
Retrosynthesis of a Newly Discovered Molecule in Betula Alnoides
Achieved by Considering a Similar Molecule,
3-Farnesyl-2,4,6-Trihydroxybenzophenon
Jihang Wu
1
, Rowena Ge
2,*
and Shu Liu
3
1
High School Attatched to Northeast Normal University, 377 Boxue St, Jingyue District, Changchun, Jilin, 130117, China
2
Lafayette College, 111 Quad Drive, Easton, PA, 18042, U.S.A.
3
Shanghai Experimental Foreign Language School, No.99 Yanji Dong Road, Yangpu District, Shanghai, 200093, China
Keywords:
Retrosynthesis, Benzophenone, 3-Farnesyl-2,4,6-Trihydroxybenzophenon.
Abstract: A recent found type of benzophenone (molecule A), a component of the alcohol extract of Betula alnoides,
has shown antiausterity activity against PANC-1 human pancreatic cancer cells. The molecular structure of
this new type of bezophenone is very similar to that of an already found bezophenone, 3-farnesyl-2,4,6-
trihydroxybenzophenon (molecule B), which demonstrates the availability of examining the production
method of this new bezophenone (molecule A) via considering the molecular structure of the already found
bezophenone (molecule B). In this paper, retrosynthesis (a theoretical analytic method) is applied to these two
bezophenones, in order to determine the types of molecules of the raw materials and a few efficient ways of
production.
1 INTRODUCTION
In a study published in the Journal of Nature Products
on May 19th. 2021, an ethanol extract of Betula
alnoides showed antiausterity activity, a method used
to discover lead compounds by using unprecedented
anticancer activities which target the tolerance of
cancer cells to nutrition starvation, against PANC-1
human pancreatic cancer cells under nutrient-
deprived conditions. According to the study, the
Phytochemical investigation of this active extract led
to the isolation of eight benzophenones (1−8), in
which 6 of them (2-7) are newly discovered and three
xanthones (9−11) (Omar, 2021).
The unknown molecule A (Figure1), one of the
main components of ethanol extract of Betula
alnoides, demonstrates great similarities in molecular
structure with 3-farnesyl-2,4,6-
trihydroxybenzophenone (Figure 2). Therefore, we
intend to use the concept of retrosynthesis to break
down the unknown molecule A (Figure1) through
such similarities.
*
Corresponding author
Retrosynthesis is an analytic approach based on
the desired product. When synthesizing this planning
process, the following rules should be clearly
understood. First of all, fragment ions produced
should be stable. Secondly, fragments are always in a
form of cations and anions so that they will be able to
switch between each other. Thirdly, the relationship
between the functional groups plays a role in deciding
the order of disconnection. For example, a functional
group that would boost the other to connect should be
disconnected last. Fourthly, electron-withdrawing
groups should be the first ones to be disconnected.
Fifthly, a balance of applying these rules should be
achieved in order to make the entire process as simple
as possible (Tutor, 2020).
Wu, J., Ge, R. and Liu, S.
Retrosynthesis of a Newly Discovered Molecule in Betula Alnoides Achieved by Considering a Similar Molecule, 3-Farnesyl-2,4,6-Trihydroxybenzophenon.
