
Use of Oncolytic Vaccinia Virus Armed with Certain Cytokines
(IL-7, IL-12, IL-15, IL-21) in Combination with Checkpoint
Inhibitors to Treat Non-Small Cell Lung Cancer
Yuqian Xu
1,*
, Yuantong Li
2
, Ziyang Yin
3
, Zihao Jin
4
, Wendao Li
5
and Zhaoyang Che
5
1
School of Pharmaceutical Sciences, Tsinghua University, Beijing, 100084, China
2
Department of Biology, Brandeis University, Waltham, MA, U.S.A.
3
Concordia International School Shanghai, Shanghai, 201206, China
4
Western International School Shanghai, Shanghai, 201206, China
5
Shenzhen Foreign Languages School, Shenzhen, 518083, China
Keywords:
Oncolytic Virus, Checkpoint Inhibitors (Cpis), Cytokines, Non-Small Cell Lung Cancer.
Abstract:
A variety of oncolytic viruses (OVs) have been reported in treating different cancers and several have entered
clinical trials. This study proposes the combination of cytokines IL-7, IL-12, IL-15, IL-21, and checkpoint
inhibitors (CPIs) co-expressed on an oncolytic vaccinia virus JX-594 to treat non-small cell lung cancer
(NSCLC) with the aim of tumor regression. The effectiveness of the modified JX-594 vaccinia virus is
measured both in H460 cell lines and in LSL-KrasG12D mouse model, including the production of cytokines
and CPI, cytotoxicity, tumor growth, overall survival and tumor-infiltrating lymphocytes (TILs). The
increased overall survival rate and tumor size regression in mouse model are predicted.
1 INTRODUCTION
1.1 General Overview to Oncolytic
Viruses
In 1991, a genetically engineered herpes simplex
virus-1 (HSV-I) was found to be a potential therapy
in malignant glioma (Martuza, 1991). Since then,
increasing attention was paid to the role of virus in
treating cancer. Nowadays, oncolytic virus (OV) is a
promising cancer immunotherapy. OV could kill
cancers in two main ways: direct cell lysis as well as
increased anti-tumor immune response (Abd-Aziz N,
2021). OVs are genetically engineered to selectively
infect cancer cells via some receptor-mediated
pathways, therefore minimizing potential tissue
damage (Ferguson, 2012). OVs could also stimulate
anti-tumor immune responses, such as presentation of
TSAs (tumor specific antigens) and TAAs (tumor-
associated antigens) to APCs to activate effector T
cells (Ferguson, 2012). As the knowledge of the
therapy advanced over years, OVs are genetically
modified in diverse ways to enhance antitumor effect.
*
Corresponding author.
These armed OVs could deliver cytokines, CPIs,
tumor suppressors, etc (Marintcheva, 2018). Besides,
a wider variety of OVs were tested clinically,
including adenovirus, reovirus, measles, HSV-I,
vaccinia virus, and Newcastle disease virus
(Fukuhara, 2016).
Despite the extraordinary advantages, current
oncolytic virus treatments face several challenges,
including physical barriers, antiviral immunity, and
immunosuppressive tumor microenvironment
(TME). Multiple strategies that enhance OV delivery,
infiltration, and oncolysis have been developed to
increase the efficacy of OV therapy. Besides,
recombinant OVs might cause some unexpected
toxicities so safety analysis must be considered
carefully. Currently, emerging genetic engineering
techniques, combined with other therapies, like
adoptive T cell therapy (ACT) and CPI are applied in
OVs. These will make OV therapy one of the most
promising immunotherapies in the future (Zheng,
2019).
Xu, Y., Li, Y., Yin, Z., Jin, Z., Li, W. and Che, Z.
Use of Oncolytic Vaccinia Virus Armed with Certain Cytokines (IL-7, IL-12, IL-15, IL-21) in Combination with Checkpoint Inhibitors to Treat Non-Small Cell Lung Cancer.
DOI: 10.5220/0012032500003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 457-466
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
457

1.2 General Functions of Cytokines and
CPIs in Regular and Antitumoral
Immune Responses
Cytokines are polypeptides or glycoproteins secreted
by diverse immune cells including T cells,
neutrophils, and macrophages and could regulate
immune responses (Cohen, 1996). Some cytokines
have been discovered to have potent anti-tumor
properties, which makes cytokine a monotherapy or
potentiator of other therapies in cancer treatment
(Berraondo, 2019). IL-7, IL-12, IL-15, and IL-21 are
chosen in this article. IL-7 is needed in B-and T-cells
development and could diminish cancer cell growth
(Alderson, 1991). IL-7 treatment was also reported to
enhance long-term CD8+ T-cell responses in mouse
model (Colombetti, 2009). IL-12 can activate effector
Th1 response, thus serving as a link between innate
and acquired immunity. This also further induces
activation of T-cell, NK-cells, and tumor clearance.
