
Trypsin-Assisted Cell Depletion Method for Wound Healing Assay
Di Yin
1
, Shihmo Yang
2
, Hongbo Zhang
1
and Wenjun Zhang
1,3,*
1
School of Mechanical and Power Engineering, East China University of Science and Technology, Shanghai, China
2
Biomedical Science and Technology Research Center, School of Mechatronic Engineering and Automation,
Shanghai University, Shanghai, China
3
Division of Biomedical Engineering, University of Saskatchewan, Saskatoon, Canada
Keywords:
Wound Healing Assay, Cell Depletion, Cell Patterning, Trypsin.
Abstract:
Wound healing assay is a commonly used method in the laboratory to study cell migration ability. Among the
methods used to create cell-free zone, the widely used method, called cell depletion, will leave a certain
amount of injured cells in the migration regions, which will have an impact on the subsequent healing
experiments. To this end, we present a trypsin-assisted cell depletion method in wound healing assay to create
cell-free zone without dead cells. This method could rinse the dead cell after applying depleting process
without interfering in the attachment of the living cells. All the operation process is accomplished by
commonly used equipment and drugs in biological experiments. The effect of the enzyme is controlled by the
ambient temperature and processing time. The debris of dead cells are easily detached and removed to avoid
the impact on wound healing assay. This method is expected to combine with other 2D and 3D cell patterning
methods to form a more reliable cell processing technique.
1 INTRODUCTION
The investigation of wound healing assay would
provide more information about cell migration and
cell-cell interaction (HE, 2020) for biologist to study
cellular mechanisms (Grada, 2017), tumor formation
and metastasis (Teleanu, 2019), and inflammation
models (Biglari, 2019). The first step in wound
healing assay requires creating an artificial cell-free
zone which have been well developed by researchers.
And most of these methods can be categorized as cell
depletion that inevitably causes damage to the cells
(Monfared, 2021). Meanwhile, the injured cells may
remain on substrate, which seriously affect the wound
healing assay of the rest living cells. In this article, we
developed a trypsin-assisted method to remove these
injured cells by controlling the efficacy of trypsin by
temperature and time. After comparison, the most
effective processing parameters were obtained. With
experimental verification, this method will have
negligible side effect on the cells that are prepared for
the subsequent wound healing assay. This approach
not only addresses the inherent disadvantage of cell
depletion, but also makes the process of creating cell-
free zone regions more stable and reliable.
2 METHOD
The device with stamping function used to culture
cell is shown in Figure 1. The top layer of the chip is
the Polydimethylsiloxane (PDMS) with pillars
dimensions of 100 μm height and a 400μm diameter
fabricated by mold, which is made by
photolithography technology. After the PDMS is
made, the two ends of the PDMS are punched to
fabricate the outlet and inlet of the chip. The middle
layer is a spacer with a hollow cavity made of laser-
cut Acrylic(PMMA) board. And the bottom layer is
the culture dish substrate used for cell attachment.
After the oxygen plasma and ultraviolet treatment,
these three parts are aligned and bonded together by
double-sided adhesive tapes and use heavy objects to
press the device for an hour to obtain a fully sealed
channel with 1mm height and 1cm width. The
stainless steel needles are inserted at both ends of the
channel, and the liquid in the channel can be replaced
through the Teflon tube which are connected with
syringes. The PDMS layer can be pressed down so
that the pillars in the central region can touch the
bottom of the channel and realize the stamping
function.
Yin, D., Yang, S., Zhang, H. and Zhang, W.
Trypsin-Assisted Cell Depletion Method for Wound Healing Assay.