DOI: 10.5220/0012031900003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 419-424
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
419
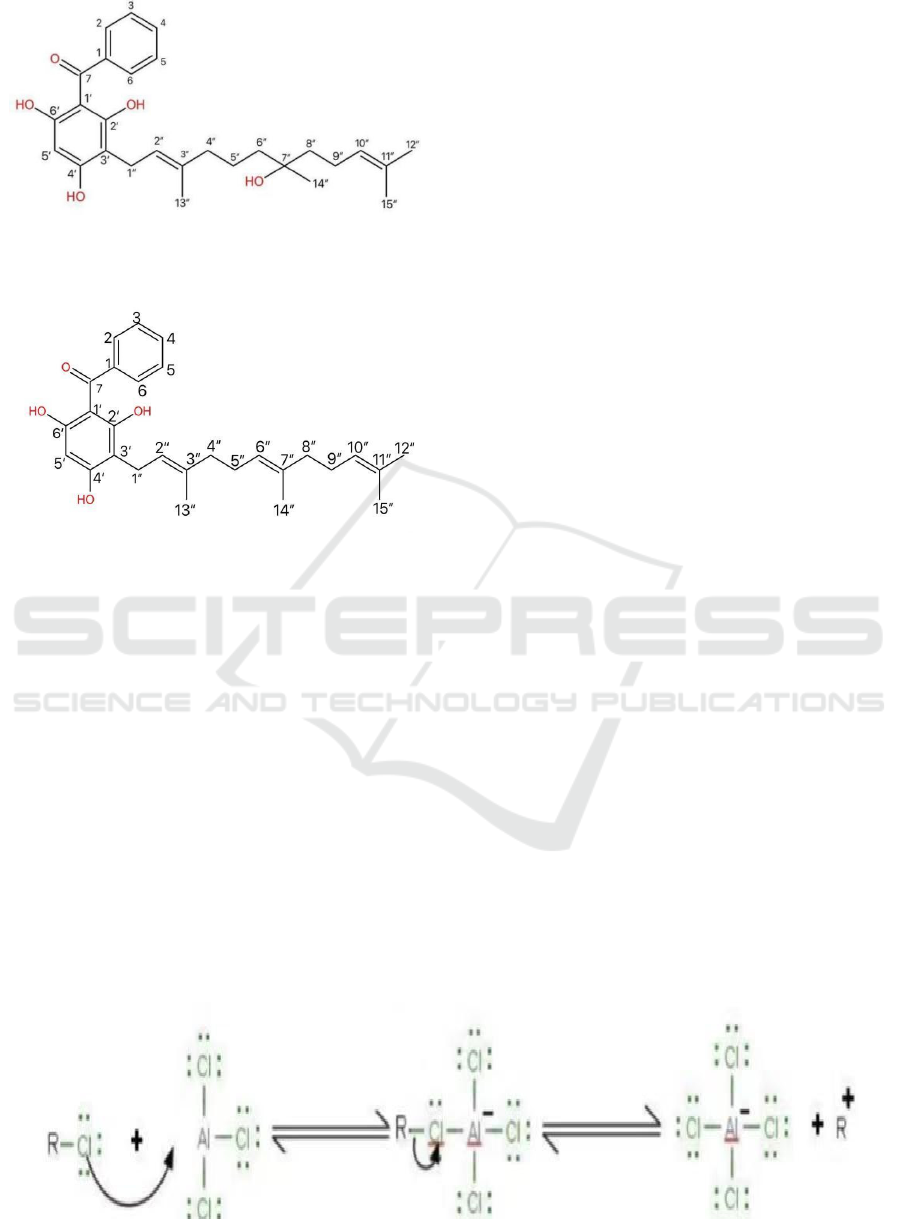
Figure 1: Molecular Structure of Molecule A.
Figure 2: Molecular Structure of Molecule B.
Considering the structures of molecule, A (Figure
1) and molecule B (Figure 2), they are both mainly
composed of benzophenone and a large chain with 15
carbons. Benzophenone is composed of two aromatic
rings, a structure with closed rings and conjugated
double bonds or lone pairs. One is a simple benzene
ring, the other is a trihydroxybenzene, called
phloroglucinol. The difference here is that between C
6''and C 7'', molecule A has a single bond whereas
molecule B has a double bond. In addition, C 7''of
molecule A is connected to a hydroxide group
whereas that of molecule B is connected to hydrogen.
2 METHODS
2.1 General Disconnecting Sequence
As regards to the rules of retrosynthesis, the general
sequence of disconnecting bonds is listed here. First,
the bond between C 3' and C 1''. Then, the bond
between C 1 and C 7 and the bond between C 1' and
C 7 could both be the second ones to be broken down.
