
Study on the Effects of the Deregulation of Drug Price Control on
Orphan Drug Price: Empirical Study with Big Data on Drug Price
Data of 16 Provinces in China
Zeyu Zhang and Ying Bian
Institute of Chinese Medicine, University of Macau-State Key Laboratory of Quality Research in Chinese Medicine, Macau
999078, China
Keywords: Orphan Drugs, Price Difference, Drug Price, Big Data.
Abstract: Objective: To analyze the price changes of orphan drugs before and after the reform. To explore the impact of
the abolition of drug price control and market liberalization on the price of orphan drugs. And to provide
reference for the formulation of policies related to orphan drugs. Method: We used drug price data monitored
by Beijing Municipal Bureau of Health from February 2014 to June 2017. 32 kinds of 12222 orphan drug
data were extracted. Based on the interrupt time-series model and the accumulation of drug price differences
frequency, this research analyzes and compares the price level of orphan drug before and after the
deregulation. And the impact of reform on the price of orphan drugs were put forward. Results: After the
deregulation, the orphan drug price level increased significantly (slope change amount β
3
=3.45×10
-1
, P <0.05).
In 2014, 1.77% of the orphan drugs were at high price differences, and 1.22% in 2015, compared with 2.57%
in 2016 and 4.25% in 2017. Conclusion: After the deregulation, the Laspeyres index level of orphan drugs
increased significantly, and the situation of high price differences drug became more serious. It is difficult to
improve the accessibility of orphan drugs by market-oriented control measures alone. A better pricing
mechanism needs to be introduced to protect the rights and interests of patients with rare diseases.
1 INTRODUCTION
The accessibility and affordability of drugs are the
important attributes of drugs, and the drug price is of
great significance for drug accessibility and
affordability. Drug prices have always been one of the
focus of medical and health undertakings. In recent
years, China's drug expenses account for about 40%
of the total health expenditure of (https
://www.who.int/medicines/publications/pharm_guid
e_country_price_policy/en/.), which is at a high level
compared with developed countries. Statistics from
the Organization for Economic Cooperation and
Development (OECD) (https://data.oecd.org/
healthres/ pharmaceutical-spending.html) show that
the drug costs of most developed countries remain at
10% to 20% or less. Therefore, reasonable regulation
of drug prices is very important to relieve the
economic burden of individuals and even the country.
Government intervention is one of the main
means of drug price control, the (Huang, 2005;
Technology Information, 1997; China
Pharmaceuticals, 1999). However, the drug price
control system did not achieve the expected effect,
but induced some adverse phenomena, such as high
price differences (Shen, 2014), "Hu Piao effect"
(Ruan, 2008) caused by drug manufacturers avoiding
price reduction, excessive competition (Shi, 2014)
manifested in anti-price competition. There is also the
"exit effect" (Zhu, 2005), which makes too low prices
drugs gradually disappear from the prescription.
In the case of the chronic failure of China's drug
price control measures, on May 4, 2015, the National
Development and Reform Commission, the Health
and Family Planning Commission, the Food and Drug
Administration and other departments issued the
Opinions on Promoting the Drug Price Reform (F R
ANCO, 2013). Since June 1, 2015, in addition to
anesthetic drugs and type I psychotropic drugs, the
regulation of drug price has been canceled, and the
actual sales price of drugs has been formed by market
competition, marking the prelude to a new round of
drug price reform.
Zhang, Z. and Bian, Y.
Study on the Effects of the Deregulation of Drug Price Control on Orphan Drug Price: Empirical Study with Big Data on Drug Price Data of 16 Provinces in China.
DOI: 10.5220/0012019900003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 285-289
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
285

Rare disease, also known as an orphan disease.
The definition of rare diseases is not unified around
the world, mainly with low prevalence, very low total
population, life-threatening, difficult treatment, low
enthusiasm for drug development and high treatment
cost of (F R ANCO, 2013). Orphan drugs are drug for
rare diseases, refer to the drug (Liu, 2019) used in the
treatment, diagnosis, or prevention of specific rare
diseases.
