
The Whole is Greater than the Sum of the Parts, Combination Use as
CABENUVA in Treating HIV: Meta-Analysis from Clinical Datasets
Xuanji Cao
1,†
, Youkai Yang
2,†
and Yiyi Zhao
3,*,†
1
School of Chemistry, University of Nottingham, Nottingham, U.K.
2
Faculty of Engineering, China Pharmaceutical University, China Pharmaceutical University, Nanjing, China
3
WUT-AMU Institute, Wuhan University of Technology, Wuhan University of Technology, Wuhan, China
Keywords:
CABENUVA, HIV-1, Cabotegravir, Rilpivirine.
Abstract:
CABENUVA is a 2-drug co-packaged product for the treatment of HIV-1 infection in adults to replace current
antiretroviral therapy. The development of more effective and convenient medication can improve the lives
of HIV patients. This article mainly focuses on the drug profile, clinical trial and comparative analysis of
CABENUVA with other newly approved HIV-1 drugs. There have been several HIV-1 drugs approved since
2018, but the route of administration of them is still oral. The appearance of CABENUVA indicates the
possibility of introducing and developing injection HIV medications.
1 INTRODUCTION
1.1 Introduction of HIV-1
HIV is a type of retrovirus that infects cells of the
human immune system and causes the spread of
diseases in the human body by destroying the body's
T-lymphocytes, thereby blocking cellular and
humoral immune processes. That causes the immune
system to become paralyzed, thus creating the
environment for AIDS. Because HIV mutates so
rapidly, it is difficult to produce a specific vaccine.
The fact that is no effective treatment for the disease
made it a great threat to human health.
The Joint United Nations Programme on
HIV/AIDS estimates that in 2019, 38 million people
worldwide will be living with HIV, 1.7 million will
be newly infected and 690,000 will die from HIV
disease. Compared with their estimation in 2010, the
overall HIV incidence in 2019 has decreased by 23%
and mortality by 37%. However, age stratification
shows a 52% reduction in new infections among
children, and only a 13% reduction among adults.
With mortality declining but HIV incidence and
population continuing to grow, the total number of
people living with HIV in 2019 is 24% higher than in
2010. (De Cock, 2021)
†
These authors contributed equally
In the nearly 40 years since the discovery of HIV,
great progress has been made in developing effective
treatments. However, a vaccine that prevents HIV
infection remains elusive. Most licensed vaccines
protect through the induction of antibodies. In the
case of HIV, the antibodies induced by the vaccine
must be able to protect the human immune system
against the effects of the multiple variants of HIV that
are prevalent worldwide, known as broadly
neutralizing antibodies. Recent advances in the
identification and characterization of such antibodies,
as well as progress in the design of candidate
antibodies to stimulate cellular immunity and the
results of recent clinical trials, are driving efforts to
develop an HIV vaccine that can eradicate the virus
once and for all. (Koff, 2016)
However, the development of an HIV vaccine has
not been successful, the number of people living with
HIV is still huge and continues to grow, and the need
for HIV suppressant drugs gets stronger every day.
Antiretroviral therapy (ART), is the treatment of
HIV with a combination of several antiviral drugs.
Although ART is effective in suppressing the onset of
HIV, it is time-consuming and has serious side
effects. There is an urgent need to find new and easier
ways to cure HIV infection or suppress the onset of
AIDS.
440
Cao, X., Yang, Y. and Zhao, Y.
The Whole is Greater than the Sum of the Parts, Combination Use as CABENUVA in Treating HIV: Meta-Analysis from Clinical Datasets.
DOI: 10.5220/0012032200003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 440-447
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
1.2 CABENUVA
CABENUVA is a 2-drug co-packaged product for the
treatment of human immunodeficiency virus type 1
(HIV-1) infection in adults to replace current
antiretroviral therapy in patients who are
virologically stable and suppressed. (Surve, 2020)
CABENUVA kept HIV-1 viral load at a suppressed
level.
CABENUVA is the world's first complete and
long-acting HIV treatment regimen, administered by
intramuscular injection (IM) once a month. The
approval of the drug marks a major milestone that will
revolutionize HIV treatment by shifting from oral
administration 365 days a day throughout the year to
monthly injections for only 12 days of treatment
throughout the year. CABENUVA (Cabotegravir and
Rilpivirine), developed by ViiV Healthcare is also the
latest drug currently approved by the FDA for the
treatment of AIDS, with approval scheduled on
January 20, 2021.