(Mirlekar, 2021; Zundler, 2015). IL-15 can promote
differentiation and expansion of T-cells, B-cells, and
NK cells, which leads to enhanced tumor response.
Moreover, IL-15 is important in the ontogeny of NK
and CD8+ cells (Isvoranu, 2021). IL-21 is involved in
co-stimulation of B-cell differentiation and
immunoglobulin production, stimulation of NK and
CD8+ cytotoxic function and co-mitogen of T-cells
(Sondergaard, 2009).
Checkpoint inhibitors (CPIs), on the other hand,
are important in enhancing T cell activation to combat
tumors (Zheng, 2019). T cell exhaustion,
characterized by loss of effector function and other
properties, arises during chronic exposure to antigens,
which limits tumor control (Wherry, 2011). Several
inhibitory pathways including PD-1 and PD-L1 play
important roles in this process. This led to the
development of CPIs to recover dysfunctional T cells,
including PD-L1 inhibitors, PD-1 inhibitors and
CTLA-4 inhibitors (Vaddepally, 2020). Nevertheless,
CPIs are ineffective for 'cold' tumors with low
infiltration of T cells. OV’s infecting and lysing the
tumor cells could improve intra-tumoral infiltration
and solve the limitations of CPI; thus, co-treatment of
OVs and CPI is a natural trend.
1.3 The Non-Small Cell Lung Cancer
Non-small cell lung cancer (NSCLC) is a
heterogeneous disease accounting for about 84% of
all lung cancer diagnoses in the United States
(Molina, 2008). Current treatment of NSCLC
includes surgeries, chemotherapy, radiation therapy,
and therapies targeting cell cycle control and
apoptosis (Molina, 2008). The immune checkpoint
inhibitors have been used recently to treat
unresectable stage III NSCLC, using anti PD-1/PD-
L1 antibodies (Onoi, 2020). Studies have also
reported the improved treatment of NSCLC with
several cytokines, such as IL-7 and IL-12. The
cytokine induced killer cells and chemotherapy can
effectively increase the overall survival of patients
with advanced stages of NSCLC. IL-7 can aid in the
sensitivity of NSCLC towards chemotherapy drug
cisplatin. IL-12 is also shown to directly target human
lung adenocarcinoma cells as well as adjacent normal
bronchial epithelial cells (NBEC) (Airoldi, 2009).
However, the primary and acquired resistance to PD-
1/PDL1 blockade mechanisms in NSCLC have been
reported, which might arise from components in the
immunosuppressive tumor microenvironments that
leads to inefficient activation and infiltration of T
cells (Pathak, 2020).
2 NEW TREATMENT
A new therapy called JX-594alpha is designed, which
consists of JX-594 (an oncolytic vaccinia virus) that
expresses IL-15, IL-12, IL-7, IL-21 and PD-L1
inhibitor (iPDL1). Oncolytic vaccinia virus (VV), JX-
594 is chosen as the delivery platform for several
reasons. VV, compared to other types of oncolytic
viruses, has a large genome size that allows it to
accommodate multiple foreign genes (Breitbach,
2013). This makes it possible to carry a combination
of the genes encoding IL-7, IL-12, IL-15, and IL-21.
VV also exhibits features such as rapid replication, a
wide tropism, and easy recombination for making
viral mutants (Hawkins, 2002). Besides, JX-594 with
granulocyte-macrophage colony-stimulating-factor
(GM-CSF) gene and deletion in thymidine kinase
(TK) gene could enhance immune responses and
selectively replicate in cancer cells with mutated RAS
or p53 genes (Merrick, 2009).
The cytokines (IL-7, IL-12, IL-15, IL-21) could
boost T-, B-, and NK cell performance. while CPIs
allow tumor recognition by T cells. Therefore, we
believe that these two subjects working in tandem
could substantially enhance antitumor effect. We
hypothesize that the JX-594alpha is able to initiate
antitumor immune responses that would eventually
lead to tumor regression in NSCLC mouse model.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
458

3 EXPERIMENT
3.1 Generation and Characterization
3.1.1 Generation of 4 Engineered OV
The generation of the JX-594alpha is achieved by
inserting genes encoding different ILs(including IL-
7, IL-12, IL-15, and IL-21) and iPDL1 into vaccinia
virus. During the experiment, five groups will be
generated, including VV-iPDL1/IL, VV-IL, VV-
iPDL1, VV-empty, and the control group. The VV
shuttle vector pSel-DsRed2N1 will be used to deliver
iPDL1 into the VV (Wang, 2020). The VV shuttle
vector pCMS1-IRES will be used to deliver the ILs
into the VV (Ge, 2020).
3.1.2 iPDL1 Expression and Secretion in
Infected H460 Cells
24 h after infection, supernatants of H460 cells are
collected. iPDL1 concentration will be detected by
mice iPDL1 ELISA kit in control, VV-iPDL1/IL,
VV-IL, VV-iPDL1, and VV-empty groups.
