
Prediction of Sepsis Using Light Gradient-Boosting Machine
Classifier in Comparison with Adaboost Classifier Based on Accuracy
Chindukuru Naga Sai Sreedhar and Loganayagi S.
Department of Computer Science and Engineering, Saveetha School of Engineering,
Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, 602105, India
KeywoRds: Sepsis, Machine Learning, Adaboost Classifier, Innovative Novel LightGBM Technique, Clinical Data,
Health.
Abstract: This study introduces a method to forecast sepsis employing the innovative LightGBM classifier model,
juxtaposing its improved accuracy against the Adaboost Classifier model. The dataset was sourced from
PhysioNet/Computing in Cardiology Challenge 2019's training set. The G power software informed the
sample size decision, suggesting 10 participants for each group, adopting a pretest power of 80%. A 95%
confidence interval was applied, and a significance level was established at 0.05%. Remarkably, the
LightGBM Technique achieved 96.41% accuracy, surpassing the AdaBoost Classifier's 77.58%. A significant
difference was observed between the two, evidenced by a P value of 0.019. In conclusion, the Light Gradient-
Boosting Machine classifier offers superior accuracy in predicting sepsis events.
1 INTRODUCTION
Sepsis is a severe condition that can become fatal
when the body overreacts to an infection. It can affect
people of all ages and may be triggered by various
infections, including bacterial, viral, and fungal
(Pravda, 2021). An exaggerated immune response to
these infections can damage healthy tissue. Using
machine learning for early sepsis detection is crucial
as it can enhance patient outcomes and potentially
save lives (Liu et al., 2019; G. Ramkumar et al.,
2021). Diagnosing sepsis can be challenging due to
its varied symptoms, which can resemble other health
issues. Progress in sepsis prediction through clinical
data analysis can help identify patients at high risk of
developing sepsis, facilitating quicker treatment
initiation (Reyna et al., 2019). Improved sepsis
prediction can not only better patient outcomes but
also facilitate bespoke treatment plans based on
individual risk (Wong et al., 2015). The research's
applications involve machine learning algorithms
capable of identifying patients at high risk for sepsis
during clinical trials, encouraging early intervention,
enhancing outcomes, and bolstering overall health
(Deepak et al., 2020; Sivakumar et al., 2022). These
algorithms can also craft predictive models gauging
sepsis probability using factors like demographic
data, health histories, and laboratory results.
Healthcare providers can then assess the situation
using the proposed model. Numerous research
articles have been published on sepsis prediction due
to its grave implications. Over 60 research articles on
sepsis prediction are available on ResearchGate, and
75 are found on Google Scholar. In a study by Taylor
et al. (2016), a random forest model was employed,
and its efficacy was compared with a classification
and regression tree (CART) model and a logistic
regression model. The random forest model
registered an AUC of 0.86 with a 95% confidence
interval, whereas the CART and logistic regression
models recorded AUCs of 0.69 and 0.76,
respectively. Bloch et al. (2019) reported that the
pooled area under the receiving operating curve
(SAUROC) for predicting sepsis 3 to 4 hours prior to
onset was 0.89, while the pooled SAUROCs for
SIRS, MEWS, and SOFA stood at 0.70, 0.50, and
0.78, respectively. In their research, Shrestha et al.
(2021) recommended a solution employing Gradient
Boosting that achieved a classification accuracy of
97.67%, compared to the typical 91.12% accuracy. X.
Peng and his team in their 2018 paper suggested a
methodology using a mixture-of-experts framework
to individualise sepsis treatment. This model
selectively oscillated between kernel (neighbour-
based) and DRL (Deep reinforcement learning)
methods. The paper by Shrestha et al. (2021) remains
Sreedhar, C. and S., L.
Prediction of Sepsis Using Light Gradient-Boosting Machine Classifier in Comparison with Adaboost Classifier Based on Accuracy.
DOI: 10.5220/0012570200003739
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Artificial Intelligence for Internet of Things: Accelerating Innovation in Industry and Consumer Electronics (AI4IoT 2023), pages 595-600
ISBN: 978-989-758-661-3
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
595

the pinnacle in this area, with its model performance
being considerably superior to others.