On May 11,2018, the National Health
Commission, the National Drug Administration and
other five departments jointly formulated China's
First Batch of Rare Diseases List, clearly listing 121
rare diseases, aiming at safeguarding the health rights
and interests of patients with rare diseases, which is a
milestone in the management of rare diseases in
China. However, there are few studies on the drug
price of rare diseases in China. China's population
base is large, and although the incidence of rare
diseases is low, its patient population still cannot be
ignored. This study is based on the drug price data
from 16 provinces from 2014 to 2017. It aims to
analyze the price changes of orphan drugs before and
after the reform, explore the impact of abolishing
price control and market liberalization on the price of
orphan drugs, and provide a basis for the policy
formulation related to orphan drugs.
2 DATA AND METHODS
2.1 Data Sources
The research data originated from the national drug
price monitoring data of the Beijing Municipal
Bureau of Health from February 2014 to June2017,
and the cooperative research institution is the Peking
University School of Pharmaceutical Sciences. In this
study, 32 kinds of 12222 orphan drug data from 16
provinces including Gansu, Shandong, Heilongjiang,
Shanghai, Jiangsu, Zhejiang, Hubei, Jilin, Liaoning,
Inner Mongolia, Ningxia, Shandong, Shanxi,
Sichuan, Yunnan and Chongqing were selected. The
types of orphan drugs refer to the research of Liu Xin
(Liu, 2019) on the status quo of orphan drugs in
China. The drug data include the general name,
specification, production unit, monitoring unit, sales
volume, price and other data.
2.2 Data Analysis
This study used daily dose (DDD) as the unit of
measurement to compare drugs of different
manufacturers, dosage forms and specifications, with
the total DDDs of each drug as the amount of drugs
every two months. The DDD data for the drugs were
obtained from the WHO Collaborating Centre for
Drug Statistics Methodology
(https://www.whocc.no/).
2.3 Drug Price Differences
The drug price differences is the ratio of the
difference between the actual retail price and the
factory price and the factory price, and its expression
is as follows:
Price Diference cs
PD
=
Actual retail price − factory price
factory price
×100%
According to Shen Hongtao's research (Shen,
2014) on drug price structure in many countries, he
proposed that the factory price of less than or equal to
50% retail price is unreasonable, that is, the drug price
is artificially high. More than 50% of the average
evaluation price ratio is relatively reasonable, that is,
the drug price is not artificially high. Therefore, drugs
with a price differences above 100% were high price
difference drug.
2.4 Laspeyres Index
Laspeyres index can measure the overall price level
of a drug (Ye, 2016), that is, keep the weight of each
drug unchanged, and compare the drug price level
after the calculated period with the drug price of the
base period. Collect drug prices for the calculated and
base periods (P
0
, P
1
) and Basal usage (Q
0
). The
Laspeyres index was calculated using the following
formula:
𝐿=
∑
𝑃
𝑄
∑
𝑃
𝑄
In this study, the drug price data from February
2014 was used as the base period data to calculate the
pull price index of each period.
2.5 Interrupt Time Series Model
Interrupt time-series (ITS) design is designed to
collect outcome data from multiple time points before
and after the intervention, and evaluate the effect of
the intervention with statistical models, including
level changes and trend changes, which is mostly
used to evaluate policy (Shao, 2015). Its nature is a
linear regression (Lagarde, 2011) for a piecewise fit.
Set up X
1
For the time variable of the count, X
1
=
1,2,3,…,n; X
2
Represents the intervention, and the
ICBB 2022 - International Conference on Biotechnology and Biomedicine
286

pre-intervention X
2
=0. After the intervention X
2
=1;
X
3
Represents the slope, and set X
3
=0 represents the
pre-intervention observations, X
3
=X
1
Represents the
observations after the intervention, and εt is a random
error term. The fit level and slope change models
were performed as follows:
Y
t
=β
0
+β
1
X
1
+β
2
X
2
+β
3
X
3
+∑β
j
β
j
+εt, ∑β
j
X
j
Represents a set of covariates which is not considered
here. Generation the variables X
1
, X
2
, and X
3
into the
formula, the pre-intervention model is: Y
t
=β
0
+β
1
X
1
+
εt; The post-intervention model is: Y
t
=β
0
+β
1
X
1
+β
2
X
2
+β
3
X
3
+εt =β
0
+β
1
X
1
+β
2
×1+β
3
X
3
+εt
=(β
0
+β
2
)+(β
1
+β
3
)X
1
+εt =β
0
*
+β
1
*
X
1
+ t; β
0
*
and β
1
*
are
Called adjust parameters. β
1
is the slope of pre-
intervention, β
2
is the amount of horizontal change, β
3
is the amount of slope change, (β
1
+β
3
) Is the slope
after the intervention. The hypothesis test of the
regression coefficient is the significance test of
horizontal change and slope change. The interrupt
point of this study was in June 2015. Analysis using
the Durbin-Watson test found that there was
autocorrelation in the pre-intervention regression
equation. Use generalized least-squares estimation to
solve the first-order autocorrelation bias (Shao,
2015).