3.1.3 Characterization of iPDL1
Supernatants of the tumor cells infected with VV-
iPDL1/IL are harvested and then purify the
supernatant to get iPDL1. Then the binding of
purified iPDL1 to PD-L1+/+ or PD-L1 -/- cells is
determined. The infected cells are incubated with
purified iPDL1 or IgG before they are stained with
antibodies against PD-L1 or IgG Fc.
3.1.4 Expression, Secretion and
Characterization of IL
To test the expression and secretion of ILs in the
H460 cells, harvesting the culture supernatants is
planned to measure IL-7, IL-12, IL-15, IL-21 using
ELISA. The presence of these ILs will be observed in
the control, VV-iPDL1/IL, VV-IL, VV-iPDL1, and
VV-empty groups, respectively.
3.1.5 Cytotoxicity
To test cytotoxicity of the immune checkpoint, the
H460 cell line with KRAS mutation is used. Tumor
cells will be plated in well-plates. Two groups are
planned to be used to perform the experiment, with
each group containing 5 subgroups with varying gene
insertions. The PD-L1 +/+ tumor cells will contain
control, VV-iPDL1/IL, VV-IL, VV-iPDL1, and VV-
empty cells. The PD-L1 -/- tumor cells will be
generated using the CRISP-Cas9 technique and will
contain the same subgroups as that in PD-L1 +/+. The
cell viability will be determined 48 hours after
infection with cell-counting kit or nonradioactive cell
proliferation assay (Ge, 2020).
3.2 Antitumor Activity in Mice NSCLC
Model
3.2.1 Model Construction
The mouse mutant LSL-KrasG12D is proposed to be
used as our mouse model. This mouse model was
generated by Xu and others where they crossed mice
carrying CC10-CreER allele to Lox-stop-Lox (LSL)
K-RasG12D mice. They expressed oncogenic codon
12 mutant K-Ras in CC10- and Sftpc-expressing cells
in the adult mice lung using knock-in CreER driver
mouse lines. The researchers found that CC10+ type
II cells are one of the origins of adenocarcinomas in
response to K-Ras activation. The phenotype of the
mice shows adenomas and adenocarcinomas with
SPC-Cre, which works for our experiment to test the
oncolytic viruses in NSCLC (Xu, 2012).
3.2.2 OV Injection
Continuing from the last step, the antitumor activity
of the oncolytic virus is evaluated in the established
model. The mice are divided into five groups
randomly: control group, VV-iPDL1/IL, VV-IL, VV-
iPDL1 and VV-empty group. When their tumors
reach a volume of 100mm
3
(day 0), they receive intra-
tumoral injections of 50μL indicated VV three times
on 0,3,7 days post-transplantation and the control
group receive an equal amount of PBS at the same
time and site.
3.2.3 Characterization of iPDL1 and IL
After the previous step, the expression of iPDL1 and
IL levels in tumor-bearing mice are detected. Serum
samples are collected from mice without treatment,
injected VV-iPDL1/IL, VV-IL, VV-iPDL1 or VV-
empty 3 days after the infusion of indicated VV. The
serum levels are determined using mouse PD-1
ELISA kit and mouse IL-7, IL-12, IL-15 and IL-21
ELISA kit. To explore how long the iPDL1 could
maintain in tumor-bearing mice, both the serum
sample and tumor tissue are collected from the mice
1, 2, 4, 7, 10, 15, 20 …days after VV-iPDL1/IL
injection. iPDL1 level is determined using mouse PD-
L1 ELISA kit until iPDL1 level is too low to be
detected. The kinetics curve of iPDL1 level is made
Use of Oncolytic Vaccinia Virus Armed with Certain Cytokines (IL-7, IL-12, IL-15, IL-21) in Combination with Checkpoint Inhibitors to
Treat Non-Small Cell Lung Cancer
459
accordingly. There are three independent samples in
a group and each experiment is repeated twice.
3.2.4 Tumor Growth and Survival
The tumor growth is monitored after the injection of
VV to observe the progression of NSCLC. At 3, 7, 10,
13, 16, 20, 25, 30 days, each mouse in five groups
receives microCT scan, which is a relatively accurate
and easy method for tumor volume measurement
(Jensen, 2008). MicroCT images are analyzed by
certain softwares to get tumor volumes. Kaplan-
Meier survival curve is made within 100 days. There
are five independent individuals in a group.
3.2.5 Tumor Infiltration and Immune Cell
Ativation
Five days after VV injection, tumor tissues are
collected from mice without treatment, injected VV-
iPDL1/IL, VV-IL, VV-iPDL1 or VV-empty and
digested with collagenase type I and DNase. Then the
tissues are filtered to prepare single-cell suspensions,
which are then subjected to antibodies staining and
analyzed by FACS. Antibodies against CD45, CD8,
CD4, CD11c, CD11b and Gr-1 are used. Then the
plots are drawn based on the percentage of infiltrating
CD45+ immune cells, dendritic cell (DC; CD11c+),
Myeloid-derived suppressor cells (MDSCs;
CD11b+Gr-1+), CD4+ T cells, CD8+ T cells in tumor
tissues. Besides, we assume that the virus could also
activate the infiltrating effector T cells. The IFN-γ
and TNF-α expression of CD8+ T cells can be
measured by intracellular staining. There are five
independent samples in a group and each experiment
is repeated twice.