Machine learning algorithms' efficacy heavily
hinges on the quality of the clinical data they're
trained upon. Should this data be skewed or
incomplete, the algorithm's performance could suffer,
highlighting a research gap. The research thus
discussed methods for deriving balanced data from an
imbalanced dataset. This research's objective is to
predict sepsis using the innovative novel LightGBM
classifier model and contrast its accuracy against the
Adaboost classifier.
2 MATERIALS AND METHODS
The preliminary research was undertaken at the
Machine Learning laboratory of Saveetha School of
Engineering, affiliated with the Saveetha Institute of
Medical and Technical Sciences, situated in Chennai.
The study used two groups, with 10 samples in each.
Group 1 made use of the innovative light GBM
classifier, whereas Group 2 adopted the Adaboost
classifier. For the desired accuracy, samples were
sourced from the device and underwent ten
repetitions with an 80% G power, a significance level
of 0.05%, and a 95% confidence interval
(Kakaraparthi & Karthick, 2022). The dataset, a
compilation of Sepsis Clinical Data from patients,
was accessed via the Physionet Repository.
The Jupyter Notebook served as the coding
platform for testing, whilst SPSS version 26.0.1 was
deployed for statistical data analyses. The operations
ran on a laptop equipped with an Intel Core i5
processor and a 16GB RAM. The Physionet
repository supplied the dataset (Reyna et al., 2019).
Out of the acquired training set, 20,336 psv files,
encompassing patient clinical data in pipe-delimited
text formats, were subsequently converted to CSV.
The dataset exhibited an imbalance, with nearly 60%
of its data being null values. Resampling techniques
were used to balance the dataset. Initially containing
41 columns, post feature engineering, it was trimmed
down to 14 columns. These columns represented vital
signs, lab results, demographics, and outcomes.
Among the 14, 13 were independent whilst one was
dependent. The 'Sepsis Label' column indicates
whether a patient has sepsis.
Innovative Novel LightGBM Classifier
The innovative LightGBM classifier stands as a
machine learning model tailored for binary or
multiclass classification assignments. It's a gradient-
boosting platform known for its high performance,
which employs tree-based learning algorithms to craft
a decision tree ensemble. Owing to its design,
LightGBM is adept at efficiently handling large
datasets and real-time tasks. The model incorporates
a distinctive technique named "Gradient-based One-
Side Sampling" (GOSS) to refine the gradient
boosting procedure. This not only slashes memory
consumption but also accelerates the training phase.
Additionally, LightGBM is equipped with advanced
functionalities such as managing categorical features,
early termination, and bespoke loss functions,
rendering it an invaluable asset for classification
undertakings that demand speed and precision
(Hecht-Nielsen, 2020).
Algorithm 1.
Input: The clinical dataset of patients.
Output: Predicted label of sepsis.
Step 1: The necessary packages are imported.
Step 2: Load the dataset and store it as a data frame
using pandas.
Step 3: Calculate the null values and eliminate
them using imputation.
Step 4: Evaluate the component significance and
extract the important features using XGBoost.
Step 5: Make the labels normalized by using Label
Encoder.
Step 6: Create the training and testing datasets
using Sklearn libraries.
Step 7: Find the parameter combinations by
performing the hyper parameter tuning Boost
Machine Classifier.
Step 8: Using the parameters found by hyper
parameter tuning, Initialise the Light Gradient
boosting machine classifier.
Step 9: Commence the training of the Light
Gradient Boost Machine Classifier utilizing the
provided training data.
Step 10: The performance of the model is evaluated
by validating it with the provided testing data.
Step 11: Using Sklearn metrics compute the
accuracy and plot using matplotlib
Adaboost Classifier
AdaBoost, short for Adaptive Boosting, is a
supervised machine learning technique suitable for
both classification and regression tasks. Acting as a
meta-algorithm, it amalgamates the predictions from
numerous weaker classifiers to forge a robust and
more precise classifier. AdaBoost functions
iteratively, sequentially training weaker classifiers,
with each new one focusing on amending the errors
of the preceding one. A pivotal attribute of AdaBoost
AI4IoT 2023 - First International Conference on Artificial Intelligence for Internet of things (AI4IOT): Accelerating Innovation in Industry
and Consumer Electronics
596
is its proficiency in managing imbalanced datasets,
wherein certain classes might be underrepresented
compared to others. The flexibility to integrate with a
plethora of base classifiers bolsters its versatility,
rendering it a favoured choice in the realm of machine
learning. On the whole, AdaBoost is celebrated for its
prowess to augment classification model accuracy
and provide considerable interpretability across
diverse domains. (Hao & Huang, 2023).
