
Retrosynthesis
Yilang Zheng
1,*
and Yilin Gong
2
1
North Cross School Shanghai, Shanghai, 200940, China
2
Department of Shanghai, Fairmont international school, Shanghai, 201100, China
Keywords: Bond, Synthon, Break, Pathway.
Abstract: This essay focuses on a very important part of organic chemistry, the retrosynthesis reactions. The essay goes
from the shallow to the deep, starting with the most basic organic synthesis and transitioning to the
retrosynthesis reactions. It is described in relatively simple and understandable terms, so that all readers can
gain an understanding and insight after reading the paper.
1 INTRODUCTION
1.1 Why We Choose Retrosynthesis as
Our Paper Topic?
At the very beginning we were all new to organic
chemistry and had no previous in-depth knowledge of
this area. It was with this enthusiasm and curiosity
that we joined Prof. Brian's research project and spent
almost a month and a half in lectures to learn more
about organic chemistry. During one of the lectures,
we heard the professor talk slowly about
retrosynthesis reactions, which immediately caught
our attention and curiosity. This was our first
introduction to the subject, and as we studied and
researched it, we became more and more curious
about the retrosynthesis reaction and wanted to
document it in writing.
1.2 Briefly Introduction
The full synthesis of complex molecules requires a
thorough understanding of the reactions that form
carbon-carbon bonds and the reactions that change
one functional group into another. The largest number
of chemical reactions used in synthesis involve the
manipulation of functional groups. Furthermore,
unless all aspects of chemical reactivity, functional
group interactions, conformation and stereochemistry
are fully understood, the synthesis of molecules is
rarely successful.
Today, the term organic synthesis encompasses a
large number of chemical reactions. The planning and
use of organic transformations to piece together a
molecule is of course an important aspect of organic
synthesis. In order to achieve this, a thorough
understanding of the many organic reactions, reagents
and chemical transformations that are now known is
required. As mentioned earlier, the practice of organic
synthesis requires an understanding of the chirality
and stereochemistry of molecules, both to develop
synthetic strategies and to select the reactions and
reagents to be used in the various chemical
transformations. (Corey, 1988) It is essential to
understand the conformational analysis of each
molecule, from the starting material to the final
product, as chemical reactivity and stereochemistry
are often influenced by conformation.
Perhaps the most important component of
planning an organic synthesis is a thorough and in-
depth knowledge of chemical reactions and reagents.
If one knows only one reagent that converts an
alcohol to a ketone, and if that reagent does not work
for a particular system, then there is no alternative.
On the other hand, if one knows of 30 different
reagents for such conversions, there are many
alternatives if one of them does not work. Perhaps
more importantly, knowing these 30 reagents allows
one to better plan the synthesis and use a certain
reagent to maximise the chances that the synthetic
sequence will go as planned. The same comments
apply to making carbon-carbon bonds. (Corey, 1991)
Presumably, a synthesis starts with a starting material
of a few carbon atoms and the reaction will add
carbon fragments to increase the complexity of the
molecule as it is transformed into the final target in
many steps. It is therefore essential to understand the
different reactions and reagents used to form different
128
Zheng, Y. and Gong, Y.
Retrosynthesis.
DOI: 10.5220/0012003500003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 128-133
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

types of carbon-carbon bonds. (Corey, 1995)
2
BACKGROUND
INFORMATION
Before I talk about retrosynthesis, I would like to start
with an introduction to having synthesis. Synthesis is
a subject that is usually introduced in organic
chemistry and is introduced after you have studied the
alkyne reaction. You will make use of it time and time
again as the number of reactions you learn builds up.
2.1
What Is Retrosynthesis in Organic
Chemistry?
The definition is in its name: organic synthesis simply
means making organic compounds from scratch in the
laboratory or in industry. (Leah4sci, 2016)
Well, organic synthesis is a field where the
principles of organic chemistry are applied.