3.3 Anticipated Results
Below are the idealistic results of the experiments
based on conjecture.
3.3.1 Characterization of VV in H460 Cells
In our assumptions, high levels of iPDL1 can be
detected in VV-iPDL1/IL and VV-iPDL1 infected
H460 cells, while IL-7, IL-12, IL-15 and IL-21 levels
of VV-iPDL1/IL and VV-IL infected cells are
significantly higher than other groups. The pictures
are not shown here. Besides, it is hypothesized that
VV-iPDL1/IL-infected cells release a higher level of
iPDL1 than VV-iPDL1-infected ones and a higher
level of the interleukins than VV-IL-infected ones.
This could be explained by the synergy between the
interleukins and immune suppressive pathways.
Moreover, iPDL1 protein purified from the
supernatant of VV-iPDL1/ IL infected H460 cells
should bind to PD-L1+/+ tumor cells, but not to PD-
L1 -/- tumor cells in vitro. Expected results are shown
in figure 2. Flow cytometry is used to characterize
PD-L1 expression on different H460 cells first
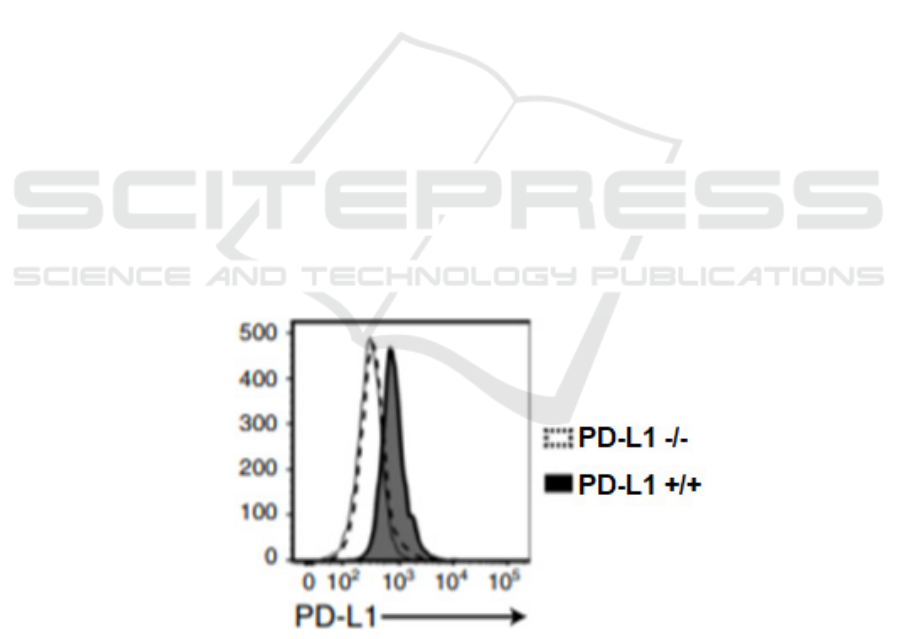
(Figure 1). These could attest to the the successful
expression of iPDL1 that can specifically bind to PD-
L1+/+ tumor cells.
Figure 1: Expression of PD-L1 on PD-L1+/+ H460 cells and PD-L1 -/- H460 cells (Wang, 2020) Flow cytometry is used to
show the PD-L1 expression on both wild type H460 cells and PD-L1-knocked out H460 cells.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
460

Figure 2: iPDL1 secreted by infected H460 cells could bind to PD-L1 +/+ cells (Wang, 2020) PD-L1 +/+ and PD-L1 -/- H460
cells are incubated with purified iPDL1 or IgG (used as a negative control) before being stained with anti-iPDL1 or anti-IgG
antibodies. Flow cytometry shows the percentage of iPDL1 that binds to PD-L1 +/+ H460 cells.
3.3.2 Enhanced Cytotoxicity in H460 Cells
As is shown in figure 3, for the PD-L1 +/+ H460 cells,
the viability of the cells infected with VV-iPDL1/IL
should be lower than that of those infected with VV-
iPDL1, VV-IL and VV-empty (the cell viability of
control group is 1). As is shown in figure 4, for the
PD-L1 -/- H460 cells, although we hypothesize that
the overall cytotoxicity is higher due to the lack of
immune checkpoint pathway, there should be so
significant difference between VV-iPDL1/IL and
VV-IL, VV-iPDL1 and VV-empty. In conclusion, the
secretion of the iPDL1 from JX-594 alpha is assumed
to lead to cell killing, in a PD-L1 dependent manner.