Algorithm 2.
Input: The clinical dataset of patients.
Output: Predicted label of sepsis.
Step 1: The required packages are imported.
Step 2: Load the dataset using pandas and store it
in a dataframe.
Step 3: The dataset is checked to ensure whether it
is balanced or not and perform resampling.
Step 4: Find the correlation between labels and
compute component significance.
Step 5: Extract the most important features using
XGBoost.
Step 6: Normalize the dataset using label encoder.
Step 7: Use the hyper parameter tuning to identify
the best optimal parameter combinations.
Step 8: Using the parameters found in the last step,
initialize the Adaboost classifier.
Step 9: Train the Adaboost classifier with training
data.
Step 10: Determine the Accuracy and Log loss for
test data.
Statistical Analysis
The statistical examination of the suggested and
counterpart algorithms was conducted utilising the
IBM SPSS 26.0.1 software. In the clinical dataset,
'Sepsis Label' serves as the dependent variable,
whereas the independent variables encompass HR,
O2SAT, Temp, SBP, MAP, DBP, RESP, EtC02,
BaseExcess, HCO3, FiO2, pH, PaCO2, SaO2, AST,
BUN, Alkalinephos, Calcium, Chloride, Creatinine,
Bilirubin_direct, Glucose, Lactate, Magnesium,
Phosphate, Potassium, Bilirubin_total, Troponin,
Hct, Hgb, PTT, WBC, Fibrinogen, Platelets, Age,
Gender, Unit1, Unit2, HospAdmTime, and ICULOS.
An independent sample T-test was employed for both
the proposed and the contrasting algorithms. Post
analysis, metrics like mean accuracy, standard
deviation, and standard error were documented
(Hussain et al., 2022).
3 RESULTS
In this research study, two algorithms – the
Innovative Novel LightGBM Technique and the
AdaBoost Classifier – were utilised, with accuracy as
the primary performance metric. The AdaBoost
Classifier's performance rendered an accuracy of
77.58%, which is comparatively lower than that of the
Innovative Novel LightGBM Technique, which
achieved an impressive accuracy of 96.41%.
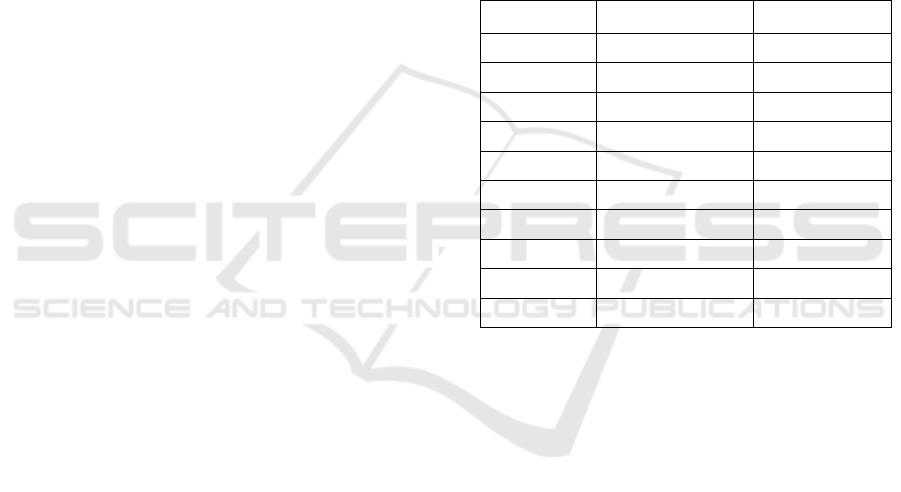
Table 1: The precise scores of both Light GBM and
Adaboost Classifier models, based on a sample size of 10
each, are presented. The LGBM classifier model exhibits
accuracies ranging from 98.63% to 93.45%, while the
Adaboost classifier model displays accuracies ranging from
79.82% to 76.43%.
S. No LGBM Adaboost
1 93.45 74.58
2 94.56 75.67
3 95.23 76.43
4 96.46 76.81
5 96.45 77.23
6 96.83 77.77
7 97.21 78.63
8 97.61 78.92
9 98.63 78.94
10 98.76 79.82
Table 1 enumerates the accuracy rates acquired
across 10 iterations for both Group 1 and Group 2.