CABENUVA, an easier and more effective
treatment, is a huge improvement of the quality of life
for people with HIV. Simplified regimens for the
treatment of HIV-1 infection may increase patient
satisfaction and facilitate adherence. (Surve, 2020)
2 DRUG PROFILES
2.1 Highly Active Antiretroviral
Therapy
In 1996, the Chinese-American scientist D.Y. Ho
proposed the highly active antiretroviral therapy
(HAART), in which three or more antiretroviral drugs
are used in combination to treat AIDS. The HAART
combines protease inhibitors with a variety of
antiviral drugs, resulting in effective control of AIDS.
The application of this therapy reduces the drug
resistance caused by unitary medication, maximizes
the inhibition of viral replication, and restores some
or even all of the body's immune function.
Eventually, it slows the progression of the disease,
prolongs the patient's life ,and improves their quality
of life. CABENUVA (Cabotegravir and Rilpivirine)
also uses the principle of highly active antiretroviral
therapy to achieve better HIV suppression through a
combination of drugs.
2.2 Cabotegravir and Rilpivirine
The first part is a separate analysis of Cabotegravir
and Rilpivirine. The analysis illustrates Rilpivirine, a
long-term active, non-nucleoside reverse
transcriptase inhibitor; and Cabotegravir, a long-term
active HIV-1 integrase strand transfer inhibitor.
The target of Rilpivirine is NNRTIs, and the target
of Cabotegravir is HIV integrase. Rilpivirine
(TMC278) is a next-generation non-nucleoside
reverse transcriptase inhibitor (NNRTI) that
vigorously represses wild-type and NNRTI-resistant
HIV-1. activity. NNRTIs work by binding to and
blocking HIV reverse transcriptase, an HIV enzyme.
Cabotegravir (GSK744, GSK1265744) is an HIV
integrase inhibitor that is effective within a broad
range of HIV subtypes and inhibits the chain transfer
reaction catalyzed by HIV-1 integrase with an IC50
of 3 nM.
The following two figures (Figure1 and Figure2)
will show each chemical structure formula of two
important components of CABENUVA.
Figure 1: Chemical structure of Cabotegravir.
Figure 2: Chemical structure of Rilpivirine.
The Whole is Greater than the Sum of the Parts, Combination Use as CABENUVA in Treating HIV: Meta-Analysis from Clinical Datasets
441
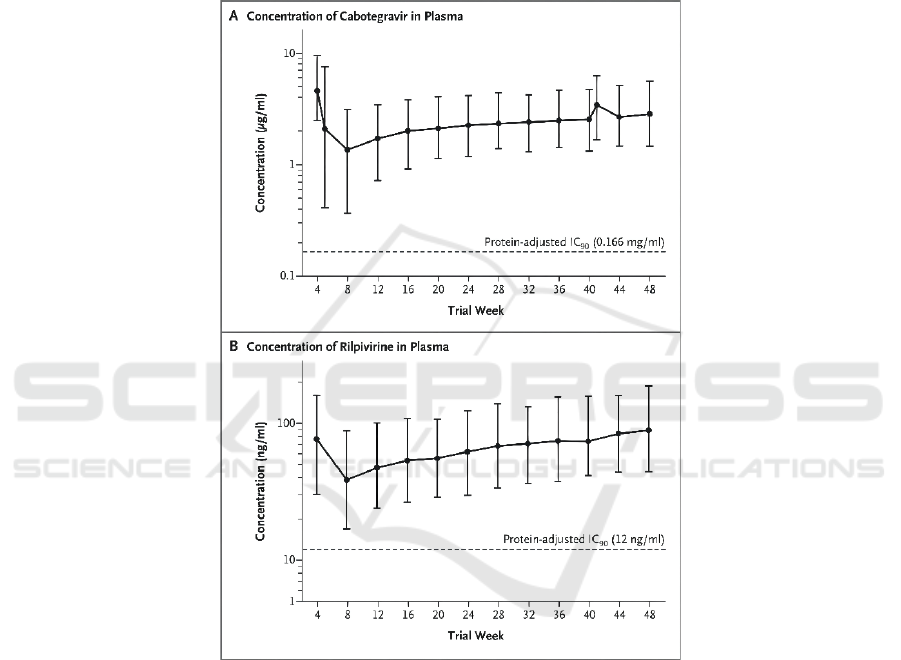
2.3 Pharmacokinetics and Treatment
Satisfaction
In terms of PHARMACOKINETICS, the research
team of the University of Nebraska’s Medical Center
confirmed that during therapy, Cabotegravir and
Rilpivirine concentrations in plasma were similar to
those reported during oral therapy (Figure 3). Both
drugs from the first trough at week 8 to the trough at
week 48 showed a cumulative accumulation of
approximately 2.3-fold, close to steady-state drug
concentrations. The geometric mean plasma
concentrations of Cabotegravir and Rilpivirine at
week 48 (2.84 micrograms per milliliter and 90.3
nanograms per milliliter, respectively) were each 17
and 7.5 times their respective protein-adjusted
concentrations, respectively, required for 90% viral
suppression, similar to the results obtained after
monthly dosing in the phase 2 study. (Swindells,
2020)
Figure 3: Plasma Concentration–Time Profiles.