DOI: 10.5220/0012032800003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 483-487
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
483
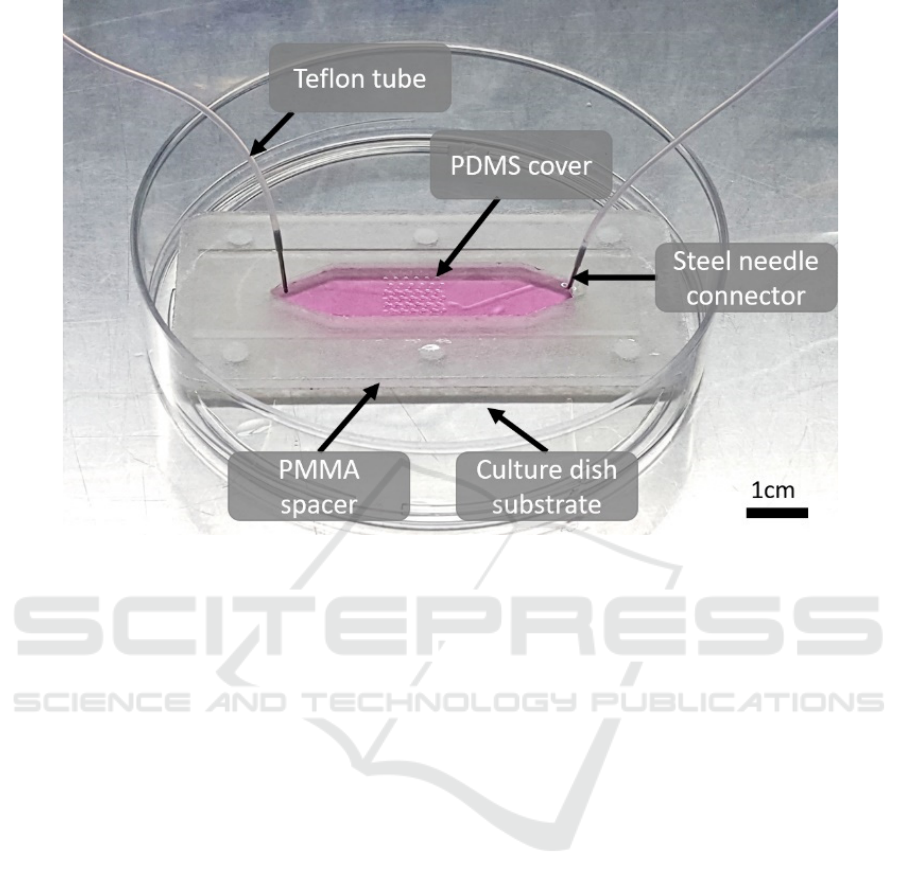
Figure 1: Schematic diagram of cell culturing chip with stamping function. Three layers of the chip are bonded and sealed by
two double-sided adhesive tapes. The two ends are connected by tubes to load the nutrients and trypsin that are necessary for
the experiment. And both ends of the tubs should be sealed with syringe to prevent leakage of liquid. The device will remain
in an incubator except for the trypsin experiment which requires the temperature of the chip to be adjusted.
Before the experiment, the device was filled with
liquid 75% ethanol and exposed to ultraviolet light
overnight. After disinfection, 5mL phosphate
buffer(PBS) was injected into the channel for
cleaning. And the PBS was then evacuated from the
channel by air, and the device was placed in a sterile
vessel to be vacuumed as much gas as possible from
the chip. After 30 minutes, the device was moved to
a sterile environment, and 75% alcohol was re-
injected to drain the air bubbles in the channel. After
that, the channel was washed again with PBS, and the
chip is ready for use. In the process of the experiment,
in addition to trypsin, other liquid injected into the
chip, must be put into the incubator in advance, so as
not to create bubbles in the chip.
The principle of the trypsin-assisted method is
shown in Figure 2 by appending weakened trypsin
digestion steps to the traditional cell depletion. In our
device, Cells were seeded into the channel as shown
in Figure 2(a). After incubation for 24 hours, the cells
reached 80% confluent without any liquid flow. The
DMEM in the device was replaced by PBS. Then, the
pillars were pressed down towards the substrate as
shown in Figure 2(c). After 5 minutes, the stamp was
reset to the original position. And the trypsin was
injected into the channel to replace the PBS and let
stand for several minutes as shown in Figure 2(e).