Most organic compounds come from living
things. For example, ethanol comes from the
fermentation of biomass. Ethanol is a simple and rich
example of organic compounds, but there are some
more complex organic compounds that are important
but less common in nature.
One good example is the drug aspirin. Aspirin
originated from willow bark. However, extracting
aspirin from it is too time-consuming and wasteful
because willow bark contains very little aspirin.
Some scientists have developed steps to
synthesize aspirin from laboratory compounds such
as salicylic acid. In this way, aspirin can be produced
in a high-volume and low-cost manner (Dr. Pere
Romea., 2014).
2.2
What Is Retrosynthesis in Organic
Chemistry?
If you look at this word in its simplest form, retro
means backwards, and synthetic means synthetic. Put
them together, this is what we call a retrosynthetic
reaction. Take an example from the results of the
discoveries of E.J. Coe of Harvard University in the
20th century.
As shown in Figure 1, this simple example of a
reverse synthetic analysis, the target molecule is
envisaged to be produced by hydrolysis of 2-bromo-
2-methylpropane. 2-Bromo-2-methylpropane is in
turn envisaged to be produced from methyl propane
via radical bromination. (https://www.chem.
ucla.edu/~harding/IGOC/R/retrosynthesis.html#:~:te
xt=Inthissimpleexampleofretrosyntheticanalysis%2C
,toariseviahydrolysisof2-bromo-2-methylpropane).
2.3
Why Is It Important in Organic
Chemistry
Retrosynthetic analysis can be used to get a clear idea
about the structure of naturally available compounds.
Also, it is a powerful tool to prepare compounds in
order to analyze the mechanism of the reaction. There
is a good example, which is the labeled compound.
Furthermore, the novel problems present everyday
requires advanced solutions that trigger
developments of modern chemistry.
3
DISCONNECTION
As previously mentioned, retrosynthetic analysis is to
disconnect target molecule into less complex
structures. This procedure is repeated until form
simple, easily available starting material. To
accomplish this goal, it usually takes known or
reasonable reactions as the account for the
disconnections used in the analysis.
Figure 1: Simple example of retrosynthetic analysis.
Retrosynthesis
129
The overall synthesis of any complex organic
molecules requires a thorough knowledge of
reactions. In the synthesis reactions, there are two
main categories of reaction types. One is called
carbon-carbon bond forming reaction. The other one
changes one functional group into another, this is
functional group exchange reaction.
Disconnection means breaking the bonds of the
molecule to produce simpler fragments.
Disconnection is actually a mental activity (we don't
actually break bonds chemically), if we break a bond
we must have a chemical process in mind to make that
bond.
As a reverse reaction to synthesis, retrosynthesis
disconnects the bonds and simplifies the target
molecule.
3.1 Carbon-Carbon Bonds
Disconnections
First and perhaps the most important retrosynthetic
rule is related to the C–C bond, electronic structure
and electronic charges of synthons that are designed
by the disconnection of those C-C bond. This rule can
be revealed in the products of disconnections which
are ionic fragments or radicals. To be synthons, those
particles should exist and be seen as reagents or
synthetic equivalents.
Before considering the electronic structure and
properties of synthons, there is a general scheme of
retrosynthetic analysis.