The third step would be to break down the other
aromatic ring. Finally, the three hydroxide groups
could be considered to be disconnected, but not
necessarily. The following content includes the
considerations of the sequence and the mechanism.
2.2 Breaking Farnesyl Group
(Explanation)
The farnesyl group is a nucleophile that would attract
electrons, or withdraw electrons, from the aromatic
ring. As regards to the fourth rule listed in the
introductions, an electron-withdrawing group should
be disconnected first so the farnesyl group would be
the first one to disconnect. In addition, when
considering whether the first disconnecting functional
group would be the farnesyl group or the hydroxide
group, rule number three gives an answer. The
linkage of nucleophiles for aromatic rings would
deactivate the aromatic ring whereas the hydroxide
group would activate the ring. Here, a deactivating
step could be achieved after there is something that
has already activated the aromatic ring. Therefore, the
hydroxides on the benzene rings should not be
removed before the farnesyl. Besides, when removing
the farnesyl group, a more symmetrical structure with
two aromatic rings would be present, which allows
the following step to be more conveniently presented
(fifth rule). All of these rules support that the farnesyl
group should be disconnected first (Kolesnikov,
2016).
The following steps show Benzene alkylation
(Libretexts, 2020), where the R group represents the
farnesyl group.
Figure 3: Benzene Alkylation.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
420
As shown in Figure 3, firstly, the Cl, linked to the
farnesyl group, link to AlCl3, forming a farnesyl
cation.
Figure 4: Farnesyl Cation Formation.
As shown in Figure 4, secondly, the cation link to
the trihydroxybenzene by electrophilic addition
reaction.
Figure 5: HCl Formation.
As shown in Figure 5, finally, the AlCl4 pushes
the electrons back, splitting the hydrogen, forming a
by-product of HCl (Libretexts, 2020).
2.3 The Separation of the Two
Aromatic Rings
After removing the farnesyl group, the rest of the