Figure 3: Cell viability of wild type H460 cells infected with indicated VVs H460 cells are infected with VV-iPDL1/IL, VV-
IL, VV-iPDL1 and VV-empty respectively. Control group is incubated with the same amount of PBS. The cell viability is
determined 48 hours after infection using nonradioactive cell proliferation assay.
Use of Oncolytic Vaccinia Virus Armed with Certain Cytokines (IL-7, IL-12, IL-15, IL-21) in Combination with Checkpoint Inhibitors to
Treat Non-Small Cell Lung Cancer
461
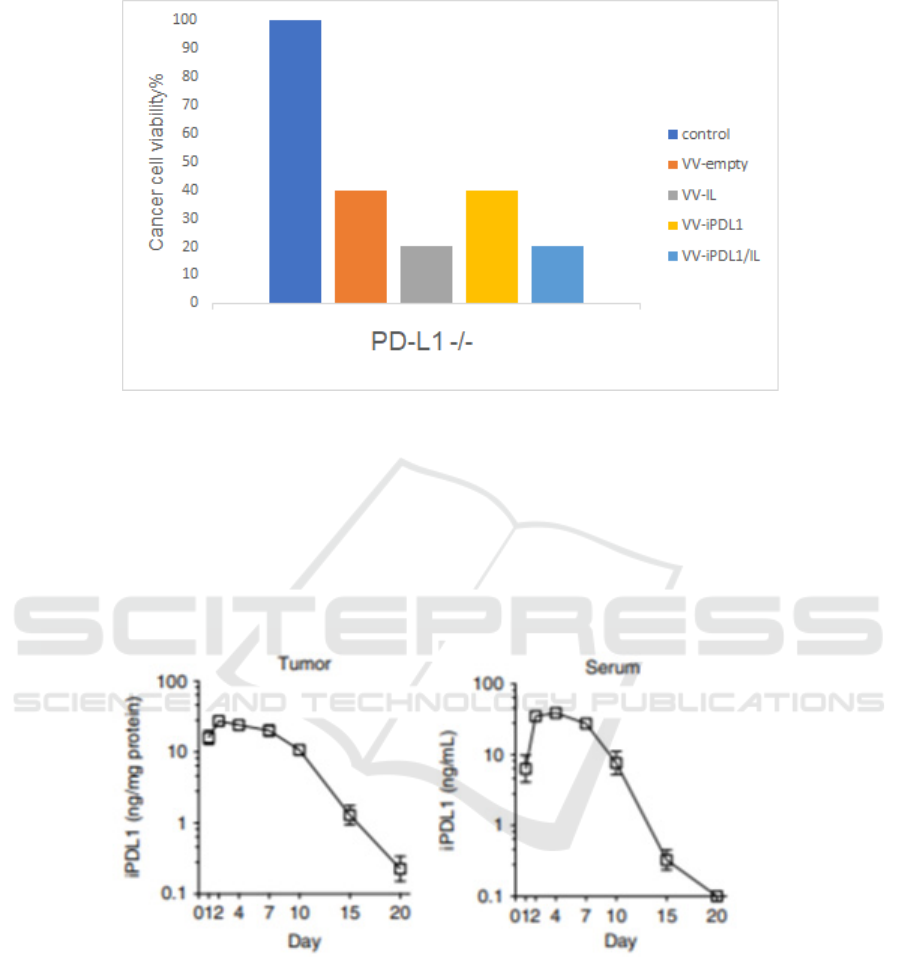
Figure 4: Cell viability of PD-L1-knocked out H460 cells infected with indicated VVs CRISPR/Cas9 is used to generate PD-
L1 knocked out H460 cell lines. The following procedures are parallel to those in wild type H460 cells.
3.3.3 Characterization of VV in LSL-
KrasG12D Mouse Model
High levels of iPDL1 should be detected in mice
infected with VV-iPDL1/IL and VV-iPDL1, while
IL-7, IL-12, IL-15 and IL-21 levels in mice infected
with VV-iPDL1/IL and VV-IL group should be
significantly higher than other groups. In a word, the
anticipated results are similar to that of in vitro studies
and the figures are omitted here. Additionally, iPDL1
levels in both the tumor and serum of mice injected
with VV-iPDL1/IL are assumed to reach their peak
several days after injection and could last for about 20
days (Figure 5), which is of great clinical importance.
Figure 5: Kinetics of iPDL1 levels in both tumors and sera of the VV-iPDL1/IL-treated mice (Wang, 2020). iPDL1
concentrations are determined using PD-L1 ELISA kit 1, 2, 4, 7, 10, 15, 20 days after intra-tumoral injection.