Last, DMEM was gently introduced into the channel
to rinse the dead cells and resupply the cells with the
nutrients that are necessary for the subsequent
experiment as shown in Figure 2(f).
ICBB 2022 - International Conference on Biotechnology and Biomedicine
484

Figure 2: The procedure of the trypsin-assisted method. The dark blue boundary represents the structure around the cell culture
chamber, the black arrow represents the flow direction of the different liquid, and the yellow arrow represents the direction
of the stamping movement. The stamping process can be performed manually or with other devices. It should be noted that
the pressure cannot be too large, otherwise the PDMS will touch the cells out of the target cell-free zone.
3 RESULTS AND DISCUSSIONS
The micrographs of the cells during the procedure
was shown in Figure 3. Before stamping, PDMS was
washed away with PBS. And the morphology of cells
is shown in Figure 3(a). After stamping, it can be
observed that the morphology of cells out of cell-free
zone was not significantly different from that of the
cells before stamping. Compared to the
aforementioned cells, the cells below the stamp were
heavily stressed, and most of them were dead and thus
detached the substrate. But there were still a few
injured cells attached to the substrate, which are
mainly concentrated in the center and the edge of the
cell-free zone, as shown in Figure 3(b). The regions
and quantities of these cells are not constant, which is
not reliable for studying injured cells. It also can
affect other living cells around. Therefore, it needs to
be processed immediately. Subsequently, trypsin was
slowly injected into the channel and remained at a
certain temperature and time. These injured cells
would fall off naturally, which were rinsed out of the
channel with re-injecting DMEM. And the remaining
cells form a well-defined cell-free zone as shown in
Figure 3(c).
Figure 3: bright-field micrographcs of the cell morphology before and after stamping(a)(b) and after being treated with
trypsin(c). The scale bar is 100 μm.
To access the effect of trypsin, which was affected
by the ambient temperature and the standing duration,
a series of experiments were carried out. Cell activity
and cell density at different temperatures are shown
Trypsin-Assisted Cell Depletion Method for Wound Healing Assay
485

in Figure 4(a). With the increase of temperature, the
effect of Trypsin became stronger and a large number
of dead cells fell off, leading to the increase of cell
viability. But at the same time, the cell density
decreased with the increase of temperature. The effect
of different standing duration on cells is shown in
Figure 4(b). With the increase of standing duration,
the shedding of dead cells also leads to the increase
of cell viability, while the cell density decreases
greatly.
Figure 4: The effect of the trypin in different conditions such like ambient temperature(a) and standing time(b). The cell
density is represented by bars and the cell viability is represented by dotted lines.