As assessed by HIVTSQs: after 44 weeks,
participants in the long-term active treatment group
showed a substantial promotion in treatment
satisfaction compared to those in the oral treatment
group. The adjusted mean score for the long-term
active treatment group was 5.68 points (95% CI, 4.37
to 6.98) higher than that of the oral treatment group.
This difference met the threshold of a minimal
clinically important difference according to a
distribution-based approach. In a within-group
comparison conducted at week 48 in the long-term
active treatment group, 97% of participants who
responded to the questionnaire and 86% of
participants in the intention-to-treat exposed
population chose injectable therapy over daily oral
therapy as their preferred HIV treatment. (Swindells,
2020)
Eventually, Nebraska’s research team concluded
the comparative trial of long-term active
Cabotegravir and Rilpivirine with oral induction
therapy for HIV-1 infection. The conclusion is that
monthly injections were non-inferior to standard oral
therapy for maintaining HIV-1 suppression.
Injection-related adverse events were familiar but
infrequently led to medication withdrawal.
(Swindells, 2020)
ICBB 2022 - International Conference on Biotechnology and Biomedicine
442

3 STUDY RESULTS
3.1
Pharmacology Property
CABENUVA was proved very effective to treat
single-cycle HIV-1 infections and HIV-1 mutants with
site-directed mutations (Elvitegravir-resistant
integrase mutants, raltegravir-resistant integrase
mutants, and raltegravir-resistant integrase mutants).
When using it for dolutegravir-resistant integrase
mutants and raltegravir-resistant integrase mutants,
the medication was less effective. The EC50 value was
shown below in Table.1.
Table 1: CABENUVA EC50 value of HIV mutants.
Mutants EC50 (nmol/L)
92UG029, NL4-3,
LAI, MJ4, 92UG001,
MVP5180-91
1.3-2.0
Y143C, T97A+Y143C 1.2
E92Q+N155H 5.9
E138K+G140S+Q148R 15
E92Q+Y143C 2.4
E92Q+NQ155H 12
G140A+Q148R 11
Participants were randomly allocated to IM
cabotegravir 800 mg after 14 days of receiving 30 mg
oral cabotegravir once a day, followed by three 200
mg SC doses, three 200 mg IM doses, or three One
400 mg IM doses every four weeks, or a second
cabotegravir 800 mg IM treatment after 12 weeks. At
month 3 (1200 mg) and month 4, those in the 200 and
400 mg IM cohorts received IM doses of long-acting
rilpivirine (900 or 600 mg). For all four regimens,
treatment-related plasma cabotegravir concentrations
(mean > 4 PA-IC90) were seen 3 days after injection
and lasted until the conclusion of the dosing interval.
The cabotegravir 800 and then 400 mg IM regimens
showed accumulation until the fourth month, whereas
the other regimens appeared to be stable after three
months.