3.3.4 Enhanced Antitumor Activities in
LSL-KrasG12D Mouse Model
As is shown in figure 6, we assume that tumors in
mice treated with VV grow slightly before shrinking
and the tumors all disappear finally. But the tumor
volume of mice treated with PBS should increase
substantially. Among the mice treated with VV, the
tumors disappear earliest in VV-iPDL1/IL group
within 30 days, the second is VV-iPDL1 group and
following are the VV-IL and VV-empty groups. As is
the survival curve in figure 7, we hypothesize that
mice in VV-iPDL1 group have the highest survival
rate 100 days after injection. These all demonstrate
that intra-tumoral injection of VV-iPDL1/IL could
significantly inhibit tumor growth in KrasG12D
mouse model although other VV are also potent
tumor inhibitors.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
462
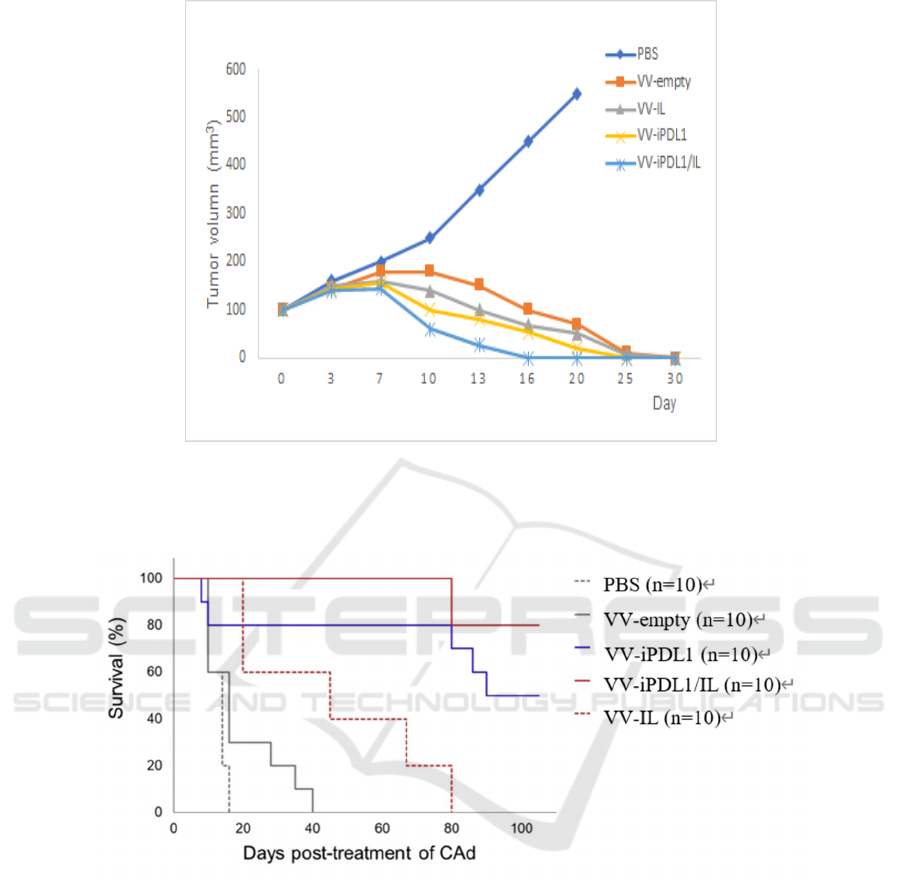
Figure 6: Tumor volume of mice injected with indicated VVs within 30 days LSL-KrasG12D mice are intratumorally injected
with indicated VVs when their tumors reach a certain size. At 3, 7, 10, 13, 16, 20, 25, 30 days, each mouse receives microCT
scan to determine tumor volume.
Figure 7: Kaplan-Meier survival curve of tumor-bearing mice injected with indicated VVs within 100 days (Caroline, 2020)
LSL-KrasG12D mice are intratumorally injected with indicated VVs and monitored in 100 days. Each group has five
independent samples.
3.3.5 Enhanced Tumor Infiltration and
Immune Cell Activation
We hypothesize that FACS analysis (Figure 8) could
indicate a higher level of overall lymphocytes marked
by CD45+ and a higher percentage of CD4+, CD8+
T cells and dendritic cells in VV-iPDL1/IL group
compared to VV-IL and VV-empty group. For
MDSC cell, VV-IL and VV-iPDL1/IL should both
enhance its composition compared to PBS group.
However, we assume the MDSC cells in VV-
iPDL1/IL group doesn't increase as much as the other
two groups, indicating the ability of VV-iPDL1/IL to
inhibit immune suppressive cells. Besides, VV-
iPDL1/IL should activate effector T cells by
enhancing the expression of IFN-γ, CD107a and
TNF-α in CD8+ T cells (Figure 9). In a word, it's
speculated that VV-iPDL1/IL could enhance the
infiltration of lymphocytes, inhibit the suppressive
cells and activate CD8+ effector T cells.
