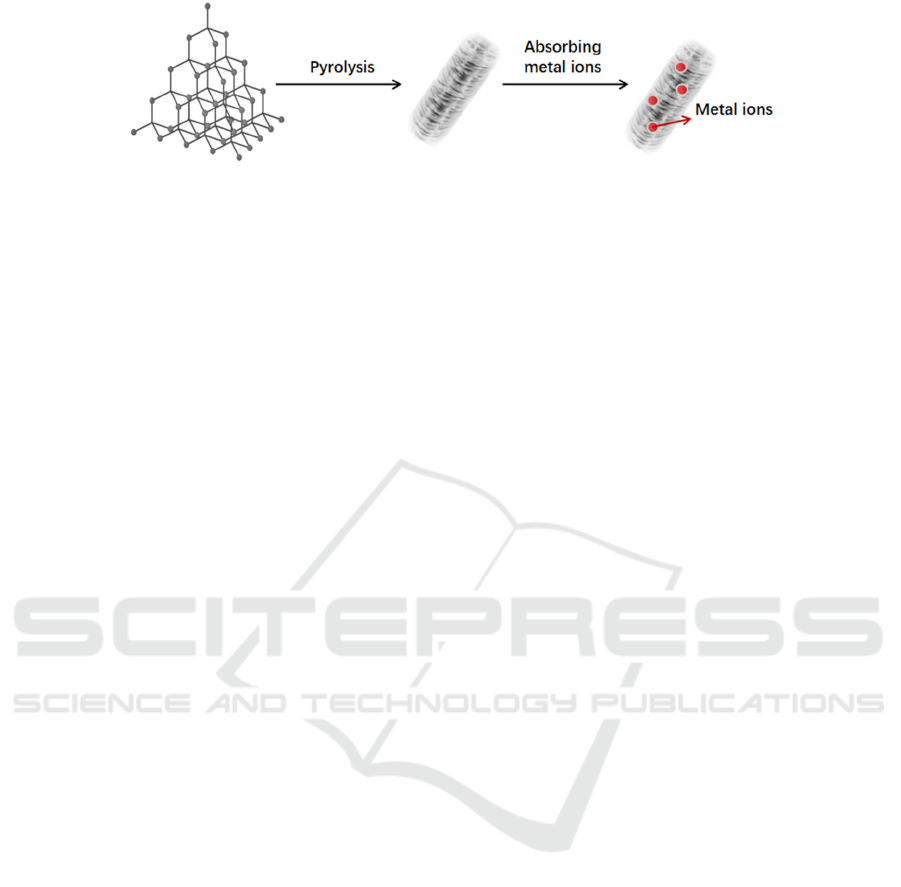
Figure 3: Schematic illustration of the preparation of mesoporous metal-organic framework materials.
The currently reported strategy is to select
suitable MOFs. The organic ligand of the template
contains amino groups and the metal center is Al
3+
.
After high-temperature pyrolysis, the carbon support
of the MOFs can be obtained. The aluminum catalyst
carrier should be pyrolyzed into a carbon skeleton at
high temperature, immersed in Fe (II)-phenanthroline
solution, then removed and dried after adsorbing iron
atoms, and then pyrolyzed again to obtain zero-valent
iron atoms.
3.3
Electrochemical Properties
First, we used cyclic voltammetry to evaluate the
electrochemical performances of Fe-S
1
N
3
, Cu-S
1
N
3
,
Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-P
1
N
3
. The onset
potential, half-wave potential, and maximum limited
current are three important indicators for ORR. We
expect that we could select the best electrochemical
performances of ORR catalysts from the small library
of Fe-S
1
N
3
, Cu-S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-P
1
N
3
single-atom solid catalysts based on the
indicators of onset potential, half-wave potential, and
maximum limited current. We would pay much
attention to the overpotential of ORR. This indicator
is highly related with the voltage efficiency of fuel
cells. To overcome the potential differences of ORR
thermodynamically controlled values and
experimentally measured values, the single-atom
solid electrocatalysts were decorated on the cathode
electrode. The as-synthesized novel Fe-S
1
N
3
, Cu-
S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-P
1
N
3
single-atom solid catalysts could reduce the Gibbs
free energy greatly. As a result, the onset potential
and half-wave potential would be reduced as well as
maximum limited current would be increased. More
heat loss would be avoided due to the excellent ORR
performance in the fuel cells.
To explore the electrical properties of Fe-S
1
N
3
,
Cu-S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-P
1
N
3
single-atom solid catalysts, we also plan to use
electrochemical impedance measurements. The
proposed equivalent circuits would support the
analysis of nanostructured materials and be expected
to be in line with a series of materials charac-
terizations. (Small, 2017) We would pay much
attention to the value of charge-transfer resistance. If
the Fe-S
1
N
3
, Cu-S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-P
1
N
3
single-atom solid catalysts have an
excellent electrochemical performance, the value of
charge-transfer resistance fitting from a proposed
equivalent circuit would be reduced greatly. Since the
oxygen has a faster diffusion rate, the diffusion-
related parameters would be analyzed in our research.
