
Immune Checkpoint Inhibitor Therapy: Application in
Non-Small Cell Lung Cancer
Yumeng Liu
Chengdu University of Technology, Chengdu, China
Keywords:
Immune Checkpoint Inhibitor, Non-Small Cell Lung Cancer, Therapy.
Abstract: Lung cancer is the most deadly disease in the world. The common treatment options include surgery,
chemotherapy and radiotherapy, and targeted therapy can also be used selectively for those with positive
driver genes. However, the 5-year survival rate of lung cancer patients is still low. Non-small cell lung cancer
(NSCLC) is the most common type of lung cancer, and there is an urgent need for new treatment methods in
clinic. Immune checkpoint inhibitors have changed the treatment landscape for advanced NSCLC, showing
advantages in first-line, second-line and even multi-line therapy for patients with NSCLC. In recent years,
immunotherapy has provided a possibility for patients with NSCLC as a new and effective tumor treatment
method. This review reviewed the mechanism of action, clinical application, immune escape mechanism and
adverse reactions of immune checkpoint inhibitor therapy in non-small cell lung cancer.
1 INTRODUCTION
Lung cancer has become a global problem
endangering human health, causing more than 1.6
million deaths every year. In recent years, targeted
therapy has been proposed for patients with genetic
dysfunction of NSCLC, but many patients do not have
oncogenic factors such as epidermal growth factor and
anaplastic lymphoma kinase, and drug resistance is
inevitable (Low, et al., 2019). It has become a trend to
study new therapeutic directions, and the development
of immunotherapy has become a harbinger of the era
of personalized medicine with the deepening of tumor
immunology.
The application of Immune checkpoint inhibitors
(ICIs) is a great leap forward in the immunotherapy of
non-small cell lung cancer, such as antibodies to
CTLA-4 or PD-1 or its death-ligand 1(PD-L1).
Immune checkpoint inhibitors have been developed to
target immune escape and immunosuppression of
tumors (Galluzzi, Zitvogel, Kroemer, 2016). Unlike
chemotherapy or targeted drugs that directly target
malignant cells, immune checkpoint inhibitors are
thought to stimulate immune-associated cell-mediated
immune recognition and clearance, and work by
modulating T-cell function and mechanisms related to
targeted immune resistance, such as
immunosuppressive factors in the tumor
microenvironment. The interaction between PD-1 and
the PD-L1/PD-L2 ligand inhibits T cell proliferation
and promotes the secretion of cytokines related to
immune response. Pd-l1 and PD-L2 ligands are
expressed by antigen-presenting cells (APC) and can
be expressed by tumor cells or other cells in the tumor
microenvironment to promote tumor cell proliferation
(Wang, et al., 2014). It is the monoclonal antibody
against PD-1 or PD-L1 that blocks their interaction
and rejuvenates T cells to eliminate cancer cells. Ctla-
4 is mainly expressed in dendritic cells and inhibits the
activation of CD28-dependent T cells, resulting in
decreased levels of IL-2, IL-4, TNF-α and IFN-γ, and
decreased proliferation of CD8+ and CD4+T cells. In
addition, the interaction of CTLA-4 with CD80 and
CD86 expressed by conventional T cells increases
their inhibition of Treg mediation (Xiao, et al., 2020).
In recent years, targeted therapy has proposed new
treatment options for patients with genetic dysfunction
of NSCLC, but many patients do not have carcinogens
such as epidermal growth factor and anaplastic
lymphoma kinase, and drug resistance is inevitable
(Low, et al., 2019). Approximately 60% of patients
develop primary drug resistance during anti-PD-1
/PD-L1 therapy, and effective patients will also
develop secondary drug resistance as NSCLC patients
receiving targeted therapy. At present about the
resistance mechanism of the immune checkpoint
inhibitors may have the following several aspects:
448
Liu, Y.
Immune Checkpoint Inhibitor Therapy: Application in Non-Small Cell Lung Cancer.
DOI: 10.5220/0012032400003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 448-456
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

first, the effect of T cell activation depends on the local
immune of the tumor microenvironment, first of all,
the cancer cells of the immune cells to identify cancer
cells around, so after lifting immunosuppression, these
identifiable immune cells can effectively play a role of
anti-tumor, i.e. thermal tumor. If the tumor is cold, due
to the low degree of immune resistance in the immune
microenvironment and the immunogenicity of the
tumor itself is lower than that of the thermal tumor,
there will be no immune cells to kill the tumor after
the removal of immunosuppression (Arbour, Riely,
2019). Second, the TMB value of tumors. Studies
have confirmed that low mutation load and poor
immunogenicity of tumors will affect the development
and maturity of effector T cells, affect the activation
of T cells, and lead to the occurrence of drug resistance
(Suresh, et al., 2018). Third, effect closely associated
with APCs and T cell activation antigen presenting
cells, dendritic cells, for example, by taking the tumor
antigens, and to the effect of T cells, a process that
strong induction and activation of the effects of tumor
antigen specific T cells, which mediates antitumor
effect, disrupting any link in the process is likely to
lead to the occurrence of immune resistance. Fourthly,
there are many immunosuppressive pathways in
human body. In addition to pD-1 /PD-L1 signaling
pathway and CTLA-4 signaling pathway, there are
also TIM3 and LAG3, etc. It is assumed that these
immunosuppressive pathways coexist in tumor
tissues. If only one immunosuppressive pathway is
blocked, the other immunosuppressive pathways will
still be normal transduction. Even with compensatory
enhancement, immunotherapy does not achieve ideal
efficacy (Champiat, et al., 2017).