The pharmacokinetic characteristics of the two
medications have low to moderate inter-subject
variability (cabotegravir AUC CV percent is 23-52
percent, and ripavirine AUC CV percent is 34-35
percent). At week 8, 4 weeks following the first IM
loading dosage (primary trough concentration), the
cumulative geometric mean (95 percent CI) plasma
concentrations for CAB (1.31 to 1.46) and RPV
(38.05 to 41.80) were 1.38 g/mL (1.31 to 1.46) and
39.88 ng/mL (38.05 to 41.80), respectively. The
geometric mean (95 percent confidence interval)
plasma concentration of CAB was 2.97 g/mL (2.85 to
3.10) at week 48, whereas the RPV was 86.42 ng/mL.
(82.87 to 90.14).
FLAIR (N = 566) and ATLAS (N = 618) trials
were conducted in HIV-1 infected adults to
demonstrate its non-inferior antiviral efficacy. The
distinction is that FLAIR enrolls ART-naive patients,
while ATLAS select ART-experienced patients who
were stable on an ARV regimen. (Clinical Review
Report)
The trials were divided into 3 stages. First of all,
the induction phase (FLAIR only) controls their
plasma HIV-1 RNA. Then in the maintenance phase,
the patients were randomly grouped in half to remain
on oral CART or switch to the CAB + RPV regimen.
Patients in the FLAIR studies continued their therapy
during the induction phase, whereas those in the
ATLAS trials continued to utilize their existing ARV
regimen. The latter group takes oral CAB + RPV (30
mg/25 mg, one tablet each) once a day for at least four
weeks, then injects the first dosage of CAB + RPV
(600 mg/900 mg) within two hours of the final oral
dose, then injects CAB + RPV (400 mg/600 mg)
every four weeks after that. Patients in the CAB +
RPV group continued their IM dose as normal
throughout the "extension phase," and those moving
from the CART group to CAB + RPV followed the
same treatment regimen.
Figure 4: Trial process.
HIV-1 immunotherapeutic vaccines, systemic
immunomodulators, acetaminophen (if acute viral
hepatitis is present), chronic use of systemic
glucocorticoids, HCV therapy, certain antibiotics,
consistent administration of medications that lower
the concentration of any study drug component, and
other exploratory agents, ARV drugs (not otherwise
specified), cytotoxic chemotherapy, or radiation
therapy were not allowed at any time during the study.
Over 90% of the patients in both trials were
continuing through or completed the maintenance
phase. However. 22% of patients in FLAIR and 12%
patients in ATLAS were considered screening
failures since lack of efficacy (5%), AE or withdraw
The Whole is Greater than the Sum of the Parts, Combination Use as CABENUVA in Treating HIV: Meta-Analysis from Clinical Datasets
443
from the trials (approximately 5% to 7% after
entering the long-term follow-up phase).
The results existed at week 48 as the virologic
responses became significant, which is the standard
time frame used in the HIV-1 trial and is consistent
with the shortest analysis duration recommended by
the FDA for virological endpoints. The trial is
ongoing and the planned duration is 120 to 148
weeks.
In FLAIR, virologic failure was reported in 2.1
percent and 2.5 percent of patients in the CAB + RPV
and CART groups, respectively, with HIV-1 RNA
levels of 50 copies/mL or above at week 48. In
CART, 1.6 percent of patients in the CAB + RPV
group and 1.0 percent in the CART group. In FLAIR,
CAB + RPV and CART scored 94 percent and 93
percent, respectively, while in ATLAS, CAB + RPV
and CART scored 93 percent and 95 percent,
respectively. At week 48, HIV-1 RNA was less than
50 copies/mL. In FLAIR and ATLAS, treatment
differences were 0.4 percent (95 percent CI, 3.7 to
4.5) and 3.0 percent, respectively. It shows that there
isn't much of a difference between CAB+RPV and
CART effectiveness. The 96-week results confirm the
48-week findings, indicating that long-acting
cabotegravir and rilpivirine are non-inferior to
maintaining a standard care regimen in individuals
with HIV-1 for viral suppression maintenance. These
findings suggest the long-acting cabotegravir and
rilpivirine as a treatment choice for virally suppressed
people with HIV-1 over a nearly 2-year period.