Meanwhile, Table 2 highlights the mean accuracies,
standard deviation, and standard error mean derived
from group statistics. The LightGBM model
registered a mean accuracy of 96.41%, while the
AdaBoost classifier marked a mean accuracy of
77.58%. Table 3 showcases the results of the
Independent Samples T-test performed in SPSS,
revealing a significance value of p=0.019 (p<0.05).
This signifies a statistical difference between the two
groups under study. Figure 1 offers a bar graph
juxtaposing the Innovative Novel LightGBM
Technique and the AdaBoost classifier, plotting the
variables of mean accuracy and loss on the Y-axis.
The Innovative LightGBM Technique exhibits
superior relevance compared to its AdaBoost
counterpart. Furthermore, the error bars within the
graph facilitate an assessment of the error rate,
highlighting that the Innovative LightGBM
Technique possesses a notably reduced error rate in
contrast to the AdaBoost Classifier.
Prediction of Sepsis Using Light Gradient-Boosting Machine Classifier in Comparison with Adaboost Classifier Based on Accuracy
597
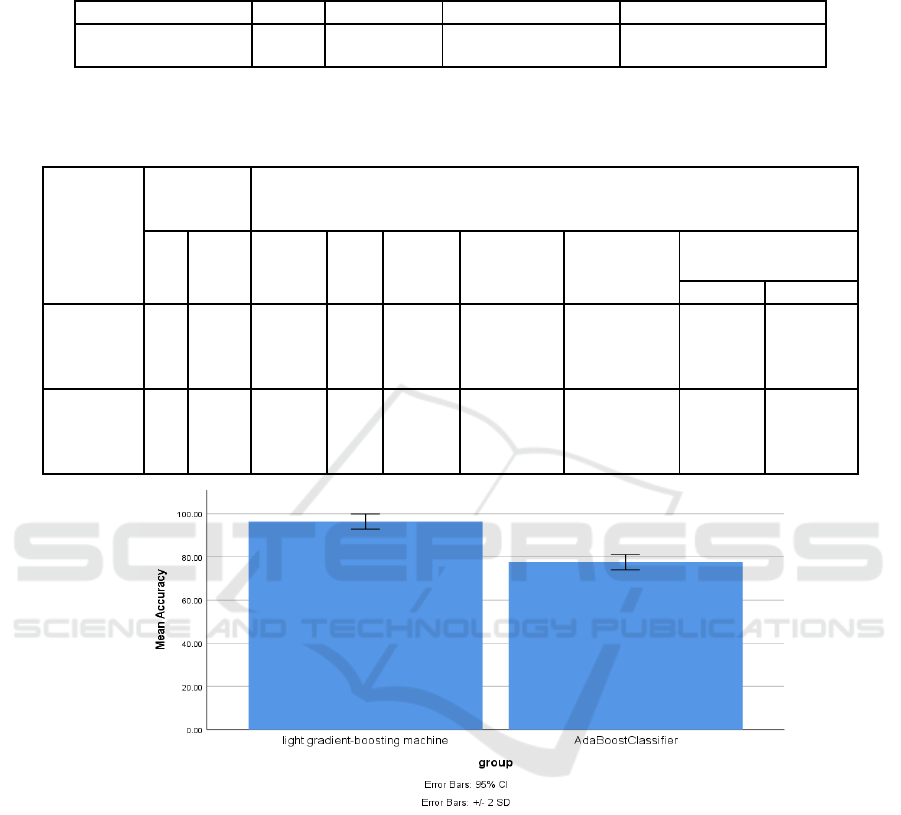
Table 2: The Innovative LightGBM Classifier has a mean accuracy of 96.41%, while the Adaboost Classifier's is 77.58%.
Standard deviation and standard error were calculated for both groups. LightGBM showed a higher standard deviation than
Adaboost.
Algorith
m
N Mean Standard Deviation Standard Error Mean
LGBM
ADABOOST
10
10
96.41
77.58
1.73804
1.7
0.54962
0.52429
Table 3: The Independent Samples T-test shows that the p-value is p=0.019 (p<0.05), which indicates that there is a significant
difference between the two groups. The mean accuracy of the two groups was compared assuming equal variances, and a
95% confidence interval was used.