At present, immunotherapy for NSCLC has
achieved good results in phase I /II clinical trials:
improved tumor response rate, small toxic and side
effects, and easy tolerance by patients, which will
develop a new field for NSCLC treatment. Immune
checkpoint inhibitor therapy achieves anti-tumor
effect by inhibiting immune checkpoint activity,
releasing immune brake in tumor microenvironment
and reactivating the immune response effect of T cells
to tumor, which also makes it a new weapon against
tumor. Clinically, some patients can achieve lasting
clinical results and remain free of any tumor-related
clinical symptoms for several years.
This review mainly includes the following parts:
mechanism of action, clinical application, immune
escape mechanism and main adverse reactions of
immune checkpoint inhibitor therapy in non-small cell
lung cancer.
2 MECHANISM OF IMMUNE
CHECKPOINT INHIBITORS IN
THE TREATMENT OF
NON-SMALL CELL LUNG
CANCER
Lung cancer can be divided into primary lung cancer
and secondary lung cancer, according to etiology,
while NSCLC is the most common type of primary
lung cancer. Among them, non-small cell lung cancer
(NSCLC) accounts for about 85% of primary lung
cancers (Brahmer, et al., 2018). The overall five-year
survival rate of lung cancer patients in the past 40
years is still less than 21% (Lu, Yang, Huang, et al.,
2019). Unfortunately, about 60% of patients are
diagnosed at an advanced stage and lose the
opportunity for surgical treatment. The only treatment
options for these patients are chemotherapy,
radiotherapy and targeted therapy. It is reported that
the 5-year survival rate of NSCLC is only 17%, and
the 5-year survival rate of advanced NSCLC is less
than 5% (Siegel, Miller, Jemal, 2017).
Table 1: Basic information on non-small cell lung cancer.
Type Adenocarcinoma Squamous cell carcinomas Large cell carcinoma
Incidence rate About 50% About 30% About 5%
Features
The most common type of
lung cancer, especially in
non-smokers. Targeted
drugs are suitable for most
p
atients in China.
Squamous cell carcinoma
usually grows slowly.
Surgery is an option for
early detection.
Relatively rare, but
malignant degree is
generally higher, easy to
metastasize.
Metastatic Moderate Moderate Strong
Main treatment
modalities
Surgery, chemotherapy,
radiation, targeted drugs
Surgery, chemotherapy,
radiation
Surgery, chemotherapy,
radiation
Immune Checkpoint Inhibitor Therapy: Application in Non-Small Cell Lung Cancer
449
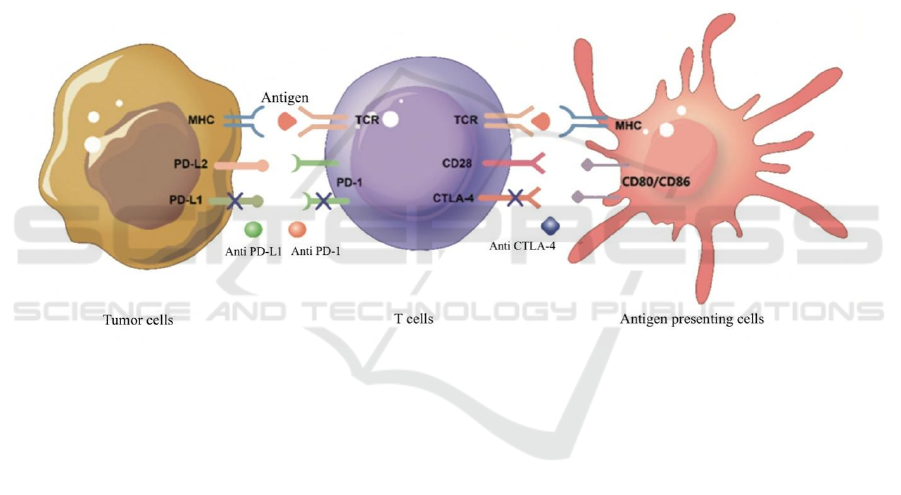
The body resists tumor formation through
acquired immunity. Tumor cells avoid detection and
attack by the body's immune system through allowing
tumor cells to escape the immune killing impact and
advance to growth and metastasis, according to
immunology. Antigen uptake, processing, and
presentation by antigen-presenting cells start the
immune response. APC binds to major
histocompatibility complex (MHC) molecules and
transfers antigen to the t-cell surface receptor.
