
A Proposal for the Synthesis Route of Lyaline
Shuang Wu
1*
, Xu Han
2
and Jiedong Li
3
1
Xiwai International School, Shanghai 201600, China
2
Shenzhen College of International Education, Shenzhen 518043, China
3
University of California, Santa Barbara, CA 93106, U.S.A.
Keywords: Lyaline, Synthesis Route, Dials-Alder Addition, Fischer-Indole Mechanism.
Abstract: Lyaline is a monoterpene indole alkaloid first isolated in 1974. After attempts of proposing a synthesis route
of this compound, researchers found its structure to be unstable and that the proposed structure to be incorrect.
Later researchers revised the structure and set lyaline as the first naturally occurring nacycline analogue.
While most previous research examine the spectroscopic data of lyaline and came up with a structure, this
work assess the possibility of retrosynthesizing this compound and propose a theoretical synthesis pathway
for the revised structure of lyaline. Through online research and books, we decided to retrosynthesize this
molecule by first separating it into a cyclohexene and an indole. And with 3 starting materials—molecule 1
and 2 presented below, and an indole—it is possible to synthesis lyaline through a series of nucleophilic
substitutions and Dials-Alder cyclic addition. This proposal will finally make the molecule of lyaline, though
not completed. The following researches can be done by experimenting and resolving the stereoselectivity of
the specific Dials-Alder reaction and using a better protection to increase the yield of the amine formation.
1 INTRODUCTION
The molecule lyaline was first isolated from the root
of the plant named Pauridiantha paucinervis. Its
structure was proposed by researchers based on
limited spectroscopic data and was later proven
incorrect through attempts in retrosynthesising this
compound. Significant difference from the original
spectroscopic data and the fragile nature of the
synthesized compound indicates that the initially
proposed structure was incorrect. Through thorough
analysis of the 1H NMR spectrum by analyzing the
peaks produced and through comparison with the
array of MIA structural variants, a new structure of
lyaline was proposed. Though there is possibility that
degradation of the sample analyzed may have
occurred, which may invalidate the tests done,
experiments have been done to proved the stability of
MIA collections. Since most research done on lyaline
was examination of the spectroscopic data of the
sample containing lyaline, this work aims to propose
a synthesis route for the revised the structure of
lyaline so that this molecular structure can be
synthesized and further test can be carried out to
examine its property.
Figure 1: The revised structure of lyaline.
2 GENERAL OUTLINE
As shown in Figure 2, the first step starts with
breaking the N-C21 bond and C14-C15 bond to
divide the huge molecule into an indole-containing
molecule and a cyclohexene containing molecule.
Next, a Dials-Alder mechanism to break the
cyclohexene into smaller molecules. We believe that
the indole containing molecule is purchasable, but in
case it cannot, we can synthesize it using a Fischer-
Indole mechanism.
Figure 2: The3 two parts that the original molecule is broken
into.
134
Wu, S., Han, X. and Li, J.
A Proposal for the Synthesis Route of Lyaline.
DOI: 10.5220/0012003600003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 134-137
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
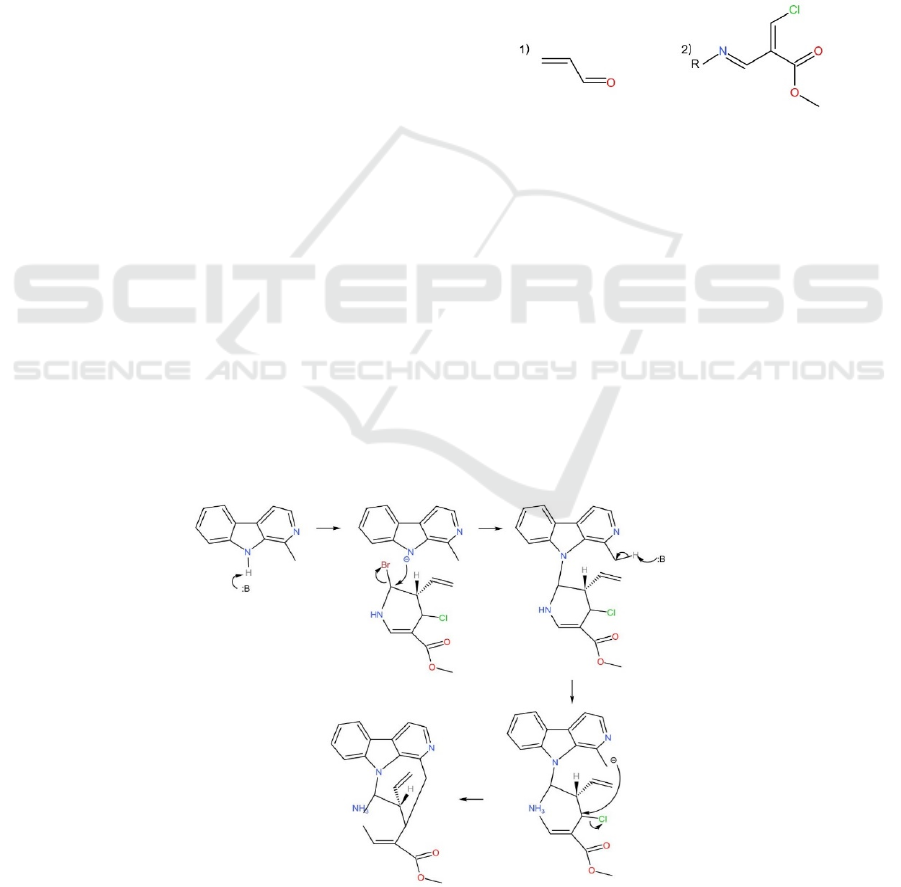
3 METHODS
3.1 Breaking N-C21 Bond and
C14-C15 Bond
Upon considering the procedure to connect C-21 and
nitrogen in the pentagon, and the connection of C-14
and C-15, the basic logic was to make form the easier
bond and then the other bond. This logic allows us to
avoid the influence of the problem of choosing the
point to connect. This means that with the application
of a sequential procedure rather than a spontaneous
reaction, we can form the desired bond first.