structure is mainly composed of benzophenone with
two aromatic rings. The reason why the hydroxides
should still not be separated is that it could also boost
the following linkage of the aromatic ring and the acyl
group. Moreover, steps should be as simple as
possible. The next retrosynthesis step is to separate
the two aromatic rings. As there is a ketone (-R-C=O-
R-) in the middle of this complex, there will be two
pathways of disconnection: the benzene ring or the
trihydroxy benzene ring.
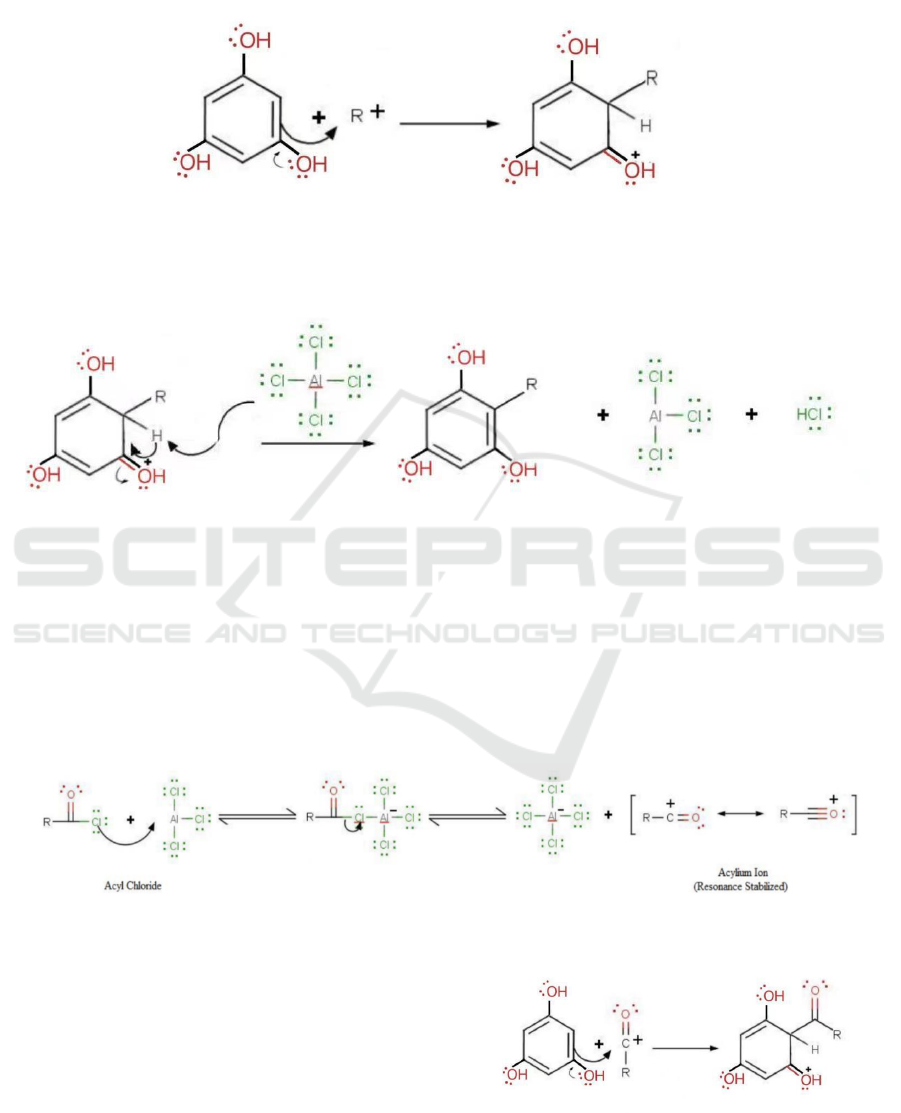
Figure 6: Formation of Acylium Ion and AlCl4-.
As shown in Figure 6, firstly, the reverse reaction
of both of the disconnections would start with a
formation of an Acylium ion and AlCl4, where the
AlCl3 acts as a catalyst. Here the R group would be
either trihydroxybenzene or benzene, which depends
on the pathway retrosynthesis is performed.
i. Breaking benzophenone into Benzene and
Benzoyl chloride, 2,4,6-trihydroxy (acyl chloride)
The reverse reaction mechanism would be a
simple electrophilic substitution.
Figure 7: Linking of the Aromatic Ring.
Retrosynthesis of a Newly Discovered Molecule in Betula Alnoides Achieved by Considering a Similar Molecule,
3-Farnesyl-2,4,6-Trihydroxybenzophenon
421
In Figure 7, the R group refers to the benzene ring.
In this mechanism, the acylium ion acts as a
nucleophile while the tri hydroxybenzene acts as an
electrophile. The inductive effect of the hydroxide
groups could "push" the electron onto the conjugated
double bond in the trihydroxybenzene which will
attach to the nucleophile. The move of electrons
allows the acylium ion and the aromatic ring to link
together.
Then, a deprotonation step formed by AlCl4-
would produce a by-product HCl and target molecule
benzophenone.
Figure 8: Formation of HCl.
Again, in figure 8, here the R group refers to the
benzene ring. In this mechanism, the AlCl4 act as an
electrophile, attaching the hydrogen on the
trihydroxybenzene, "pushing" back a pair of
electrons along the same way back, finally to the
oxygen. And the hydroxide and chloride are split,
forming a molecule of HCl (Libretexts, 2020).