The schematic Zinc air fuel cells are exhibited in
the Fig. 4. The zinc-air fuel cells get the energy via
zinc oxidation of oxygen in the air. The oxygen
reduction reaction is the rate-limiting step. The as-
synthesized asymmetric single-atom electrocatalysts
would boost this reaction rate, as a result, the zinc-air
battery based on asymmetric single-atom electro-
catalysts is expected to get better performances.
(Chen, 2018) Many advanced studies have been
explored in the Zinc-air fuel cells. For example, in
recent Science paper, non-alkaline rechargeable zinc
air Batteries was reported. The stable circulation in
air could persist for 1600 hours amazingly. (Sun,
2021) For the first time, a new non-alkaline
rechargeable zinc-air battery was reported, and the
reaction mechanism of reversible generation and
degradation based on zinc peroxide (ZnO
2
) was
successfully analyzed.
There is no doubt that this breakthrough work not
only provides new understanding and research ideas
for the subsequent development of highly reversible
secondary metal-air batteries. However, in addition
to the excitement, there is a question worth
wondering: Has the problem of zinc-air batteries
really been solved? The answer is negative. In
addition to problems such as electrolyte evaporation
and dendrite growth, another issue that requires the
most attention is the charge-discharge rate. This work
takes up to 20 hours for a charge-discharge cycle. So,
we would select our as-synthesized Fe-S
1
N
3
, Cu-
S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-P
1
N
3
single-atom solid catalysts based on the time of the
stable circulation in air. Since the interaction of single
atoms and carbon support is coordination bond. This
kind of chemical bond has a much better stability than
the metal bond that traditional nanoparticles have. In
Preparation of Asymmetric Single-Atom Electrocatalysts for High-Performance Oxygen Reduction Reaction
103

Figure 4: Zinc–air fuel cells.
the addition, the as-synthesized carbon support based
on MOF templates has the porous and conductive
nanostructures. The reliable stability of carbon
supports is also expected in the stability testing of
Zinc air fuel cells.
3.4 Applications and Comparisons
The search for high-performance ORR reaction
catalysts is the "Holy Grail" catalyst for
electrochemical energy conversion devices.
(Mohamed Fathi Sanad, 2021) In catalytic reactions,
it is generally believed that the electronic properties
of the catalyst determine how the reactants and
intermediates bind to the catalyst surface. Density
functional theory (DFT) calculations show that the
single-atom catalyst can effectively activate and
dissociate the O-O bond, reducing the energy barrier
of O-O bond breaking and improving the ORR
activity. In addition, the DFT calculations would
provide plenty of information about the energy of
adsorption and desorption. DFT calculations would
give us scientific guidance about the ORR catalysts
design and preparation. (Shang, 2020) Since the Fe-
S
1
N
3
, Cu-S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-
P
1
N
3
single-atom solid catalysts have different metal
centers and ligands, make quantitative analysis of
oxygen adsorption and desorption would inspire us
the design of more variety of asymmetric single-atom
catalysts.
Single atom catalysts (SACs) have a high
utilization rate of metal active center and adjustable
coordination structure. However, because of the lack
of group sites connected by multiple atoms, single
atom sites are difficult to be used in complex and
multi-step catalytic reactions. (Xie, 2021) For
example, monatomic Pt catalyst in THE ORR
catalytic process, isolated Pt atom cannot destroy the
O-O bond through lateral adsorption, so it is difficult
for monatomic Pt catalyst to effectively catalyze
ORR by four-electron mechanism.
There are literature reports on the synthesis of Cu-
Co bimetallic ORR catalysts via Cu-Co MOFs.
(Mohamed Fathi Sanad, 2021) Through XPS analysis
and DFT calculations, we demonstrate the forceful
electronic coupling between Cu-Co, which triggers
an efficient electron transfer process, and it is the key
to achieving high-performance ORR-active catalysis.
The synthesis process of Co-Cu bimetallic metal-
organic framework is based on a low-temperature
hydrothermal method. (Mohamed Fathi Sanad, 2021)
The catalytic activity is superior to that of the noble
metal Pt in alkaline environments because of the
specific electronic collaboration existing in the Co-
Cu bimetallic center in the MOF. (Mohamed Fathi
Sanad, 2021)
In addition, the common products of ORR
reactions are H
2
O, which is a four-electron reaction
product of oxygen reduction reaction, and H
2
O
2
,
which is a two-electron product of oxygen reduction
reaction. So, we need to analyze the products of ORR
carefully. We should avoid the generation of H
2
O
2
in
the ORR based on the design and selection of Fe-
S
1
N
3
, Cu-S
1
N
3
, Mn-S
1
N
3
, Cu-P
1
N
3
, Fe-P
1
N
3
and Mn-
P
1
N
3
single-atom solid catalysts. Because the H
2
O
2
product would make the maximum limiting current
become much smaller and H
2
O
2
product has the
oxidative activity that would damage the fuel cells
devices if we want to pursue an actual application
with a long time duration.