In this case, the bond between C-21 and that
particular nitrogen is considered to be formed through
formation of a trisubstituted amine. The reaction
starts with the central nitrogen in a substituted indole.
We will use a base to deprotonate the nitrogen,
producing a nitrogen anion. In the lower part, there
are initially two carbons connect with halogens, that
are C-21 and C-15. In this case, with the contribution
of the lone pair electron from the nearby nitrogen of
C-21, the amine formation of C-21 is more likely to
perform rather than C-15. The reason is that the lone
pair nitrogen is likely to attack C-21 due to the
inductive effect of bromine’s negativity, which makes
C-21 more electropositive. The negatively charged N
will then perform a SN2 nucleophilic substitution to
connect to C-21, forming the target bond between C-
21 and nitrogen in the indole (ABU-SHANAB, 2010).
Attempt to make the C-14, C-15 bond:
With a treatment of a base, a carbon anion could
form at C-14 on the indole structure of the upper
section. The base deprotonates the methyl group of C-
14 and forms a carbon anion. This anion again
performs an SN2 nucleophilic substitution on the
electropositive C-15 that is bonded to a Cl. Upon
attacking C-15 with the carbon anion and ejecting Cl-,
the desired C15-C14 bond is formed, so is the desired
product (Figure 3).
It is worthy noticing that benefiting from the
amine alkylation of the previous step, the strong base
added to the reaction in this stage only deprotonates
C-14 instead of forming anion on the nitrogen that is
to connect with C-21 (Hunt, 2021).
3.2 Breaking the Cyclohexene
To form the cyclohexene, use 2 smaller molecules:
Figure 4: The starting materials to make the cyclohexene.
With the starting ingredients shown in Figure 4,
the initializing step here uses a double-bonded O
instead of C on molecule 1 because the O can
mesmerically withdraw electrons, which makes the
left most C more electropositive; the reason why not
separate the ester out from part 2 and name it as part
3 is that the ester can also draw electrons toward the
O in the ester through resonance, which also helps
make C bonded to Cl more electropositive; and
connect N to a R-group because it can help stabilize
the electrons on N. All of these helps the Dials-Alder
reaction proceed (Wilson, 2001; Juhl M, 2009).
Figure 3: The mechanism for making C-14, C-15 bond.
A Proposal for the Synthesis Route of Lyaline
135
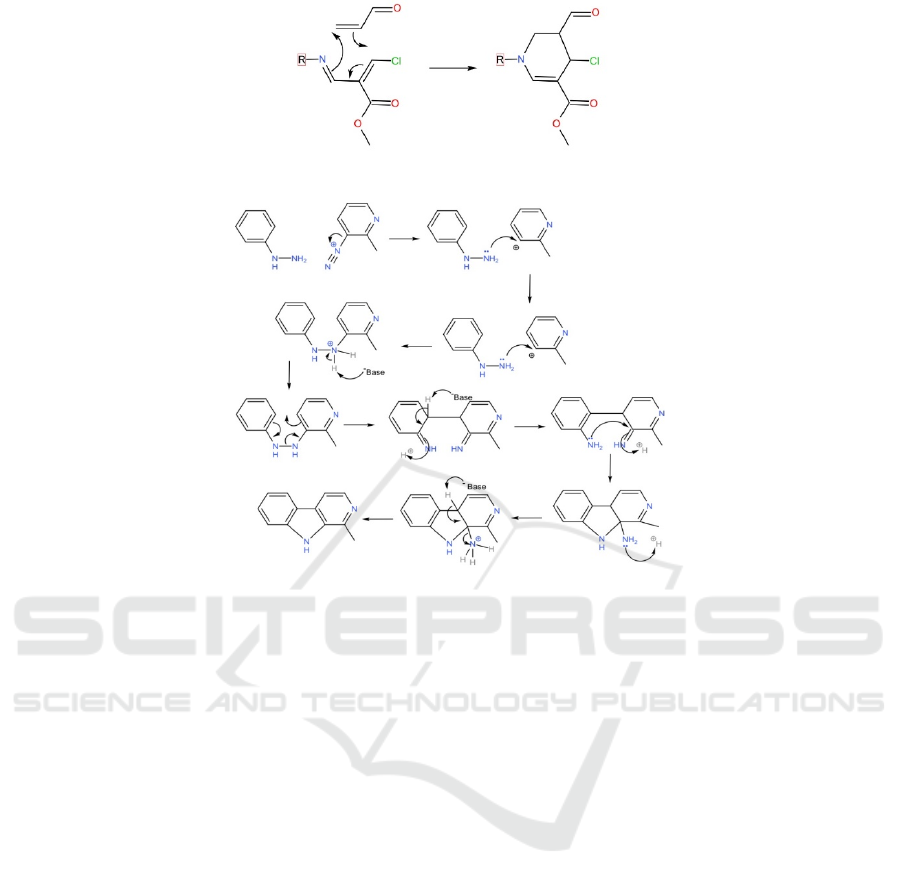
Figure 5: The mechanism for the Diels-Alder reaction to form the cyclohexene.