Levene’s test
for equality o
f
variances
T-test for Equality of Means
F Sig. t df
Sig. 2-
tailed
Mean
Difference
Std. Error
Difference
95% confidence interval
of the difference
Lowe
r
Uppe
r
Accuracy
Equal
variance
Assumed
0.010 0.921 24.148 18 0.019 18.83900 0.78013 17.200000 20.47800
Accuracy
Equal
variance not
Assume
d
24.148 17.999 0.019 18.83900 0.78013 17.200000 20.47800
Figure 1: A graphical comparison of LGBM and Adaboost Classifiers based on mean accuracy and loss. LGBM outperforms
with higher accuracy and lower loss. Both classifiers are plotted on the x-axis against accuracy and loss on the y-axis, with a
95% confidence interval of +/-2 SD.
4 DISCUSSION
The findings of this research study highlight the
superior performance of the Innovative LightGBM
Technique over the AdaBoost Classifier in predicting
sepsis. The significance value, calculated using the
independent sample T-Test, stood at 0.019 (p<0.05),
marking the research as statistically significant. The
Innovative LightGBM Technique recorded a
commendable accuracy rate of 96.41% coupled with
a log loss of 0.064, clearly outshining the AdaBoost
Classifier which posted an accuracy of 77.58% and a
loss of 0.6731.
This study resonates with the contention that a
lower rate of loss is invaluable as it attests to the
efficiency of the approach (Elith et al., 2008). In their
discussion on the innovative technique known as
boosted regression trees, researchers pointed out the
application of boosting algorithms like AdaBoost for
challenges like two-class classification. This model
accentuated the significance of applying weights to
AI4IoT 2023 - First International Conference on Artificial Intelligence for Internet of things (AI4IOT): Accelerating Innovation in Industry
and Consumer Electronics
598

observations, underscoring those that are weakly
modelled. Several published research articles
corroborate these findings. For instance, Nesaragi &
Patidar (2021) conceptualised an ensemble model
amalgamating LightGBM, XGBoost, and Random
Forest, marking their best performance with an AUC
of 0.792 and an ACC of 0.727. Meanwhile, Bhavekar
& Goswami (2022) employed the five-fold-cross-
validation method to achieve an average normalized
utility score of 0.4314. L. Peng et al. (2022)
constructed seven diverse models, with the light
GBM model emerging as the best, recording an
accuracy of 0.96 on the test dataset. Chami &
Tavakolian (2019) benchmarked the Light Gradient
Boosting Machine Classifier against a hybrid of
survival analysis and neural networks, demonstrating
the supremacy of LGBM with a score of 0.172.
However, contrary perspectives are also evident.
Tarif et al. (2018) and Neelagandan (2012) observed
superior accuracy and efficiency with AdaBoost
classifiers, especially when juxtaposed with gradient-
boosted tree classifiers.
Nonetheless, this study isn't without limitations.
While the Innovative LightGBM Technique offers
impressive performance, it can be more time-
consuming during training phases and can consume
more memory compared to other classifiers. This
becomes more evident with extensive datasets or
when using specific high-category categorical
variables. There's also a susceptibility to overfitting,
especially with noisy data or over-extended training
durations. Looking ahead, the aspiration is to refine
the research by incorporating deep learning models.
Despite their promise of potentially unparalleled
accuracy in sepsis prediction, these models demand
extended training durations and necessitate advanced
computational infrastructure. Enhancing accuracy is
pivotal, for it directly impacts the mortality rate,
thereby ensuring better patient outcomes.
5 CONCLUSION
Drawing from the extensive analysis and findings of
this research, it becomes unequivocal that machine
learning models, especially the ones tailored for
specialized tasks, have the potential to revolutionize
the medical diagnostics sector. The comparative
analysis between the Innovative LightGBM
Technique and the AdaBoost classifier in the context
of sepsis prediction is a testament to this. Based on
our comprehensive discussions, the following six key
points emerge:
Performance Metrics: The Innovative LightGBM
Technique, with an impressive accuracy of 96.41%,
starkly outperformed the AdaBoost classifier, which
only managed to secure an accuracy of 77.58%.
Accuracy being a critical metric in medical diagnosis,
this difference in performance can translate to
tangible improvements in patient care.