3.2 AE and Model Health-Related
Quality of Life
In both studies, the CAB + RPV group had higher
AEs than those in the CART group in the process of
the maintenance. In ATLAS and FLAIR, more than
90% of patients in the CAB + RPV group had at least
one AE across trials, whereas in ATLAS and FLAIR,
the overall prevalence of AEs in the CART group was
71 percent and 80 percent, respectively. Most of AEs
were classified as grade 1 or 2. The revealed higher
occurrence of AEs was due in part to a variety of ISRs
caused by the monthly IM injections. Another trial
suggests that the observed AE profile with CAB +
RPV LA treatment was identical to that in the CAR
arm, omitting ISRs, and is consistent with the
previously oral therapy. Despite the fact that ISRs are
widespread (they account for 25% of all injections),
the majority of them are moderate (99 percent grade
1 or 2). Throughout the trial, the overall incidence of
ISRs rapidly declined from 70% at week 4 to 16% at
week 48, and the length of ISRs was brief (median 3
days). These findings show that the initial high rate of
ISRs was due to the introduction of a novel treatment
administration method (IM injection), with a lower
rate over time as participants got more comfortable
with the injection process.
HRQoL can be a valuable data for us to evaluate
the patients’ prognosis. In that case, HIVTSQ, PIN,
ACCEPT, HAT-QoL, NRS, and SF-12 are used to
evaluate a variety of characteristics of HRQoL, such
as acceptance of an injectable regimen and issues that
may arise as a result of it. In ATLAS, the HIVTSQs
and PIN results were statistically significant in favor
of the CAB + RPV group, but this was not the case in
FLAIR (after adjusting for multiplicity). The other
measurements revealed that CAB + RPV had a
numerical advantage in terms of patients' HRQoL.
None of these analyses were multiplicity-adjusted,
except the total score of HIVTSQs and PIN, hence the
results should be regarded with care. (Clinical
Review Report)
4 PHARMACOECONOMIC
RESULT
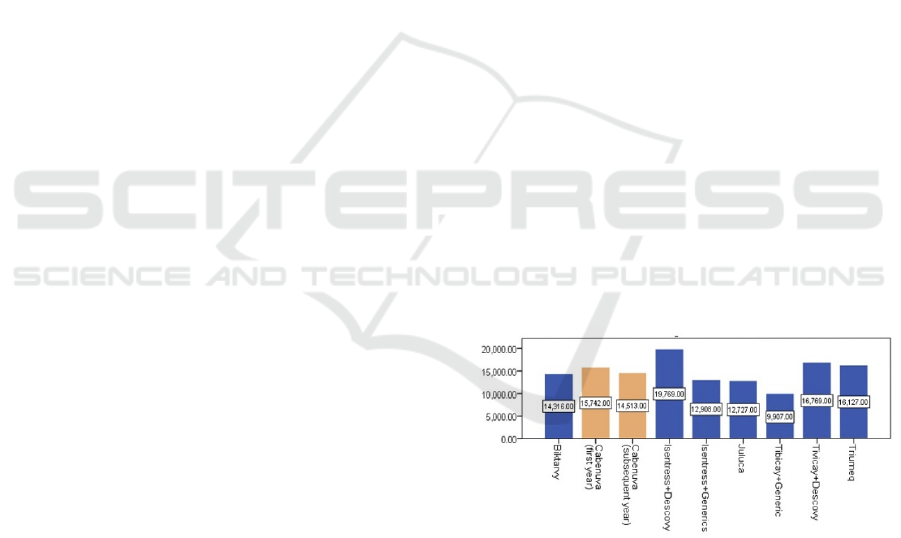
Pharmacoeconomic research of CABENUVA has
been made by ViiV and CADTH using the Markov
cohort state transition model to discover its cost and
QALY to evaluate the value of this drug as a
treatment to HIV, especially comparing with oral
ART administration. The result was shown below in
Figure 5.
Figure 5: Annual Cost comparison between HIV treatments.
In comparison to combined oral ART, CAB+RPV
has lower overall costs and less total QALYs, as
indicated in the figure, indicating that it is not cost-
effective. The overall estimated cost of CAB + RPV
during the patient's lifetime is $647,491, whereas the
total estimated cost of oral contraceptives ART is
$646,865. The two comparisons generated similar life
years (CAB + RPV = 24.33; oral contraceptives ART
= 24.21) and QALY (18.05 and 17.96). The major
cause of the discrepancies in predicted QALYs was
ICBB 2022 - International Conference on Biotechnology and Biomedicine
444

found by CADTH as adherence. The sponsor model
projected no drop in adherence among CAB + RPV
users, but increased adherence (measured as
treatment disruptions) in the oral ART method
(decline in adherence = 8.12 percent).