SPSS 25.0 statistical analysis software was used,
and P <0.05 was set to indicate statistical significance.
3 RESULT
3.1 The Impact of Deregulation on the
Price Level of Orphan Drugs
A total of 32 orphan drugs were included in this study,
including 12,222 drug price data. Take the time as the
horizontal coordinate and take the fixed Roche price
index as the ordinate. Fig. 1 shows the impact of the
deregulation on the overall price of orphan drugs.
Figure 1: Effect of drug price deregulation on orphan drugs prices.
The slope (β
1
) of the orphan drug price index
before the deregulation is 0.006. The level change
after the deregulation is -0.394 and the amount of
slope change is 0.345 (P <0.05). The difference before
and after was significant, indicating that the price
level of orphan drugs increased significantly after the
deregulation.
3.2 Drug Price Differences Before and
After the Deregulation
The drug price increase rate was taken as the abscissa,
and the cumulative frequency was taken as the
ordinate for drawing.
In Fig. 2, when the drug price differences was at
the highest price increase rate set by the government,
the cumulative frequency curve increased almost
vertical. After more than 15%, the growth rate slowed
down, and the price differences of most drugs was
0.8
0.9
1
1.1
1.2
1.3
1.4
1.5
1.6
2014 2015 2016 2017
Laspeyres index
Year
Study on the Effects of the Deregulation of Drug Price Control on Orphan Drug Price: Empirical Study with Big Data on Drug Price Data of
16 Provinces in China
287
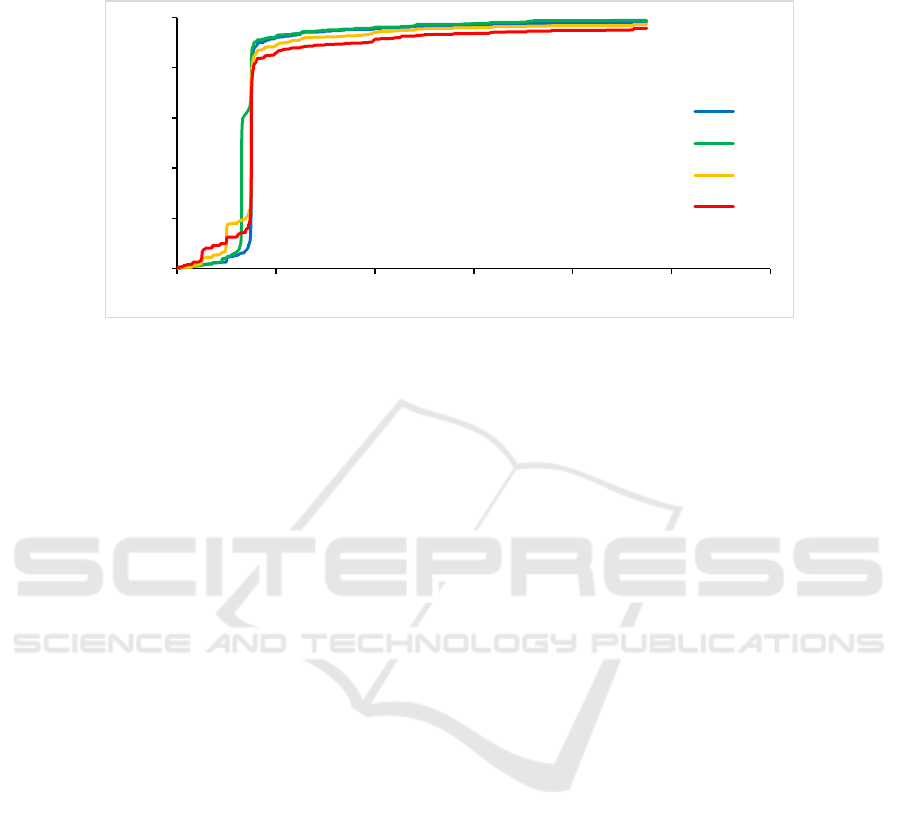
Figure 2: Cumulative frequency of orphan drug price increase rate from 2014 to 2017.