Furthermore, T cells' CD28 receptor binds to APC's
CD80/CD86 ligand, causing both signals to activate
T cells (Reck, Heigener, Reinmuth, 2016). As
demonstrated in Figure 1, lung cancer cells'
immunological escape weakens the immune response
primarily through the immune checkpoint pathway,
which includes CTLA-4 and PD-1.
2.1 Anti-CTLA-4 Antibody
CTLA-4 is a CD28 homologous analogue. CTLA-4
is more compatible with CD80/CD86 than CD28,
hence CTLA-4 preemies CD80/CD86 by competitive
action. CTLA-4 can also suppress the production of
CD80 / CD86 on APC or remove it via
cytoendocytosis, preventing CD28 from attaching to
T cells and inhibiting T cell activation (Qureshi,
Zheng, Nakamura, et al., 2011). In the process of T
cell activation and immune response activation,
CTLA-4 acts as a negative regulator. Antibodies
against CTLA-4 can disrupt inhibitory signals,
causing T cells to activate and proliferate, thus
restoring their function.
Fig. 1 T cell activation mechanism and immune checkpoint inhibitor action mechanism.
2.2 Anti-PD-1 /PD-L1 Antibody
PD-1, a type I transmembrane glycoprotein, was first
identified in isolation of mouse T cells involved in
programmed cell death. PD-L1 and PD-L2 are the
two ligands for PD-1. In normal tissues, the
expression of PD-L1 regulates the expression of the
tissue's own immune response after a prolonged
inflammatory response to tissue injury. T cells, B
cells, macrophages, vascular endothelial cells, islet
cells, and other cells all express PD-L1. PD-L2 is
mostly expressed in macrophages and B cells, with a
low level of basic expression (Sharpe, Pauken, 2018).
Tyrosine residues in the cytoplasmic domain are
phosphorylated after PD-L1 interacts to ligands, and
protein-tyrosine phosphatase (PTP) is recruited.
Signal kinases in signaling pathways are
dephosphorylated as a result of this PTP. The signal
transduction of CD28 receptor positive activation is
inhibited (Akinleye, Rasool, 2019). PD-1 signaling
inhibits T cell activation and proliferation by
lowering activating transcription factors (TFs) such
as activating protein 1 (AP1), activating T cells
(NFAT), and NF-κβ (Wu, Gu, Chen, et al., 2019).
3 CLINICAL APPLICATION OF
IMMUNE CHECKPOINT
INHIBITORS IN NON-SMALL
CELL LUNG CANCER
3.1 Immune Checkpoint Inhibitor
Monotherapy
As the rise of immunotherapy and multiple ICI
approved for monotherapy, some studies reported that
CTLA-4 antibodies (Ipilimumab, Tremelimumab)
and human anti-PD-1 / PD-L1 antibodies
ICBB 2022 - International Conference on Biotechnology and Biomedicine
450

(pembrolizumab, Nivolumab, Atezolizumab) was
effective in the treatment of NSCLC in recent years.
Pembrolizumab monotherapy, based on
KEYNOTE024 and 042, is utilized as a first-line
treatment for PD-L1 positive NSCLC patients,
particularly those with high PD-L1 expression (Mok,
Wu, Kudaba, et al., 2019). After the CheckMate 017
and CheckMate 057 studies compared to docetaxel,
nivolumab was authorized for second-line therapy of
advanced relapsed or refractory NSCLC (Borghaei,
Paz-Ares, Horn, et al., 2015). The efficacy of
Atezolizumab in lung cancer was revealed in a Phase
II trial of POP-lar, and this benefit was particularly
pronounced in patients with high PD-L1 expression
(Fehrenbacher, Spira, Ballinger, et al., 2016). The
OAK Phase III experiment was later confirmed.
Regardless of PD-L1 expression status, atezolizumab
was approved for treatment in previously treated
patients with metastatic NSCLC (Brahmer,
Govindan, Anders, et al., 2018).