Handling of Large Datasets: LightGBM is known
for its efficiency and scalability, which makes it adept
at handling large datasets. The ability to effectively
deal with extensive data is critical in medical
applications where vast amounts of patient data are
often involved.
Boosting Techniques: LightGBM employs
advanced boosting techniques such as Gradient-based
One-Side Sampling (GOSS). This not only
accelerates the training process but also optimizes
memory usage, making the model both fast and
resource-efficient.
Mitigation of Overfitting: Overfitting is a
perennial concern in machine learning, more so in
medical diagnostics. While the LightGBM model did
show potential susceptibilities to overfitting,
especially with noisy data, its performance in this
research still overshadowed the AdaBoost Classifier.
Versatility of AdaBoost: Despite the lower
accuracy, it's important to recognize the versatility of
the AdaBoost classifier. Its iterative approach to
rectifying errors and its compatibility with a range of
base classifiers still make it a valuable tool in many
applications.
Future Direction: While the LightGBM model has
shown superior performance in this research, it also
brings forth the idea of exploring deep learning
models in the future. The aim would be to achieve
even higher accuracy levels, albeit with the
understanding that these models might require more
intensive computational resources.
In conclusion, the overarching insight is that the
Innovative LightGBM Technique provides a more
accurate and efficient means of predicting sepsis
compared to the AdaBoost classifier. This not only
has implications for the advancement of machine
learning in healthcare diagnostics but also
underscores the critical role of selecting the
appropriate model for specific challenges.
REFERENCES
Bhavekar, G. S., & Goswami, A. D. (2022). Herding
Exploring Algorithm With Light Gradient Boosting
Machine Classifier for Effective Prediction of Heart
Diseases. In International Journal of Swarm
Prediction of Sepsis Using Light Gradient-Boosting Machine Classifier in Comparison with Adaboost Classifier Based on Accuracy
599

Intelligence Research (Vol. 13, Issue 1, pp. 1–22).
https://doi.org/10.4018/ijsir.302609
Bloch, E., Rotem, T., Cohen, J., Singer, P., & Aperstein, Y.
(2019). Machine Learning Models for Analysis of Vital
Signs Dynamics: A Case for Sepsis Onset Prediction.
Journal of Healthcare Engineering, 2019.
https://doi.org/10.1155/2019/5930379
Chami, S., & Tavakolian, K. (2019). Comparative Study of
Light-GBM and LSTM for Early Prediction of Sepsis
From Clinical Data. In 2019 Computing in Cardiology
Conference (CinC).
https://doi.org/10.22489/cinc.2019. 367
Deepak., John Justin Thangaraj, S., & Rajesh Khanna, M.
(2020, October 7). An improved early detection method
of autism spectrum anarchy using euclidean method.
2020 Fourth International Conference on I-SMAC (IoT
in Social, Mobile, Analytics and Cloud) (I-SMAC).
2020 Fourth International Conference on I-SMAC (IoT
in Social, Mobile, Analytics and Cloud) (I-SMAC),
Palladam, India. https://doi.org/10.1109/i-smac49090.
2020.9243361
Elith, J., Leathwick, J. R., & Hastie, T. (2008). A working
guide to boosted regression trees. The Journal of
Animal Ecology, 77(4), 802–813.
G. Ramkumar, R. Thandaiah Prabu, Ngangbam Phalguni
Singh, U. Maheswaran, Experimental analysis of brain
tumor detection system using Machine learning
approach, Materials Today: Proceedings, 2021, ISSN
2214-7853,
https://doi.org/10.1016/j.matpr.2021.01.246.
Hao, L., & Huang, G. (2023). An improved AdaBoost
algorithm for identification of lung cancer based on
electronic nose. Heliyon, 9(3), e13633.
Hecht-Nielsen, R. (2020). LPG-model: A novel model for
throughput prediction in stream processing, using a
light gradient boosting machine, incremental principal
component analysis, and deep gated recurrent unit
network. Information Sciences, 535, 107–129.
Hussain, M. M., Mahammad Hussain, M., & Karthick, V.
(2022). Efficient Search in Cloud Storage with Reduced
Computational Cost using Token Generation Method
over Crypto Hash Algorithm. In 2022 3rd International
Conference on Smart Electronics and Communication
(ICOSEC). https://doi.org/10.1109/icosec54921.2022.