Figure 6: The mechanism for synthesizing the indole using Fischer-Indole Mechanism.
To start synthesis the ring, the electron on N will first
attack the carbon connecting to Bromine and start the
reaction as shown in Figure 5. The double bond
connecting bromine will shift one bond to form new
sigma bond with the carbon connecting Chlorine in
part 2. Then the double bond near Chlorine will also
shift the bond to left to form new double bond. And
the double bond originally connecting nitrogen will
shift one bond to help form C-N bond and finish to
form the final product (Jagora et al., 2021).
After forming the product, change the C=O in
molecule 1 back to a C=C using a Wittig mechanism.
Unfortunately, there is not a clear way to change the
N-R to an N-H in molecule 2, which could be an aim
for future research.
So, with molecule 1, molecule 2, and the indole
containing molecule as starting materials and with a
reliable way to change N-R to NH in molecule 2 after
forming the cyclohexene, the above mechanism could
be a possible way of synthesizing the molecule
lyaline.
3.3 Breaking the Pyradine Structure
into Simpler and Easier to
Purchase Ingredients
As shown in figure 6, the indole can possibly be
formed with the Fischer-Indole mechanism using the
two starting materials shown above and heated in
acidic solution. As it is unfavorable to perform an
imine formation on a benzene (because benzene is
electron dense, it therefore repels the lone pair of
electrons on the nucleophile), a N2 is used. As the N2
leaves as a gas, it leaves the C it connected to
positively charged, which makes it easier for the lone
pair on the N of the other starting material to attack.
Then the molecule would perform a [3, 3]
sigmatropic rearrangement. This step is favourable
because of 2 reasons: 1. From an orbital symmetry
perspective, according to the Woodward-Hoffman
rules, the reaction is thermally allowed as its total
number of (4q+2)s and (4r)a components is odd; 2.
From a enthalpy perspective, the original N-N bond
has lower bond enthalpy than the new C-C bond while
the original C=C has the same bond enthalpy as the
new C=N, so the reaction is also enthalpy favoured.
The last step is reforming the aromaticity of the
benzene, which gives an aminal, then expulsing
ammonia.
4 CONCLUSIONS
As shown above, the molecule lyaline can possibly be
FSB 2022 - The International Conference on Food Science and Biotechnology
136

synthesized through a series of nucleophilic
substitutions and Dials-Alder cyclic addition, using
the starting materials molecule 1, molecule 2, and the
indole. However, most research done in this work are
through the internet and books as we were not yet able
to experiment on each step of our proposal, thus we
could not give the accurate yield of each step at the
moment. In future research, we would test this
proposal in experimentation and keep making
improvements on this synthesis route.
ACKNOWLEDGEMENT
We would like to thank Professor Brian Stoltz from
California Institute of Technology, who taught us
knowledge of retrosynthesis and helped giving
insights to our research.
REFERENCES
Abu-Shanab, F. A., Wakefield, B. J., & Elnagdi, M. H.
(2010). ChemInform Abstract: Methylpyridines and
Other Methylazines as Precursors to Bicycles and
Polycycles. ChemInform, 28(31), no. https://doi.org/10.
1002/chin.199731246
Jagora, A., Gallard, J.-F. ̧., Beniddir, M. A., & Pogam, P. L.
(2021, September 15). A Reappraisal of the Structure of
Lyaline as the First Naturally Occurring Nacycline
Monoterpene Indole Alkaloid. ACS Publications.
Retrieved November 19, 2021, from https://pubs.
acs.org/doi/10.1021/acs.jnatprod.1c00572?ref=pdf&
Juhl M, Tanner D. Recent applications of intramolecular
Diels–Alder reactions to natural product synthesis. [J].
Chemical Society Reviews, 2009.
Hunt, L. (n.d.). Alkylation of Amines. University of Calgary.
Retrieved November 19, 2021, from https://www.
chem.ucalgary.ca/courses/350/Carey5th/Ch22/ch22-3 -
1.html
Wilson, R. (2001, January 24). Intramolecular Diels-Alder
Reactions. Princeton Edu. Retrieved November 19,
2021, from https://macmillan.princeton.edu/wp-conte
nt/uploads/Wilson-IMDA.pdf
A Proposal for the Synthesis Route of Lyaline
137