3.2 Immune Checkpoint Inhibitors
Combined with Chemotherapy
Pembrolizumab plus chemotherapy significantly
improved ORR compared to chemotherapy alone and
pembrolizumab, regardless of PD-L1 expression,
following KEYNOTE 021 first successful
combination of platinum-based chemotherapy with
ICI in patients with advanced NSCLC. The risk of
tumor progression and death was cut in half (Langer,
Gadgeel, Borghaei, et al., 2016), and immunotherapy
was switched to a combination treatment. The
objective response rate of Nivolumab combined with
conventional chemotherapy was as high as 47% in
CheckMate 012
(Rizvi, Hellmann, Brahmer, et al.,
2016), with a 2-year OS rate of 62% for
Nivolumab(5mg/kg) plus paclitaxel-carboplatin.
Pembrolizumab plus pemetrexed and platinum-based
patients with previously untreated metastatic non-
squamous NSCLC without EGFR or ALK mutations
had a median progression-free survival of 8.8 months
in Keynote-189 (Gandhi, Rodriguez-Abreu, Gadgeel,
et al., 2018).
Overall, 69.2% of patients survived for 12
months. IMpower132 found that Atezolizumab in
combination with chemotherapy improved PFS more
than chemotherapy alone, while imPOWER150
found that Atezolizumab in combination with
bevacizumab and standard chemotherapy improved
OS regardless of pD-L1 expression or genetic
changed status of EGFR or ALK (Socinski, Jotte,
Cappuzzo, et al., 2018). In patients with metastatic
non-squamous non-small cell lung cancer who do not
have EGFR or ALK genetic abnormalities, the FDA
has approved Atezolizumab in combination with
bevacizumab and conventional chemotherapy as a
first-line treatment.
3.3 Combination Therapy with
Immune Checkpoint Inhibitors
The CheckMate 012 experiment began attempting
dual ICI medication therapy based on the various
modes of action of the PD-1 and CTLA4 pathways.
In the first-line therapy of NSCLC patients,
Nivolumab plus Ipilimumab has a tolerable safety and
therapeutic effect (Hellmann, Rizvi, Goldman, et al.,
2017). The CheckMate 227 study also found that,
regardless of PD-L1 status, the median PFS in the
dual ICI group (Nivolumab plus Ipilimumab) was 7.2
months compared to 5.4 months in the high-TMB
chemotherapy group. The ORR was also greater, at
45.3% vs. 26.9%. 252 patients with recurrent stage
IIIB/IV NSCLC treated with Nivolumab in
combination with Ipilimumab were divided into three
groups based on pD-L1 expression levels of less than
1%, 1%, and more than 1% in CheckMate 568
(Ready, Hellmann, Awad, et al., 2019). ORR rates
were 15%, 30%, and 41%, respectively, and ORR
increased as TMB increased.
However, in a Phase Ib
trial (NCT02000947) involving 102 patients with
advanced NSCLC who received Durvalumab, an anti-
PD-L1 antibody, and Tremelimumab, an anti-CTLA-
4 antibody, 29 patients (28%) were stopped due to
treatment-related adverse events, and 37 patients
(36%) had treatment-related serious adverse events
(Antonia, Goldberg, Balmanoukian, et al., 2016).
Group according to PD-L1 expression, 118 patients
were separated into two groups (>25% and <25%) in
the phase III clinical trial (NCT02453282). There was
no statistically significant difference in OS and PFS
whether Durvalumab (or Durvalumab +
Tremelimumab) or chemotherapy was given in
addition to the machine (Rizvi, Cho, Reinmuth, et al.,
2018). D + T was administered in combination with
chemotherapy in a Phase Ib trial (NCT02537418),
and 17 of 24 patients with advanced NSCLC achieved
remission, with an ORR of 52.9%. The efficacy of D
+ T combination with platinum therapy will be further
investigated in the ongoing Phase II clinical trial
(NCT03057106) and Phase III trial (NCT02542293).
The trial's primary goal is OS, with secondary
endpoints including PFS, ORR, quality of life, and
safety, with the particular effect yet to be determined.
Immune Checkpoint Inhibitor Therapy: Application in Non-Small Cell Lung Cancer
451

3.4 Immune Checkpoint Inhibitors
Combined with Indoleamine
2,3-dioxygenase 1(IDO-1)
Inhibitors
IDO-1 is a metalloproteinase that can catalyze the
metabolism of tryptophan into canisuric acid. IDO-1
oxidizes tryptophan to N-formylcanisuric acid and
then converts it into catabolites (Prendergast,
Mondal, Dey, et al., 2018). When tryptophan is
consumed, the activity of T-cell-activated kinase
PKC-θ decreases, and the tryptophan catabolites
promote the differentiation of CD4+T cells into Treg
cells by binding to the aromatics receptor, while
limiting the differentiation of CD4+T cells into Th17
cells, inhibiting the immune system (Zhu, Dancsok,
Nielsen, 2019). Multiple quantitative
immunofluorescence was used to measure the levels
of PD-L1, ID-1, B7-H4 and different tumor-
infiltrating lymphocyte (TIL) subtypes in 552 patients
with stage I to IV lung cancer, and the increase of PD-
L1 and ID-1 was consistent with significant B and T
cell infiltration, with limited co-expression (Schalper,
Carvajal-Hausdorf, Mclaughlin, et al., 2017). The
echo-202 / Keynote-037 trial used the IDO-1 inhibitor
EpACa-dostat in combination with Pembrolizumab
in NSCLC, resulting in an ORR of 35% and a DCR
of 60% in 40 assessable patients. The most common
adverse reactions were fatigue (19%), arthralgia
(9%), and elevated AST (9%). E+P was generally
well tolerated and effective in the treatment of
advanced NSCLC (Mitchell, Hamid, Smith, et al.,
2018).