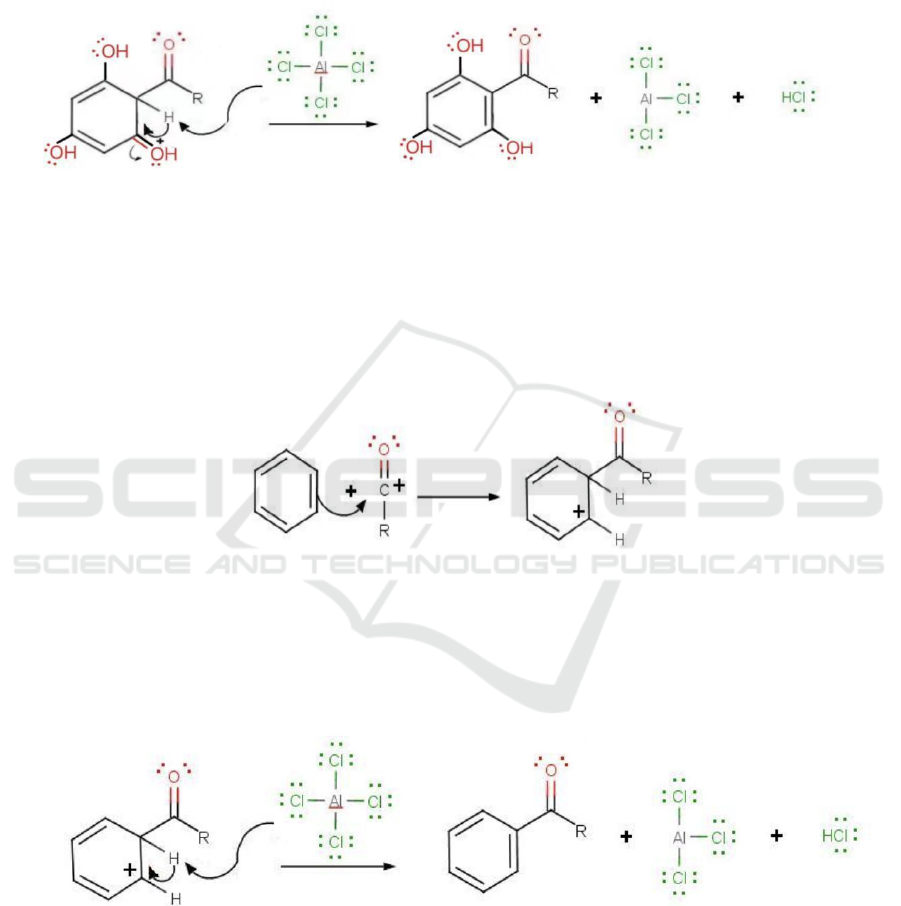
ii. Breaking benzophenone into Phloroglucinol
and Benzoyl chloride (acyl chloride)
This mechanism is very similar to the last one.
The reverse reaction mechanism is also a simple
electrophilic substitution.
Figure 9: Phloroglucinol and Benzoyl Chloride (https://www.chemistry.msu.edu/faculty/reusch/VirtTxtJml/chapt15.htm).
In the Figure 9, the R group refers to
trihydroxybenzene. In this mechanism, the acylium
ion also acts as a nucleophile while the benzene acts
as an electrophile. The difference is that there is no
hydroxide group to "push" the electron pairs to the
nucleophile. The linkage of benzene and the acylium
ion is achieved by moving an electron pair from the
conjugate double bond itself to the acylium ion.
After that, the deprotonation step is very similar.
Figure 10: Formation of the conjugated double bond and HCl
(https://www.chemistry.msu.edu/faculty/reusch/VirtTxtJml/chapt15.htm.)
As shown in Figure 10, the electron pair on the
chloride of AlCl4 passes along the same path to the
benzene ring, forming the conjugated double bond
again. The chloride also attracts the hydrogen and
form a by-product of HCl.