(Pharmacoeconomic Review Report, 2020)
Over and above what is already being done, there
is a need and potential for community pharmacists to
be used in major global health sectors. Pharmacists
may use their prescription knowledge to fill gaps in
care that fit with significant global health activities
and programs, from workforce development to drug
administration. It took decades for pharmacists in
high-income nations to move from product-centered
to patient-centered services with public health
consequences. It will take time for pharmacists in
low- and middle-income nations to make the
transition. These encouraging results highlight the
strategic necessity of enlisting the help of community
pharmacists in the Cabenuva administration. This
would be especially advantageous in many
communities where HIV services for extremely
vulnerable groups are endangered by anti-gay
legislation. Cabenuva administration at community
pharmacies provides feasible, discreet, and cost-
effective choices for patients and donor agencies
supporting ART programs in such scenarios. Finally,
authorities should ensure that pharmacists in their
communities have the resources they need to properly
support the administration of Cabenuva and other
potential public-health medications. The
aforementioned measures would complement the
many efforts being undertaken to achieve UNAIDS'
goal of ending the AIDS pandemic by 2030 by
attaining 95 percent diagnosis and 95 percent
antiretroviral treatment (ART) among all people
living with HIV (PLHIV). 95% on antiretroviral
therapy (ART) among diagnosed, and 95% virally
suppressed (VS) among treated. (Rasaq Kayode O,
2021)
The initiator created a queuing Markov state
transition model and a decision tree process hybrid
model. To represent the possibility of treatment
failure and/or interruption, the sponsor modelled
three ART lines and one remedial therapy line. The
cohort in this model migrated to the sponsor's health
condition, which was determined by the treatment
line, viral load, and CD4+ T cell count. Patients may
develop ADE, treatment-related AEs, or
cardiovascular illness in these conditions. The
transition between health condition in each monthly
cycle is determined by the cohort's viral status and
CD4+ T cell count, and the patient can reach the
absorptive state of death at any time throughout the
model cycle.
Consequently, due to the customized character of
HIV-1 therapy, particularly in terms of the timing and
reason for switching treatments, sponsors may have
overstated the cost reductions associated with CAB +
RPV. Cost reductions may or may not be obtained
depending on the setting in which CAB + RPV is
treated and if these expenses are shared by public
healthcare payers. Patients were enthusiastic about
the notion of a once-monthly injection, which is
supposed to minimize stigma by giving HIV-1
patients greater privacy and discretion. Furthermore,
patients thought that lowering pill load would
increase adherence and, as a result, viral suppression.
The experience of a patient on CAB + RPV, who
experienced less side effects and the capacity to be
more socially involved, was shared with one patient
group. (Aschenbrenner, 2021)
5 COMPARATIVE ANALYSIS
5.1 Route of Administration
Besides Cabenuva, there are other newly FDA-
approved HIV medications. Compared to other newly
approved HIV drugs, Cabenuva has its advantages as
well as its disadvantages, and the comparison will
depend on several aspects including MOA, route of
administration, indication. Although there is a large
amount of new HIV medications since the beginning
of this century. This article will mainly focus on the
comparison between Cabenuva and other new HIV
drugs since 2018.