3.5 Immune Checkpoint Inhibitors
Combined with Radiotherapy
Massive radiotherapy can destroy intracellular DNA,
effectively delay tumor metastasis, and reduce local
compression symptoms in patients with advanced
NSCLC; on the other hand, tumor cells killed by
radiotherapy will release a significant number of new
antigens to stimulate the body's immune response. As
a result, immunocheckpoint inhibitors combined with
radiation have emerged as a new effective therapeutic
strategy for advanced NSCLC. Pembrolizumab (0.2
g/kg every 3 weeks) or radiation (8 Gy 3 times)
followed by Pembrolizumab were given to 76 patients
with relapsed metastatic non-small cell lung cancer
(treatment group). The experimental group's ORR
was 36% vs. 18% at 12 weeks, their PFS was 6.6 vs.
about 2 months, and their median overall survival was
15.9 vs. 7.6 months. For patients with PD-L1 negative
tumors, a subgroup study revealed that increasing the
radiation dose was more effective.
The findings
revealed that a small amount of radiation before to
Pembrolizumab treatment improved the therapeutic
outcome (Theelen, Peulen, Lalezari, et al., 2019).
Patients with advanced NSCLC who received
intravenous Pembrolizumab following local ablation
had a median PFS of about 20 months and an overall
mean survival rate of 90.9% at 1 year in the
NCT02316002 study. Pembrolizumab after local
ablation increases PFS without impairing mass of life
in patients with advanced lung cancer, according to a
study (Bauml, Mick, Ciunci, et al., 2019). However,
several issues remain with this treatment, including
the best dose and timing for ICI-related radiation, the
impact of PD-L1 status, and how to incorporate
immune checkpoint inhibitors into these
combinations. More experimental research is needed
to find the best therapy combination.
3.6 Immune Checkpoint Inhibitors
Combined with Targeted Therapy
First-line targeted therapy with tyrosine kinase
inhibitors (TKI) is preferred for sensitive NSCLC
patients with driver gene mutations such as EGFR,
BRAF, ALK, or ROS1. Due to the drug resistance of
targeted therapies, a combination of ICI and TKI was
tested. Although there were some favorable signs in
some subgroups, overall clinical efficacy was poor,
and adverse events were common (Berghoff,
Bellosillo, Caux, et al., 2019). When TKI therapy is
ineffective or the response to TKI drugs is intolerable,
ICI is indicated.
4 IMMUNE ESCAPE AND
IMMUNOSUPPRESSION IN
NSCLC
Immune escape is a major feature of cancer, in which
tumors lose expression of their antigens, negatively
collectively regulate major histocompatibility
complex (MHC) molecules, reduce antigen
presentation ability, do not express effector T
lymphocyte costimulatory molecules or overexpress
molecules that inhibit the activity of these
lymphocytes. In addition, immunosuppressive factors
can be produced by promoting T regulation of
gonorrhoetic cell differentiation (Tregs) and
increasing the number of myeloid suppressor cells
(MDSCs) (Spagnuolo, Gridelli, 2019), expressing
immune checkpoints to inhibit immune cell function,
leading to immune cell failure and apoptosis, and thus
ICBB 2022 - International Conference on Biotechnology and Biomedicine
452

avoiding the host's immune surveillance (Theelen,
Jong, Baas, 2020). The checkpoint signals PD-1 and
CTLA-4 block T cell activation and allow
malignancies to evade the adaptive immune response
(Somasundaram, Burns. 2017).
PD-1 is a negative
costimulatory receptor, mainly expressed on the
surface of activated T cells and belonging to the
CD28 family. After binding with PD-L1 and PD-L2,
the main ligands on T cells or antigen-presenting cells
(APC), PD-1 reduces the activity of T lymphocytes to
avoid being eliminated by the immune system
(Economopoulou, Mountzios, 2018). The CTLA-4
expression on regulatory T cells causes a decrease in
T cell activity and an increase in the system's
immunosuppressive activity.