within 100%. In 2014,1.77% were high-priced drugs,
1.22% were high prices in 2015, compared with
2.57% in 2016 and 4.25% in 2017.
4 DISCUSSION
4.1 The Effect of Drug Price Control
Policy Reform Has Both
Advantages and Disadvantages
Fig. 1 results show that after the deregulation, the
price level of orphan drug rose significantly, and the
Laspeyres index rose sharply in 2017. Combined with
the results of Fig. 2, in 2016 and 2017, when the price
control was abolished, the proportion of high price
difference drugs increased year by year, indicating
that the problem of the inflated price of orphan drugs
is becoming more and more prominent, and the role
of the completely competitive market in leading the
price change is not obvious. Yang mingchun (Yang,
2018) believes that after the abolition of control, in
order to see from the production period, the drug price
will generally increase by in order to gain more
benefits. Through empirical research, Jiang Zaiduo
(Jiang, 2016) found that the drug price was still
inflated and the existed after the policy reform. This
study confirms these two conclusions that the
abolition of government pricing can not curb the
problem of inflated drug prices.
As can be seen in the previous section of the chart,
the proportion of drugs with low price differences in
2016 and 2017 also increased significantly, which has
certain benefits to its own sustainable development.
The regulation of the "zero margin" of essential drugs
is too extreme. In the absence of government health
spending, zero margin blocks hospitals' income when
using essential drugs, but causes medical institutions
to try to circumvent price controls. The same is true
for low-priced drugs in orphan drugs. The reform is
of great significance to the price rise of low-priced
drugs. Most low-priced drugs are still low-priced
drugs after experiencing price increases. The price
increase effectively alleviates the "dead standard"
situation of some low-priced drugs, guarantees the
supply of low-priced drugs, protects the interests of
low-priced drug manufacturers, and enables the
market to develop (Wang, 2020) healthily. At the
same time, for patients with rare diseases, the
accessibility of low-cost orphan drugs can also be
guaranteed to reduce the economic pressure of
patients.
4.2 The Effect of Policy Reform Still
Needs to Be Long-Term Evaluation
The reform of government pricing is aimed to
establish a market-led price formation mechanism.
Considering the operation of the market and the lag
of the reform and the emergence of volume
procurement policy, the subsequent effect remains to
be further observed.
4.3 The Letter of Drug Price
Formation Mechanism Needs to Be
Improved
The previous drug price customization mechanism is
defective, and the laws and regulations are also
0
0.2
0.4
0.6
0.8
1
0 0.2 0.4 0.8 1 1.2
Cumulative frequency
0.6
Drug price differences
2014
2015
2016
2017
ICBB 2022 - International Conference on Biotechnology and Biomedicine
288

imperfect. Due to policy support and monopoly, there
is an unreasonable price gap between patented drugs
and generic drugs, while products with significantly
better quality and efficacy are allowed to be priced
separately. However, due to the differences in
manufacturers, brands, and geographical location, the
price difference between the same drugs is also very
large (Ruan, 2008). At the same time, the government
pricing of drugs is mainly the cost price increase, and
the lack of consideration of the circulation cost is also
one of the reasons for the inflated price of (Liu, 2006).
China's population base is large, and although the
incidence of rare diseases is low, its patient
population still cannot be ignored. Orphan drugs are
very important in the treatment of rare diseases, so the
price change and inflated price of orphan drugs
should be paid more attention. At present our orphan
drug accessibility is compared with developed
countries still has a certain gap (Liu, 2019), for
orphan drugs and all the long-term benign
development of drug prices, suggest a more perfect
pricing mechanism, medical and health undertakings,
pharmaceutical enterprises, medical institutions,
patients and other interests, fully consider drugs in
addition to the production cost of circulation, storage,
and sales costs, to achieve a more reasonable balance.