2.4 Comparison of 3i. and 3ii.
There are two explanations for the effects that a
substituent exerts on the reactivity of a benzene ring:
Firstly, it depends on the inductive effect of the
ICBB 2022 - International Conference on Biotechnology and Biomedicine
422
substituent. Except for metals and carbon, most
elements have much higher electronegativity than
hydrogen. As a result, sigma bonds are formed
between substituents containing nitrogen, oxygen,
halogen atoms, and an aromatic ring that gives an
inductive electron. Secondly, the conjugation of a
substituent function with the aromatic ring is another
important result. The electron pair donates or
withdraws electrons with the help of conjugated
interaction to form a benzene ring. If the atom bonded
to the ring contains nitrogen, oxygen, and halogens
which have one or more non-bonding valence shell
electron pairs, electrons may move into the aromatic
ring according to p-pie conjugation. Finally, the
benzene ring may receive electrons from polar double
and triple bonds.
In this paper, 3-farnesyl-2,4,6-
trihydroxybenzophenone(B) only has several double
bonds on its farnesyl chain while there is a hydroxyl
group located at 3’’ on the unknown compound. The
rules for oxygen are utilized on it and the hydroxyl
group actives benzene, making the following reaction
easier to happen
(https://www.chemistry.msu.edu/faculty/reusch/Virt
TxtJml/chapt15.htm.)
2.5 The Separation Between the Other
Aromatic Ring and the Acyl Group
No matter which passway it has gone through in the
last step, the next step would be just to break down
the linkage between the acyl group with the other
aromatic ring. And the step would be basically the
same as before. Therefore, the advantage of
hydroxide boosting the linkage between the aromatic
ring and the acyl group would ultimately appear for
both sequences.
2.6 The Separation of the Three
Hydroxides
This step is optional because the trihydroxybenzene,
phloroglucinol (which is made from glucosides, plant
extracts and resins), is a common raw material, and
could simply be considered as a final molecule in the
process of planning retrosynthesis (Hoool, 2018).
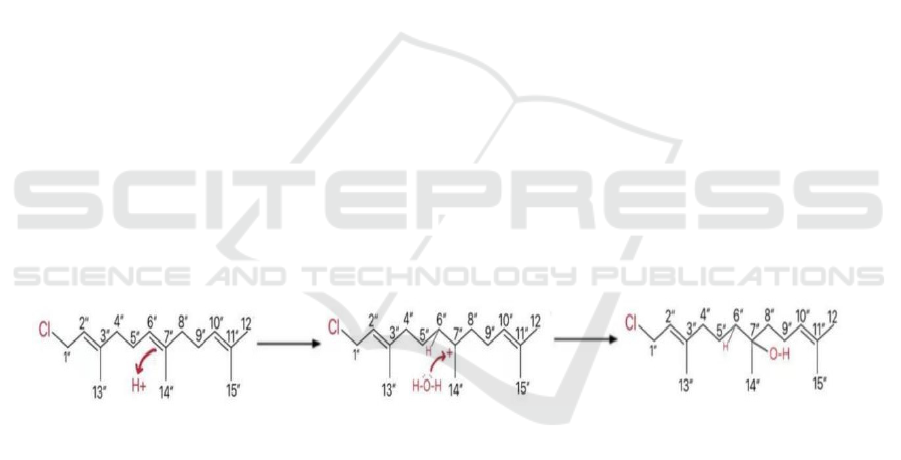
2.6.1 Converting Molecule B to A
This step could be achieved by altering the reactant of
farnesyl of the benzene alkylation
(https://www.chemistry.msu.edu/faculty/reusch/Virt
TxtJml/chapt15.htm.), which means converting the
farnesyl group into a similar one with a hydroxide on
C 7'' and no double bonds between C 6'' and C 7''. The
reason why adding the hydroxide onto the farnesyl
group first is that the reaction would be easier to target
without disruptions.
This step is an electrophilic addition reaction
Figure 11. Conversion of Molecule B to A
In Figure 11, the proton acts as a nucleophile and
the double bond between C 6'' and C 7'' acts as an
electrophile, where hydrogen is linked onto C 6''.
Then a carbocation is formed at C 7'', which acts as a
nucleophile, attacked by the lone pair of the water
molecule, and the hydroxide is linked onto C 7''.