Table 2. Route of administration and regimen of new medications
Name
Route of
Administration
Regimen Approved Time
Cabenuva Injection once a month January 22, 2021
Trogarzo Injection every 2 weeks March 6, 2018
Pifeltro Oral once daily August 30, 2018
The Whole is Greater than the Sum of the Parts, Combination Use as CABENUVA in Treating HIV: Meta-Analysis from Clinical Datasets
445
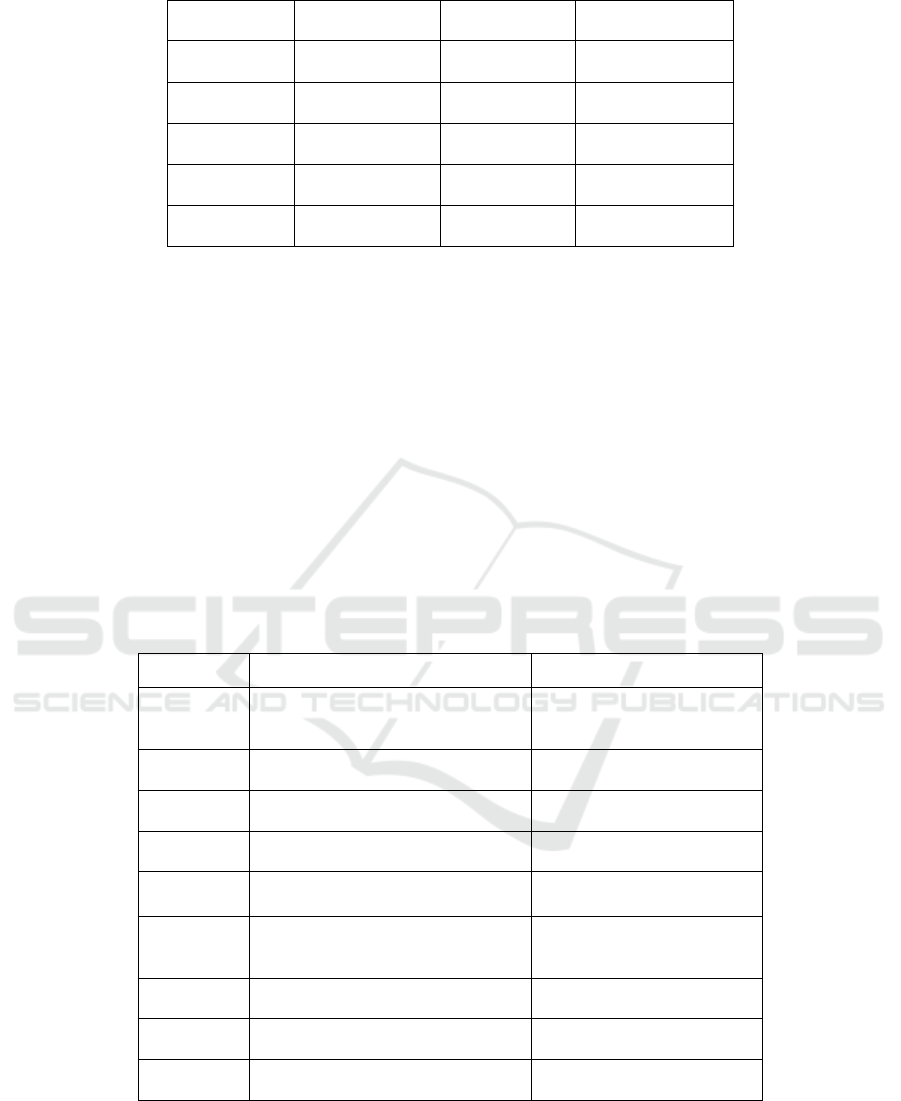
Dovato Oral once daily April 8, 2019
Biktarvy Oral once daily February 7, 2018
Symtuza Oral once daily July 17, 2018
Delstrigo Oral once daily August 30, 2018
Symfi Oral once daily March 22, 2018
Cimduo Oral once daily February 28, 2018
Table 2 shows the route of administration and
frequency of newly approved HIV drugs since 2018.
It can be concluded that the main route of
administration is oral, and the patients should take
medicine daily. Only Cabenuva and Trogarzo are
administered via injection and the regimen is once a
month and every 2 weeks, which reduces the impact
on patients’ daily life arising from taking medicines
daily. Compared to the regimen of Trogazo, injection
every 2 weeks, the regimen of Cabenuva is once a
month through injection after the patients take the
tablet for 28 days, which means only 12 days of
treatment is required for an entire year. (Villaluz,
2021)
5.2 Indication and MOA
Table 3 shows the indication and MOA of these new
medications. From Table 3, it can be concluded that
Trogarzo, Pifeltro and Cimduo should be used in
combination with other ARV. Dovato, Delstrigo and
Symfi can only be applied in the treatment of HIV-1
in adults. Only Cabenuva and Biktarvy are indicated
to be a complete regimen for all the patients.