5 ADVERSE REACTION
ANALYSIS
5.1 Completion of Immunotherapy
During the period of 70 patients receiving
immunotherapy for non-small cell lung cancer, 2
patients were discontinued due to adverse reactions.
One patient died, and the remaining 67 patients
continued treatment. Among the 70 patients, 67
patients were followed up for 6 months, with a
follow-up rate of 95%. Among them, 18 cases
received 2 cycles of treatment, 12 cases received 3
cycles of treatment, 8 cases received 4 cycles of
treatment, 14 cases received 5 cycles of treatment, 6
cases received 6 cycles of treatment, 4 cases received
7 cycles of treatment, and 8 cases received 8 cycles of
treatment (Fu, Wang, 2020).
5.2 Distribution of Species and
Classification
5.2.1 Type Analysis of Adverse Reactions
The incidence rate of anorexia and tiredness
symptoms was 100% (70 instances), which differed
from the findings of Gettinger et al (Gettinger, Horn,
Gandhi, et al., 2004). And could be due to the fact that
there were 70 patients receiving combination
treatment. With a 1% incidence of bad cardiac
reactions, one patient died, demonstrating that while
the incidence of adverse cardiac reactions is modest,
the fatality rate is significant. The other 19 cases had
a 1%-5% incidence of adverse reactions, which were
common adverse reactions to immunotherapy and
had a low incidence.
5.2.2 Distribution of Adverse Reactions
Three patients (4%) had grade 4 adverse events,
including one with liver function injury and another
with fever. All of the patients were given drug
withdrawal, and one of them died as a result of
cardiac complications. There were 17 cases of grade
1-2 adverse reactions (24%), demonstrating that
immunotherapy adverse reactions are uncommon.
Mild adverse reactions were the most common, while
severe adverse reactions were uncommon, although
severe adverse reactions had major repercussions for
patients (Fu, Wang, 2020).
The safety and observable qualities of epidematic-
free treatment have been proven in terms of the types
and amount of adverse reactions, however life-
threatening and life-threatening adverse reactions do
occasionally occur, necessitating medical
intervention (Table 1) (Fu, Wang, 2020).
Table.2: Analysis of adverse reactions to immune checkpoint inhibitor therapy in 70 patients with non-small cell lung cancer
(cases, %) (Fu, Wang, 2020).
Project Level 1 Level 2 Level 3 Level 4 Total
Incidence
rate (%)
Hypothyroidism 2 - - - 2 3
Rash 4 - - - 4 7
Fever 5 - - 1 4 7
Hepatic 5 - - 1 4 7
Interstitial pneumonia 2 2 - - 4 7
Neurotoxicity 1 - - - 1 1
Cardiac toxicity - - - 1 1 1
Loss of appetite and fatigue 70 - - - 70 100
Immune Checkpoint Inhibitor Therapy: Application in Non-Small Cell Lung Cancer
453
5.2.3 Analysis of Adverse Reaction Time
during Treatment
In the first two cycles of combination chemotherapy,
seventy patients with NSCLC experienced anorexia
and fatigue. There was a total of 20 additional adverse
events, with 18 patients (90%) having immune-
related adverse reactions in the first four cycles. All
immune-related side effects occurred within the first
six cycles of treatment, demonstrating that immune
checkpoint inhibitor-related side effects began early
in the drug administration (Fu, Wang, 2020). There
was no link between the duration of medicine and the
outcome (Table 2).
Table.3: Time of adverse reactions to immune checkpoint inhibitor therapy in 70 patients with non-small cell lung cancer
(cases, %) (Fu, Wang, 2020).
Project
The first
cycle
The second
cycle
The thrid
cycle
The fourth
cycle
The fifth
cycle
The sixth
cycle
Hypothyroidism 1 - - 1 - -
Rash - 2 - 2 -
Fever 2 - 1 1 - -
Hepatic injury - 1 2 1 - -
Interstitial
p
neumonia
- 2 - 1 1 -
Neurotoxicity 1 - - - - -
Cardiac toxicity - - - - 1 -
Loss of appetite
an
d
fati
g
ue
40 30 - - - -
Total 44 35 3 6 2 -
Incidence rate (%) 62 50 4 8 3 -
6 CONCLUSION
Immune checkpoint inhibitors have changed the
treatment landscape of advanced NSCLC, showing
advantages in first-line, second-line and even multi-
line therapy for patients with NSCLC.Despite the fact
that immunotherapy has demonstrated remarkable
efficacy in patients with non-small cell lung cancer,
there are still a number of pressing issues to be
addressed, including the lack of biomarkers that can
predict immunotherapy on their own, the difficulty of
preventing immune-related side effects, and the
emergence of drug resistance. This paper summarized
the mechanism of ICI in NSCLC, clinical application,
immune escape mechanism and the occurrence of
adverse reactions, with the purpose of summarizing
the latest progress of this immunotherapy and making
prospects. Future immunotherapy research for non-
small cell lung cancer should concentrate on
increasing sensitivity to tumor-specific antigens,
developing additional effective targets and more
reliable biomarkers, and balancing efficacy and
toxicity between monotherapy and combination
therapy.