4 CONCLUSIONS AND
PERSPECTIVES
There are main challenges in the synthesis of single-
atom catalysts and their application in energy: (1)
There are many kinds of MOFs, but only a few (ZIF-
FSB 2022 - The International Conference on Food Science and Biotechnology
104

8, ZIF-67, MIL-101-NH2, and UiO-66-NH
2
) can
synthesize SAC, and the catalytic active sites are
limited to Fe, Co, Ni, Cu, Mn, W, so looking forward
to exploring more types of MOFs with metal active
sites. (Shang, 2020) (2) Usually, the metal content of
SAC is very low because to prevent the formation of
metal agglomerates, it is necessary to increase the
metal content under the premise of preventing metal
agglomeration. (3) There is still a lack of methods for
regulating the shape of catalyst nanometers. (4) The
in-depth analysis based on DFT calculations for
asymmetrical single-atom catalysts still need to be
explored and provide more specific information for
asymmetrical single-atom catalysts design. (5) There
are many works should be explored about the
evaluation of fuel cells in real applications.
It's worth noting that in situ X-ray absorption
spectroscopy and situ electron microscopy provide
powerful tools for studying the catalytic mechanism
behind SAC in recent years. In one aspect, we expect
the commercial applications of fuel cells in the near
future if the low-cost, abundant, high active ORR
catalysts can be acquired easily. This would boost the
transitions of global energy structures from fossil
energy domination. Many key climate issues would
also be solved due to this advancement. In the other
aspect, exploration of many advanced instruments for
ORR catalysts analysis is highly desirable in the next
few years. This would help us know the underlying
scientific mechanism and ensure the sustainability of
technology developments.
REFERENCES
Benítez, A., Amaro-Gahete, J., Esquivel, D, et al. MIL-88A
Metal-organic Framework as a Stable Sulfur-host
Cathode for Long-cycle Li-s Batteries. Nanomaterials
2020, 10, 424.
Huishan Shang, Xiangyi Zhou, Juncai Dong, et al.
Engineering unsymmetrically coordinated Cu-S1N3
single atom sites with enhanced oxygen reduction
activity. Nature Communications 2020, 11, 1-11.
Haolin Tang, Shichang Cai, Shilei Xie, et al. Metal-
organic-framework-derived Dual Metal- and Nitrogen-
doped Carbon as Efficient and Robust Oxygen
Reduction Reaction Catalysts for Microbial Fuel Cells.
Advanced Science 2016, 3 1500265
Mohamed Fathi Sanad, Alain R. Puente Santiago, Sarah A.
Tolba, et al. Co–Cu bimetallic metal organic
framework catalyst outperforms the Pt/C benchmark
for oxygen reduction. Journal of the American
Chemical Society, 2021, 143, 4064-4073.
Small, Leo J, Tina M. Nenoff. Direct Electrical Detection
of Iodine Gas by a Novel Metal–Organic-Framework-
Based Sensor. ACS applied materials & interfaces,
2017, 9, 44649-44655.
Sun Wei, Fei Wang, Bao Zhang, et al. A rechargeable zinc-
air battery based on zinc peroxide chemistry. Science
2021, 371, 46-51.
Tingting Sun, Lianbin Xu, Dingsheng Wang, et al. Metal
organic frameworks derived single atom catalysts for
electrocatalytic energy conversion. Nano Research,
2019, 12, 2067-2080.
Xiaoying Xie, Lishan Peng, Hongzhou Yang, et al. MIL‐
101‐derived mesoporous carbon supporting highly
exposed Fe single‐atom sites as efficient oxygen
reduction reaction catalysts. Advanced Materials,
2021, 33, 2101038.
Yuanjun Chen, Shufang Ji, Shu Zhao, et al. Enhanced
oxygen reduction with single-atomic-site iron catalysts
for a zinc-air battery and hydrogen-air fuel cell. Nature
Communications 2018, 9, 5422.
Yazhou Zhou, Xiafang Tao, Guangbo Chen, et al.
Multilayer Stabilization for Fabricating High-loading
Single-atom Catalysts. Nature Communications 2020,
11.
Preparation of Asymmetric Single-Atom Electrocatalysts for High-Performance Oxygen Reduction Reaction
105