Use of Oncolytic Vaccinia Virus Armed with Certain Cytokines (IL-7, IL-12, IL-15, IL-21) in Combination with Checkpoint Inhibitors to
Treat Non-Small Cell Lung Cancer
463

Figure 8: FACS analysis of infiltrating CD45+ immune cells, CD4+ T cells, CD8+ T cells, dendritic cells and MDSCs in
tumors (Wang, 2020) 5 days after VV injection, tumors are harvested and digested to prepare single-cell suspensions, which
are stained by antibodies against CD45, CD8, CD4, CD11c, CD11b and Gr-1.
Figure 9: Expression of IFN-γ, TNF-α, and CD 107a in infiltrating CD8+ T cells (Wang, 2020) Intracellular staining is used
to measure IFN-γ and TNF-α expression of CD8+ T cells.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
464

4 DISCUSSION
The JX-594 alpha has large genome size which
enables it to express multiple cytokines and
checkpoint inhibitors (CPI) and thus induce T-cell
proliferation and antibody production. With the help
of several cytokines with different functions, our
modified virus can increase immune response and
target tumor cells more efficiently. In our
expectations, the overall benefits of our virus are to
induce a regression of tumor size and increase
survival rates.
Despite the benefits of combination stated above,
our experiment has some drawbacks. First, five
subgroups are designed here: VV-iPDL1/IL, VV-IL,
VV-iPDL1, VV-empty, and the control group. We
would explore how the addition of iPDL1, cytokines
and the combination of both iPDL1 and cytokines
could affect the properties of VV. However, we
regard the four cytokines as a whole rather than
explore their functions separately. This might bring
about a question of whether or not the final increase
in immune responses arises from the synergy of
cytokines. It’s possible that one or two cytokines
don’t act as immune activating agent in this condition.
Thus, more detailed studies on the function of each
cytokine in VVs are needed. Besides, oncolytic virus
therapy could be combined with other therapies in
further studies. For instance, a study has investigated
the combination effect of ReoT3D and some
chemotherapeutic agents in NSCLC cells and
demonstrated that ReoT3D and taxane could achieve
synergy through apoptosis (Sei, 2009). To make the
virus more aggressive to cancer cells, we may also
start to look for other proteins besides cytokines and
CPIs that also increase immune responses. Finally,
genetic modifications of VVs could enhance their
antitumor effect. However, some properties of viruses
might be impaired at the same time like their stability
and safety, which can cause some unexpected side
effects.
5 CONCLUSION
Based on previous studies of OV therapy, a new
remedy, JX-594 alpha is conceived and its
effectiveness in NSCLC is tested in both H460 cell
lines and KrasG12D mouse model. We hypothesize
that both VV-iPDL1 /IL and VV-iPDL1 infected
H460 cells could produce iPDL1 and the levels of IL-
7, IL-12, IL-15 and IL-21 in VV-iPDL1 /IL infected
cells were higher than those in other groups. Besides,
iPDL1 produced in vitro should be able to bind PD-
L1 +/+ tumor cells, which is tested by flow cytometry.
VV-iPDL1 /IL is also assumed to cause cell killing in
a PD-L1-dependent way. As to the in vivo
experiment, it’s also hypothesized that constructed
VVs could produce iPDL1 or the cytokines
successfully in mouse mode l. The long-term level of
iPDL1 in both the serum and tumor is also measured.
Tumor growth and overall survival of the mice are
monitored within 100 days. It is expected that the
tumor disappeared earliest in VV-iPDL1 /IL group,
and the survival rate of VV-iPDL1 /IL group is also
the highest. Other VVs should also be potent tumor
suppressors, but weaker than VV-iPDL1 /IL. FACS
should be used to characterize tumor infiltration and
immune cell activation. We assume that the VV-
iPDL1 /IL group has a higher overall CD45+
lymphocyte level and a higher percentage of DC,
CD4+ and CD8+ T cells. In addition, VV-iPDL1 /IL
should activate effector T cells by enhancing IFN-γ
and TNF-α expression. In conclusion, JX-594 alpha
is assumed to enhance lymphocyte infiltration,
activate CD8+ effector T cells and finally achieve the
goal of tumor elimination in NSCLC mouse model.
Admittedly, the experiment design has some
drawbacks discussed above. But we believe that
emerging studies on OV therapy will definitely bring
it to clinical application.
REFERENCES
Abd-Aziz N, Poh CL. (2021) "Development of oncolytic
viruses for cancer therapy". Transl Res: 237:98-123.
Alderson, M. R., et al. (1991). "Interleukin-7 induces
cytokine secretion and tumoricidal activity by human
peripheral-blood monocytes." Journal of Experimental
Medicine 173(4): 923-930.
Airoldi, I., et al. (2009). "IL-12 Can Target Human Lung
Adenocarcinoma Cells and Normal Bronchial
Epithelial Cells Surrounding Tumor Lesions." Plos One
4(7).
Berraondo, P., et al. (2019). "Cytokines in clinical cancer
immunotherapy." British Journal of Cancer 120(1): 6-
15.