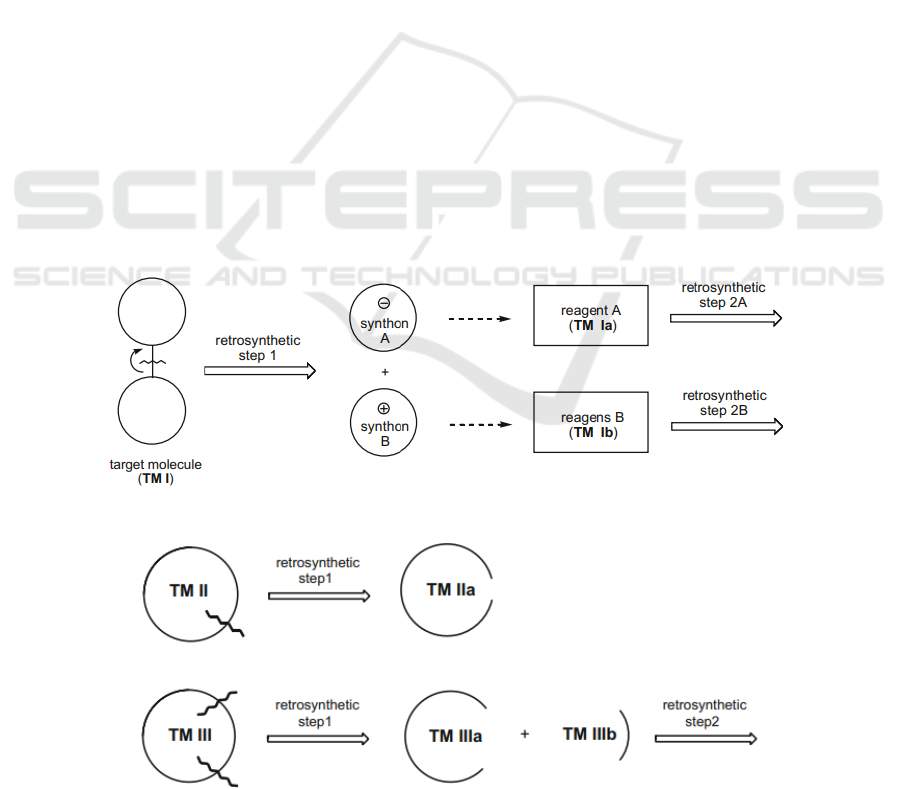
In figure 2, the curving line and arrow on the C–
C bond indicate the site of disconnection. The broader
arrow indicates the disconnection from left (target
molecule) to right (charged species). Then,
disconnection process continues until simple,
commercially available compounds are reached.
(Vitomir Šunjić, 2016).
Now consider disconnections of the C–C bond in
cyclic system in Figure 3. By disconnection of one
bond in TM Ⅱ, the ring is detached. Although there is
only one synthon, it probably provides a new vision
on how to separate the molecule in-depth. Of course,
the complex open-chain structure requires further
retrosynthetic consideration. This is just a possible
path.
In the cyclic TM Ⅲ, we disconnect two C–C
bonds at the same time. Two synthons are obtained.
The same, if one or both of this second generation still
contains complex structures, retrosynthetic
consideration continues.
To be more specific, the disconnection in TM Ⅲ
is a very well-known reaction called Diels–Alder
reaction. With two simultaneously formed carbon-
carbon bonds, Diels-Alder reaction becomes an
reliable source to form plentiful required six-
membered rings. Figure 4 reveals the most common
Diels-Alder reactions.
Figure 2: Disconnection steps.
Figure 3: Common ways to break C-C bond in the cyclic system.