5 CONCLUSION
After the abolition of drug government pricing, the
Laspeyres index of orphan drugs increased
significantly, and the inflated price of drug became
more and more serious. The proportion of drugs with
high price increase rate increased from 1.77% in 2014
to 4.25% in 2017. It is difficult to improve the
accessibility of orphan drugs by market-oriented
control measures alone, and a better pricing
mechanism needs to be introduced to protect the
rights and interests of patients with rare diseases.
REFERENCES
F R ANCO. P Orphan drugs: the regulatory environment J.
Drug Discov Today, 2013, 18(3-4):163-172.
Huang Zhiyong, “On the research of Chinese governmental
regulation problem in pharmaceutical Industry, D.
National University of Defense Science and
technology, 2005.
Jiang Zaiduo, Song Qiong, Liu Jing, Xiang Yan, Tan
Rongjun, “A fter the opening price, the purchase of
drugs and medical institution in Hubei Provinc e and the
use of the status quo a nalysis and Countermeasures,” J.
Pharmaceutical Analysis, 2016,11 (06): 25-28.
Lagarde M. How to do (or not to do) ...Assessing the impact
of a policy change with routine longitudinal data J.
Health Policy Plan, 2011,27(1):76-83.
Liu Xin, L i Jian-tao, Z hang Peng-xiao, K ong Jian, M ei
Dan, Z hang Bo. Current Status of Orphan Drugs in
China and Comparative Analysis with Foreign
Countries J. Chinese Pharmaceutical Journal
,2019,54(10):839-846.
Liu Hua, “Analysis and thinking on the problem of inflated
drug prices,” J. Chinese Health Resources, 2006(04):
151-152.
OECD , Pharmaceutical spending , EB/OL
https://data.oecd.org/healthres/pharmaceutical-
spending.html .
Ruan Jing, “Study on the government control regulation
system of d rug p rice,” D. Soochow University, 2008.
Shao Hua, Wang Qiqi, Hu Yuehua. Interrupt time-series
analysis and its application in public health J. Chinese
Journal of Epidemiology, 2015, 36(9): 1015-1017.
Shen Hongtao, “Research on the pharmaceutical price
regulation in China,” D. Harbin Institute of Technology,
2014.
Shen Hongtao, “Research on the pharmaceutical price
regulation in China,” D. Harbin Institute of Technology,
2014.
Shi HongYang, Zhou Jie, “Excessive competition, inflated
drug prices and drug price regulation,” J. Journal of
Jiangxi Open University, 2014 (03): 66-70.
“T h e National Planning Commission issued the Interim
Measures on the Administration of Drug Prices,” J.
Technology Information, 1997.
“The National Planning Commission has issued a new
regulation on drug price management: standardize the
order of drug prices to stop artificially high pricing and
high discounts.” J. China Pharmaceuticals, 1999, 008
(001): 10-10.
Wang Nuoning, Han Sheng, Fan Di, Shi Shuwen, Chen
Jing, “Study on the effect of the deregulation of drug
price control on drug price,” J. Chin a Pharmacy, 2020,
31 (03): 257-260.
WHO. WHO Guideline on country pharmaceutical pricing
policies EB/OL. https
://www.who.int/medicines/publications/pharm_guide_
country_price_policy/en/.
WHO Collaborating Centre for Drug Statistics
Methodology EB/OL. https://www.whocc.no/.
Yang Mingchun, Tian Ye, Zou Yujie, Han Sheng, Shi
Xiuwen, Guan Xiaodong, “Effect of government
regulation and deregulation on drug prices —— takes
digestive drugs as an example,” J. China Journal of
Health Policy, 2018, 11 (09): 53-58.
Ye Rendao, Liu Gan, Xue Jie. Statistics M. Xidian
University Press, 2016: 225-226.
Zhu Xiaofa, “Explore the root cause of the inflated drug
price and its governance countermeasures,” J. Price
Theory and Practice, 2005 (07): 18-19.
Study on the Effects of the Deregulation of Drug Price Control on Orphan Drug Price: Empirical Study with Big Data on Drug Price Data of
16 Provinces in China
289