REFERENCES
Akinleye A, Rasool Z. Immune checkpoint inhibitors of
PD-L1 as cancer therapeutics [J]. J Hematol Oncol,
2019, 12 (1): 92.
Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and
antitumour activity of durvalumab plus tremelimumab
in non-small cell lung cancer: A multicentre, phase 1b
study [J]. Lancet Oncol, 2016, 17(3) : 299-308.
Arbour, K.C. and G.J. Riely, Systemic Therapy for Locally
Advanced and Metastatic Non-Small Cell Lung
Cancer: A Review [J]. JAMA,2019,322(8):764-774.
Bauml JM, Mick R, Ciunci C, et al. Pembrolizumab after
comple- tion of locally ablative therapy for
oligometastatic non-small cell lung cancer: A phase 2
trial[J]. JAMA Oncology, 2019, 5(9):1283- 1290.
Berghoff AS, Bellosillo B, Caux C, et al. Immune
checkpoint inhibi- tor treatment in patients with
oncogene-addicted non-small cell lung cancer
(NSCLC): Summary of a multidisciplinary round-table
dis- cussion[J]. ESMO Open, 2019, 4(3): e000498.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus
Docetaxel in advanced nonsquamous non-small-cell
lung cancer [J]. N Engl J Med, 2015, 373 (17): 1627-
1639.
Brahmer JR, Govindan R, Anders RA, et al. The society for
immunotherapy of cancer consensus statement on
immunotherapy for the treatment of non-small cell lung
ICBB 2022 - International Conference on Biotechnology and Biomedicine
454

cancer (NSCLC) [J]. J Immunother Cancer, 2018, 6(1):
75.
Brahmer, J.R., et al. The Society for Immunotherapy of
Cancer consensus statement on immunotherapy for the
treatment of non-small cell lung cancer (NSCLC)[J]. J
Immunother Cancer,2018,6(1):75.
Champiat, S., et al. Hyperprogressive Disease Is a New
Pattern of Progression in Cancer Patients Treated by
Anti-PD-1/PD-L1[J]. Clin Cancer Res, 2017,
23(8):1920-1928.
Economopoulou, P., G. Mountzios. The emerging
treatment landscape of advanced non-small cell lung
cancer[J]. Ann Transl Med,2018,6(8):138.
Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab
versus docetaxel for patients with previously treated
non-small-cell lung cancer ( POPLAR) : A multicentre,
open-label, phase 2 randomised controlled trial[J].
Lancet, 2016, 387(10030): 1837-1846.
Fu, E., Wang, Y. and Wang, J., 2020. Analysis and nursing
countermeasures of adverse reactions of immune
checkpoint inhibitors in 70 patients with non-small cell
lung cancer. Journal of Nursing (China),.
Galluzzi, L., Zitvogel L., Kroemer G., Immunological
Mechanisms Underneath the Efficacy of Cancer
Therapy [J]. Cancer Immunology Research, 2016,
4(11):895-902.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al.
Pembrolizumab plus chemotherapy in metastatic non-
small-cell lung cancer[J]. N Engl J Med, 2018, 378
(22): 2078-2092.
Gettinger SN, Horn L,Gandhi L,et al. Overall Survival and
Long-term Safety of Nivolumab (Anti-programmed
Death 1 Antibody,BMS-936558,ONO-4538) in Patients
with Previously Treated Advanced Non-small-cell
Lung Cancer [J]. J Clin Oncol,2015,33(18):2004-12.
HellmannMD, RizviNA, GoldmanJW, etal.
Nivolumabplusipilimumab as first-line treatment for
advanced non-small-cell lung cancer (CheckMate 012):
Results of an open-label, phase 1, multicohort study [J].
Lancet Oncol, 2017, 18(1): 31-41.
Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and
pemetrexed with or without pembrolizumab for
advanced, non-squamous non-small-cell lung cancer: A
randomised, phase 2 cohort of the open-label
KEYNOTE-021 study[J]. Lancet Oncol, 2016, 17 (11):
1497-1508.
Low, J.L., et al. The evolving immuno-oncology landscape
in advanced lung cancer: first-line treatment of non-
small cell lung cancer [J]. 2019, SAGE Publications:
London, England,1758835919870360.
Lu T, Yang X, Huang Y, et al. Trends in the incidence,
treatment, and survival of patients with lung cancer in
the last four decades[J]. Cancer Manag Res, 2019, 11:
943-953.
Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus
chemotherapy for previously untreated, PD-L1-
expressing, locally advanced or metastatic non-small-
cell lung cancer (KEYNOTE-042): a randomised,
open-label, controlled, phase 3 trial [J]. Lancet, 2019,
393 (10183): 1819-1830.
Mitchell TC, Hamid O, Smith DC, et al. Epacadostat plus
Pembrolizumab in patients with advanced solid tumors:
Phase I results from a multicenter, open-label phase I/II
trial ( ECHO-202/KEYNOTE- 037) [J]. J Clin Oncol,
2018, 36(32): Jco2018789602.
Prendergast GC, Mondal A, Dey S, et al. Inflammatory
reprogramming with IDO1 inhibitors: Turning
immunologically unresponsive ‘Cold’tumors‘Hot’[J].
Trends Cancer, 2018, 4(1): 38-58.
Qureshi OS, Zheng Y, Nakamura K, et al. Trans-
endocytosis of CD80 and CD86: a molecular basis for
the cell-extrinsic function of CTLA-4[J]. Science,
2011, 332 (6029): 600-603.
Ready N, Hellmann MD, Awad MM, et al. First-Line
Nivolumab plus Ipilimumab in advanced non-small-
cell lung cancer (CheckMate 568): Outcomes by
programmed death ligand 1 and tumor mutational
burden as biomarkers [J]. J Clin Oncol, 2019, 37 (12) :
992- 1000.
Reck M, Heigener D, Reinmuth N. Immunotherapy for
small-cell lung cancer: emerging evidence[J]. Future
Oncol, 2016, 12 (7): 931-943.
Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in
combination with platinum-based doublet
chemotherapy for first-line treatment of advanced non-
small-cell lung cancer [J]. J Clin Oncol, 2016, 34(25):
2969-2979.
Rizvi NA, Cho BC, Reinmuth N, et al. LBA6Durvalumab
with or without tremelimumab vs platinum-based
chemotherapy as first-line treatment for metastatic non-
small cell lung cancer: MYSTIC [J]. Annals of
Oncology, 2018, 29(suppl_10).
Suresh, K., et al. Immune Checkpoint Immunotherapy for
Non- Small Cell Lung Cancer: Benefits and Pulmonary
Toxicities[J]. Chest,2018,154(6):1416-1423.
Siegel RL, Miller KD, Jemal A, Cancer statistics, 2017[J].
CA Cancer J Clin, 2017, 67(1): 7-30.
Sharpe AH, Pauken KE. The diverse functions of the PD1
inhibitory pathway[J]. Nat Rev Immunol, 2018, 18 (3):
153-167.
Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab
for firstline treatment of metastatic nonsquamous
NSCLC[J]. N Engl J Med, 2018, 378 (24): 2288-2301.
Schalper KA, Carvajal-Hausdorf D, Mclaughlin J, et al.
Differential expression and significance of PD-L1,
IDO-1, and B7-H4 in human lung cancer[J]. Clin
Cancer Res, 2017, 23( 2) : 370-378.
Spagnuolo, A., C. Gridelli. Combining immunotherapies to
treat non-small cell lung cancer[J]. Expert Rev Respir
Med,2019, 13(7):621-634.
Somasundaram, A., T.F. Burns. The next generation of
immunotherapy: keeping lung cancer in check[J].
Journal of hematology & oncology,2017,10(1):87-12.
Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of
Pembrol- izumab after stereotactic body radiotherapy
vs Pembrolizumab alone on tumor response in patients
with advanced non-small cell lung cancer: Results of
the PEMBRO-RT phase 2 randomized clinical trial [J].
JAMA Oncology, 2019, 5(9):1276-1282.
Immune Checkpoint Inhibitor Therapy: Application in Non-Small Cell Lung Cancer
455

Theelen, W.S., M.C. de Jong, P. Baas. Synergizing
systemic responses by combining immunotherapy with
radiotherapy in metastatic non-small cell lung
cancer[J]: The potential of the abscopal effect. Lung
Cancer,2020,142: 106-113.
Wang, C., et al. In Vitro Characterization of the Anti- PD-
1 Antibody Nivolumab, BMS-936558, and In Vivo
Toxicology in Non-Human Primates [J]. Cancer
Immunology Research,2014,2(9):846-856.
Wu X, Gu Z, Chen Y, et al. Application of PD-1 blockade
in cancer immunotherapy [J]. Comput Struct
Biotechnol J, 2019, 17: 661- 674.
Xiao, Q., et al. Genetic and Epigenetic Biomarkers of
Immune Checkpoint Blockade Response [J]. J Clin
Med, 2020,9(1).
Zhu MMT, Dancsok AR, Nielsen TO. Indoleamine
dioxygenase inhibitors: Clinical rationale and current
development [J]. Curr OncolRep, 2019, 21(1):2.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
456