FSB 2022 - The International Conference on Food Science and Biotechnology
130
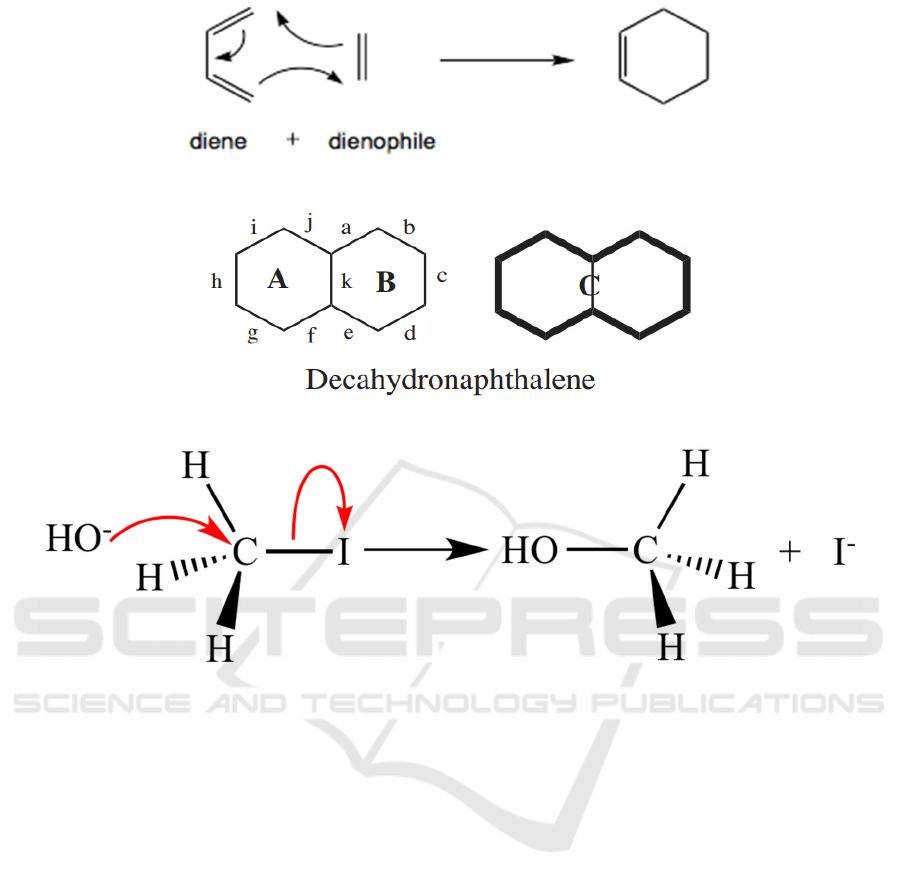
Figure 4: An usual Diels-Alder reaction model.
Figure 5: Strategic bonds.
Figure 6: Electron transfer when breaking bond.
The reverse reaction of a Diels–Alder reaction
becomes favorable at a high temperature, although
this may not be significant to most of the Diels-Alder
products. However, it makes abundant disconnection
of carbon rings possible. This reverse reaction is
known as the retro-Diels–Alder reaction.
Besides, there is another principle used to discern
strategic bonds which is that the strategic bonds are
in the primary rings, and they are not shared by two
fused rings. Since Five-, Six-, Seven-membered rings
are easy to form, whereas larger rings would be
difficult to form using synthetic ways. As a result, any
disconnections that generate rings containing more
than 7 carbon atoms is not feasible. In figure 5, the
bonds a-i are all strategic bonds except bond k. It can
be seen more directly in molecule C. If the communal
bond in the middle of the fused ring system is broken,
a ten-membered ring will be built. (Michael, 2016).
3.2 Ionic Fragments or Radicals
In the process of retrosynthesis, there is movement of
electrons that needs to be considered instead of just
thinking about the framework of the target molecule.
The arrow shown in the Figure 6 indicates the flow of
electrons. Additionally, fragmented ions can be
positively charged or negatively charged. Therefore,
they can be replaced if required.
First, the electron-
withdrawing groups should be disconnected. Then,
the group they used to attach will be positively
charged and induce another group to form reaction.
Nevertheless, it does not always obtain stable ions. In
this circumstance, FGI can be used to get the desired
ions.
There is another way to separate bonds called
homolysis (Figure 7). It produces radicals.
Comparing to polar reactions, although radical
reactions is less frequently used, it is still regarded as
a distinct genre and sometimes provides a lot of
convenience (
Abhik, 2014).
Retrosynthesis
131

Figure 7: Homolysis - separate bond equally.
Figure 8: Difference between two synthesis types.
4
GUIDELINES
4.1
Make the Pathway Short If
Possible
In the laboratory, the ideal retrosynthesis pathway
would include the highest safety and efficiency.
Based on this principle, a short plan is favorable
because it results in more yield from a given amount
of reactant.
4.1.1 Convergence
Convergence is a strategy for multistep synthesis.