Breitbach, C. J., et al. (2013). "Oncolytic Vaccinia Virus
Disrupts Tumor-Associated Vasculature in Humans."
Cancer Research 73(4): 1265-1275.
Caroline E, P. (2020). "Oncolytic Adenovirus Armed with
BiTE, Cytokine, and Checkpoint Inhibitor Enables
CAR T Cells to Control the Growth of Heterogeneous
Tumors. " Molecular therapy: the journal of the
American Society of Gene Therapy. 28(5): 1251-1262.
Cohen, M. C. and S. Cohen (1996). "Cytokine Function: A
Study in Biologic Diversity." American Journal of
Clinical Pathology 105(5): 589-598.
Use of Oncolytic Vaccinia Virus Armed with Certain Cytokines (IL-7, IL-12, IL-15, IL-21) in Combination with Checkpoint Inhibitors to
Treat Non-Small Cell Lung Cancer
465

Colombetti, S., et al. (2009). "IL-7 adjuvant treatment
enhances long-term tumor antigen-specific CD8(+) T-
cell responses after immunization with recombinant
lentivector." Blood 113(26): 6629-6637.
Ferguson, M. S., et al. (2012). "Systemic delivery of
oncolytic viruses hopes and hurdles." Advances in
virology 2012: 805629-805629.
Fukuhara, H., et al. (2016). "Oncolytic virus therapy: A new
era of cancer treatment at dawn." Cancer Science
107(10): 1373-1379.
Ge, Y., et al. (2020). "Oncolytic vaccinia virus delivering
tethered IL-12 enhances antitumor effects with
improved safety." Journal for Immunotherapy of
Cancer 8(1).
Hawkins, L. K., et al. (2002). "Oncolytic biotherapy: a
novel therapeutic platform." Lancet Oncology 3(1): 17-
26.
Isvoranu, G., et al. (2021). "Therapeutic potential of
interleukin-15 in cancer (Review)." Experimental and
Therapeutic Medicine 22(1).
Jensen, M. M., et al. (2008). "Tumor volume in
subcutaneous mouse xenografts measured by microCT
is more accurate and reproducible than determined by
18F-FDG-microPET or external caliper." BMC
medical imaging 8: 16-16.
Martuza, R. L., et al. (1991) "Experimental therapy of
human glioma by means of a genetically engineered
virus mutant." Science 252.5007:854-856.
Marintcheva, B. (2018). Chapter 9 - Virus-Based
Therapeutic Approaches. Harnessing the Power of
Viruses. B. Marintcheva, Academic Press: 243-276
Merrick AE, Ilett EJ, Melcher AA (2009). "JX-594, a
targeted oncolytic poxvirus for the treatment of cancer.
Curr Opin Investig Drugs."10(12):1372-82.
Mirlekar, B. and Y. Pylayeva-Gupta (2021). "IL-12 Family
Cytokines in Cancer and Immunotherapy." Cancers
13(2).
Molina, J. R., et al. (2008). "Non-small cell lung cancer:
epidemiology, risk factors, treatment, and
survivorship." Mayo Clin Proc 83(5): 584-594.
Onoi, K., et al. (2020). "Immune Checkpoint Inhibitors for
Lung Cancer Treatment: A Review." Journal of Clinical
Medicine 9(5).
Pathak, R., et al. (2020). "Acquired Resistance to PD-1/PD-
L1 Blockade in Lung Cancer: Mechanisms and Patterns
of Failure." Cancers 12(12).
Sei, S., et al. (2009). "Synergistic antitumor activity of
oncolytic reovirus and chemotherapeutic agents in non-
small cell lung cancer cells." Molecular Cancer 8.
Sondergaard, H. and K. Skak (2009). "IL-21: roles in
immunopathology and cancer therapy." Tissue
Antigens 74(6): 467-479.
Vaddepally, R. K., et al. (2020). "Review of Indications of
FDA-Approved Immune Checkpoint Inhibitors per
NCCN Guidelines with the Level of Evidence."
Cancers 12(3).
Wang, G., et al. (2020). "An engineered oncolytic virus
expressing PD-L1 inhibitors activates tumor
neoantigen-specific T cell responses." Nature
Communications 11(1).
Wherry, E. J. (2011). T cell exhaustion. Nature
Immunology, 12(6), 492.
Xu, X., et al. (2012). "Evidence for type II cells as cells of
origin of K-Ras-induced distal lung adenocarcinoma."
Proceedings of the National Academy of Sciences of
the United States of America 109(13): 4910-4915.
Zheng, M., et al. (2019). "Oncolytic Viruses for Cancer
Therapy: Barriers and Recent Advances." Molecular
Therapy-Oncolytics 15: 234-247.
Zundler, S. and M. F. Neurath (2015). "Interleukin-12:
Functional activities and implications for disease."
Cytokine & Growth Factor Reviews 26(5): 559-568.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
466