Considering the indication, long-acting effect,
Cabenuva has its advantages to a large extent.
Cabenuva can be applied to a wide range of patients
and also can improve the lives of these patients.
(Villaluz, 2021)
Table 3. Indication and MOA of New Medications
Name Indication MOA
Cabenuva
Long-acting regimen with the all
HIV-1 patients
INSTI/NNRTI
Trogarzo In combination with other ARV Post-attachment Inhibitor
Pifeltro In combination with other ARV NNRTI
Dovato Treatment of HIV-1 in adults INSTI/NRTI
Biktarvy
Treatment of HIV-1 as a complete
regimen
INSTI/NRTI
Symtuza
Treatment of HIV-1 in adults and
pediatric patients
NNRTI/BOOSTER/NRTI
Delstrigo Treatment of HIV-1 in adults NNRTI/NRTI
Symfi Treatment of HIV-1 in adults NNRTI/NRTI
Cimduo In combination with other ARV NRTI
6 CONCLUSION
In conclusion, HIV is a dangerous disease that affects
patients, and the daily life of them can be improved
by accepting a wild range of treatments. Therefore,
the development of more effective and convenient
medication is important. Almost all the new HIV
drugs approved since 2018 are administered orally
ICBB 2022 - International Conference on Biotechnology and Biomedicine
446

daily. However, there have been some drugs whose
route of administration is injection such as Trogazo
and Cabenuva. By reducing the frequency of
medicine dosage, the life quality of HIV patients can
be improved. Cabenuva, a newly approved HIV drug,
is the first complete, long-acting HIV treatment
regimen, administered by intramuscular injection
once a month. This drug can make changes to the life
of patients to a great extent compared to other HIV
medications. Moreover, the development of
Cabenuva indicates that it is possible to develop more
long-acting HIV drugs, which set a new development
trend not only focused on treatment efficiency but the
life quality of patients.
REFERENCES
Aschenbrenner DS. First Extended-Release Injectable Drug
Therapy for HIV. Am J Nurs. 2021 May 1;121(5):24-
25. doi: 10.1097/01.NAJ.0000751096.82989.00.
PMID: 33872258.
Clinical Review Report: Cabotegravir Tablets,
Cabotegravir Extended-Release Injectable Suspension,
and Rilpivirine Extended-Release Injectable
Suspension (Vocabria, Cabenuva)
De Cock, K.M., H.W. Jaffe, and J.W. Curran, Reflections
on 40 Years of AIDS. Emerg Infect Dis, 2021. 27(6): p.
1553-1560.
Koff, W.C., A shot at AIDS. Curr Opin Biotechnol, 2016.
42: p. 147-151.
Pharmacoeconomic Review Report: Cabotegravir Tablets,
Cabotegravir Extended-Release Injectable Suspension,
and Rilpivirine Extended-Release Injectable
Suspension (Vocabria, Cabenuva): (ViiV Healthcare
ULC): Indication: HIV-1 infection [Internet]. Ottawa
(ON): Canadian Agency for Drugs and Technologies in
Health; 2020 Sep. PMID: 33439593.
Rasaq Kayode O, Afeez Babatunde O. Cabenuva®:
Differentiated service delivery and the community
Pharmacists' roles in achieving UNAIDS 2030 target in
Nigeria. Saudi Pharm J. 2021 Aug;29(8):815-819. doi:
10.1016/j.jsps.2021.06.003. Epub 2021 Jun 15. PMID:
34408543; PMCID: PMC8360771.
Surve, D.H. and A.B. Jindal, Recent advances in long-
acting nanoformulations for delivery of antiretroviral
drugs. J Control Release, 2020. 324: p. 379-404.
Swindells S, Andrade-Villanueva JF, Richmond GJ, et al.
Long-Acting Cabotegravir and Rilpivirine for
Maintenance of HIV-1 Suppression. N Engl J Med.
2020;382(12):1112-1123.
doi:10.1056/NEJMoa1904398
Villaluz, I., & Grantner, G. (2021). Newly Approved HIV
Medications. Retrieved 18 September 2021, from
https://www.medscape.com/viewarticle/941921_5
The Whole is Greater than the Sum of the Parts, Combination Use as CABENUVA in Treating HIV: Meta-Analysis from Clinical Datasets
447