In order to evaluate the growth of cells treated
with or without trypsin, we compared our method
with the wound healing of cells in the cell exclusion
method within 24 hours. Here, the cells of the
experimental group were treated with trypsin at 20 ℃
for three minutes while the control group was treated
with DMEM for three minutes at the same
temperature. The healing area of cells was recorded
every 6 hours. As shown in Figure 5. In each time
point, the wound healing area was very close to each
ICBB 2022 - International Conference on Biotechnology and Biomedicine
486
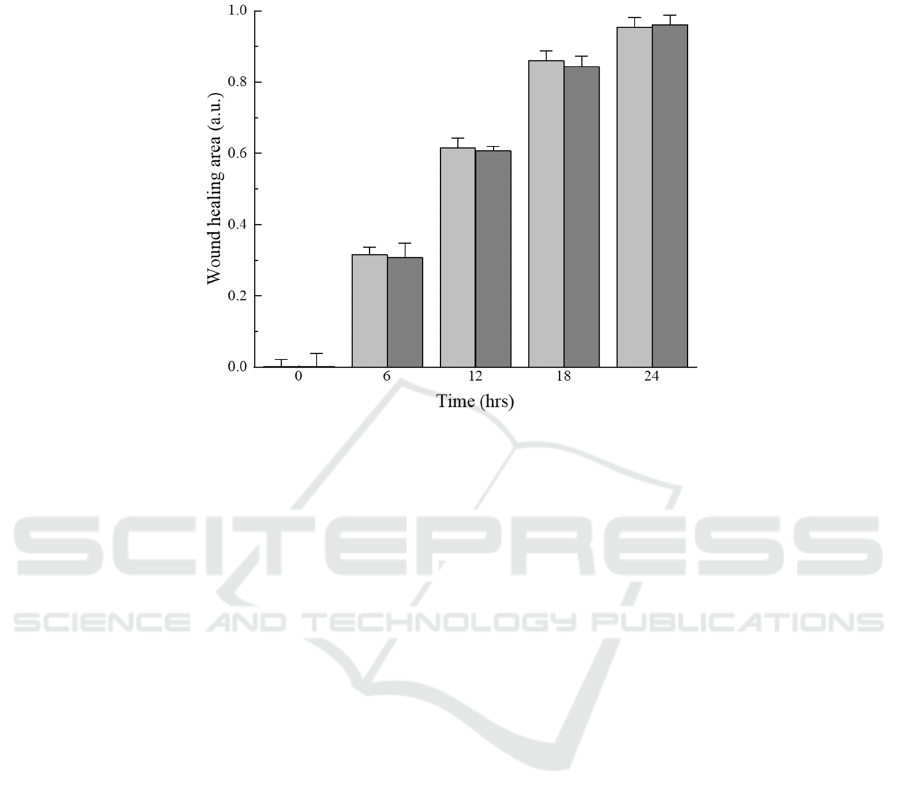
other and all achieved more than 0.95 at 24 hours.
Compared with the control group, the effect of trypsin
treatment on cells was almost negligible. And the
experimental results further verify the feasibility of
our method.
Figure 5: Wound healing area of the cells under treated or non-treated conditions. The light colored columns indicate cells
that have not been treated with trypsin, while the dark colored columns indicate cells that have been treated with trypsin. The
wound healing area at certain time points was normalized to diagram cell migration.
4
CONCLUSION
The experimental results showed that by adjusting the
temperature and time of trypsin, targeted cell
clearance could be achieved and well-defined cell-
free zone could be obtained. It also proved that the
method not only removed the negative effects of the
dead cells, but also ensured that the remaining cells
treated with the enzyme were no different from the
untreated cells in subsequent wound healing
experiments. This method overcomes the inevitable
defects in the process of cell depletion and it will
expand the application prospects of more cell
patterning methods.
ACKNOWLEDGMENTS
We are thankful to Prof. Zhang and the staff of her
Laboratory at East China University of Science and
Technology for their support. We also extend special
thanks to the staff of the Biomedical Science and
Technology Research Center at Shanghai University.
REFERENCES
A. Grada, M. Otero-Vinas, F. Prieto-Castrillo, Z. Obagi, V.
Falanga.(2017) Research Techniques Made Simple:
Analysis of Collective Cell Migration Using the Wound
Healing Assay, J Invest Dermatol, 137: e11-e16.
G. Shabestani Monfared, P. Ertl, M. Rothbauer. (2021)
Microfluidic and Lab-on-a-Chip Systems for
Cutaneous Wound Healing Studies, Pharmaceutics, 13.
H.E. Deal, A.C. Brown, M.A. Daniele. (2020)
Microphysiological systems for the modeling of wound
healing and evaluation of pro-healing therapies, J Mater
Chem B, 8: 7062-7075.
R.I. Teleanu, C. Chircov, A.M. Grumezescu, D.M. Teleanu.
(2019) Tumor Angiogenesis and Anti-Angiogenic
Strategies for Cancer Treatment, J Clin Med, 9.
S. Biglari, T.Y.L. Le, R.P. Tan, S.G. Wise, A. Zambon, G.
Codolo, M. De Bernard, M. Warkiani, A. Schindeler, S.
Naficy, P. Valtchev, A. Khademhosseini, F. Dehghani.
(2019) Simulating Inflammation in a Wound
Microenvironment Using a Dermal Wound-on-a-Chip
Model, Adv Healthc Mater, 8 e1801307.
Trypsin-Assisted Cell Depletion Method for Wound Healing Assay
487