Different from linear synthesis (also called
consecutive synthesis), convergent synthesis apply
much less steps. Since reactions cannot reach 100%
yield, as steps increases, the percent yield of the
target molecule decreases. Based on this belief,
shortening the synthesis pathway leads to a higher
efficiency using given amount of starting material.
From Figure 8, we can see a clear difference between
linear synthesis and convergent synthesis.
To summarize, the first step in the inverse
synthesis of a complex target molecule is to analyse
the oxidation level of each carbon atom. By
analysing the oxidation levels, we can convert the
binding to electronegative atoms into binding to
oxygen. As a result, we can find possible dioxygen
dioxide patterns, which indicate the type of
disconnection carried out. The disconnection will
result in simpler molecules that can be further
disconnected. Furthermore, the dioxygen dioxide
pattern guides the synthetic pathway of the target
molecule, which is a purpose of retrosynthetic
analysis as it provides known reactions that can
produce the target molecule from the disconnected
part.
4.1.2 Exploit Any Symmetrical Structure
Exploiting the symmetrical structure in the target
molecule or its intermediate can dramatically
simplify retrosynthesis. In addition, it provides
significant chance to identify a convergent pathway.
4.2
Do the Retrosynthesis with Easily
Accessible Synthons
On the practical aspect, a good retrosynthesis
pathway should make its synthons easier to approach
by buying or making in the process.
4.3 Transfer the Less Reactive
Functional Groups into Reactive
Ones
If any substructures interfere with a key process, it
can be removed using transformation to other
functional groups.
5
CONCLUSION
Our research continued for several months, and at the
beginning, we studied abundant aspects of organic
chemistry in detail. Then, we started to inquire about
retrosynthesis in depth. In the process of research, we
find it is effective to use retrosynthetic ways to
analyze chemical substances. After the development,
we group basic ideology and guidelines.
We find it with great significance that to produce
more target from easier approached raw material.
This demands a solid foundation on known chemical
reactions. In organic chemistry, we may encounter
problems that are too difficult for us to solve.
FSB 2022 - The International Conference on Food Science and Biotechnology
132

Learning reverse synthetic analysis allows us to think
critically about problems from different perspectives,
moving from forward to backward and then
backwards again. Having such chemical intuition
provides us a better understanding to more difficult
chemical problems in the future. This way of
thinking is not only applicable to our future
chemistry studies but also to our school life.
REFERENCES
Abhik Ghosh, Steffen Berg (2014), Arrow Pushing in
Inorganic Chemistry: A Logical Approach to the
Chemistry of the Main Group Elements, Wiley
Dr. Pere Romea. (2014). Intr. to Retrosynthetic Analysis.
Barcelona: universitat de barcelona.
E. J. Corey (1988). "Retrosynthetic Thinking - Essentials
and Examples". Chem. Soc. Rev. 17: 111-133. doi:
10.1039/CS9881700111.
E. J. Corey (1991). "The Logic of Chemical Synthesis:
Multistep Synthesis of Complex Carbogenic
Molecules (Nobel Lecture)" (Reprint). Angewandte
Chemie International Edition in English 30 (5): 455-
465. doi:10.1002/anie.199104553.
E. J. Corey, X-M. Cheng (1995). The Logic of Chemical
Synthesis. New York: Wiley. ISBN 0-471-11594-0.
https://www.chem.ucla.edu/~harding/IGOC/R/retrosynthe
sis.html#:~:text=Inthissimpleexampleofretrosynthetic
analysis%2C,toariseviahydrolysisof2-bromo-2-methyl
propane
Leah4sci. (2016.11.17). A Simple Approach to Retro-
synthesis in Organic Chemistry. America: https://
leah4sci.com/organic-chemistry-retrosynthesis/.
Michael B. Smith (2016), Organic Synthesis,4th Edition,
Academic Press
Vitomir Šunjić, Vesna Petrović Peroković (2016), Organic
Chemistry from retrosynthesis to Asymmetric
Synthesis, Springer
Retrosynthesis
133