According to the inductive effect, the hydroxide
group is more likely to locate on C 7'' than C 6'' as
there is more inductive effect on C 7'' that would
stabilize it. This theory is the same as the inductive
effect on C 2'' and C 10'', which means these two are
also not "favorited". In addition, when considering
the inductive effect of C 3'' and C 11'' compared with
C 7'', the inductive effect is not as symmetrical as that
of C 7''. Therefore, both of these two considerations
support that the hydroxide group would be the mostly
located on C 7'', with a small proportion of the other
Cs.
When considering the way of extracting only the
target molecule with hydroxide group located on C
7'', their slightly different boiling points would allow
fractional distillation to separate them and isolate the
wanted products.
2.6.2 Conclusion and Evaluation
In conclusion, we aim to use a known compound to
deduce a new one that obtains a similar structure by
connecting and breaking functional groups or
inducing and moving electrons. This research paper
promotes the understanding of the retrosynthesis
process of 3-farnesyl-2,4,6-trihydroxybenzophenone.
Retrosynthesis of a Newly Discovered Molecule in Betula Alnoides Achieved by Considering a Similar Molecule,
3-Farnesyl-2,4,6-Trihydroxybenzophenon
423

The results suggest that it may be a relatively simple
way to find a new medicine that targets pancreatic
cancer.
Since the retrosynthesis shown above have not
been proven in a real experiment, the feasibility of
such methods is unknown. Other factors such as
financial restrictions, potential safety hazards, and the
duration of a total experiment should be considered to
achieve practicality. Furthermore, only two routes
were analyzed so it was possible that simpler ways
were neglected. In addition, distillation should be
performed in conditions as perfect as possible so
common experimental tools may not be able to
separate the desired product because it may be mixed
with other molecules that have similar boiling points.
Overall, further testification and research are required
to determine the hypothesis provided in this paper.
REFERENCES
Aromatic Reactivity, https://www.chemistry.msu.edu/
faculty/reusch/VirtTxtJml/chapt15.htm.
Hoool. (2 July 2018) Phloroglucinol: Natural Occurrence,
Use, Dosage, AND Side-Effects: Hoool Health &
Wellness. https://www.hoool.com/phloroglucinol/.
Kolesnikov, I.M., et al. (9 Apr. 2016) Efficient Catalysts for
Benzene Alkylation with Olefins. Catalysis
Communications, Elsevier.
https://www.sciencedirect.com/science/article/abs/pii/
S1566736716301145#:~:text=Benzene%20alkylation
%20with%20ethylene%20or%20propylene%20in%20t
he,interaction%20of%20a%20benzene%20molecule%
20with%20the%20catalyst.
Libretexts. (14 July 2020) 18.5: Alkylation and Acylation
of Benzene - The Friedel-Crafts EAS REACTIONS.
Chemistry LibreTexts.
https://www.chem.libretexts.org/Bookshelves/Organic
_Chemistry/Map%3A_Organic_Chemistry_(Wade)/18
%3A_Reactions_of_Aromatic_Compounds/18.05%3A
_The_Friedel-Crafts_Alkylation.
Libretexts. (13 Sept. 2020) Friedel-Crafts Acylation.
Chemistry LibreTexts.
https://www.chem.libretexts.org/Bookshelves/Organic
_Chemistry/Supplemental_Modules_(Organic_Chemis
try)/Arenes/Reactivity_of_Arenes/Friedel-
Crafts_Acylation.
Omar, Ashraf M., et al. (2021) Benzophenones from Betula
Alnoides with Antiausterity Activities against the
PANC-1 Human Pancreatic Cancer Cell Line. Journal
of Natural Products, vol. 84, no. 5. pp. 1607–1616.
doi:10.1021/acs.jnatprod.1c00150.
Tutor. (13 July 2020) Retrosynthesis. Online Organic
Chemistry Tutor.
https://www.onlineorganicchemistrytutor.com/retrosyn
thesis/.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
424