9952137
Kakaraparthi, A., & Karthick, V. (2022). A Secure and
Cost-Effective Platform for Employee Management
System Using Lightweight Standalone Framework
Over Diffie Hellman’s Key Exchange Algorithm. In
ECS Transactions (Vol. 107, Issue 1, pp. 13663–
13674). https://doi.org/10.1149/10701.13663ecst
Liu, R., Greenstein, J. L., Granite, S. J., Fackler, J. C.,
Bembea, M. M., Sarma, S. V., & Winslow, R. L.
(2019). Data-driven discovery of a novel sepsis pre-
shock state predicts impending septic shock in the ICU.
Scientific Reports, 9(1), 1–9.
Neelagandan, R. (2012). High-Performance Face
Detection Using McT and Adaboost Algorithm. LAP
Lambert Academic Publishing.
Nesaragi, N., & Patidar, S. (2021). An Explainable Machine
Learning Model for Early Prediction of Sepsis Using
ICU Data. In Infections and Sepsis Development.
https://doi.org/10.5772/intechopen.98957
Peng, L., Peng, C., Yang, F., Wang, J., Zuo, W., Cheng, C.,
Mao, Z., Jin, Z., & Li, W. (2022). Machine learning
approach for the prediction of 30-day mortality in
patients with sepsis-associated encephalopathy. BMC
Medical Research Methodology, 22(1), 183.
Peng, X., Ding, Y., Wihl, D., Gottesman, O., Komorowski,
M., Lehman, L.-W. H., Ross, A., Faisal, A., & Doshi-
Velez, F. (2018). Improving Sepsis Treatment
Strategies by Combining Deep and Kernel-Based
Reinforcement Learning. AMIA ... Annual Symposium
Proceedings / AMIA Symposium. AMIA Symposium,
2018, 887–896.
Pravda, J. (2021). Sepsis: Evidence-based pathogenesis and
treatment. Pediatric Critical Care Medicine: A Journal
of the Society of Critical Care Medicine and the World
Federation of Pediatric Intensive and Critical Care
Societies, 10(4), 66.
Reyna, M., Josef, C., Jeter, R., Shashikumar, S., Moody, B.,
Brandon Westover, M., Sharma, A., Nemati, S., &
Clifford, G. D. (2019). Early Prediction of Sepsis from
Clinical Data: The PhysioNet/Computing in
Cardiology Challenge 2019 [Data set].
https://doi.org/10.13026/ v64v-d857
Sivakumar, V. L., Nallanathel, M., Ramalakshmi, M., &
Golla, V. (2022). Optimal route selection for the
transmission of natural gas through pipelines in
Tiruchengode Taluk using GIS–a preliminary study.
Materials Today: Proceedings, 50, 576-581.
Shrestha, U., Alsadoon, A., Prasad, P. W. C., Al Aloussi,
S., & Alsadoon, O. H. (2021). Supervised machine
learning for early predicting the sepsis patient: modified
mean imputation and modified chi-square feature
selection. Multimedia Tools and Applications, 80(13),
20477–20500.
Tarif, A. M., Raju, S. M., Al Amin Ashik, M., Islam, M. S.,
& Tahera, T. (2018). Self-Driving Car Simulation using
Adaboost-CNN Algorithm. GRIN Verlag.
Taylor, R. A., Pare, J. R., Venkatesh, A. K., Mowafi, H.,
Melnick, E. R., Fleischman, W., & Hall, M. K. (2016).
Prediction of In-hospital Mortality in Emergency
Department Patients With Sepsis: A Local Big Data-
Driven, Machine Learning Approach. Academic
Emergency Medicine: Official Journal of the Society
for Academic Emergency Medicine, 23(3), 269–278.
Wong, H. R., Cvijanovich, N. Z., Anas, N., Allen, G. L.,
Thomas, N. J., Bigham, M. T., Weiss, S. L., Fitzgerald,
J., Checchia, P. A., Meyer, K., Shanley, T. P., Quasney,
M., Hall, M., Gedeit, R., Freishtat, R. J., Nowak, J.,
Shekhar, R. S., Gertz, S., Dawson, E., … Lindsell, C. J.
(2015). Developing a Clinically Feasible Personalized
Medicine Approach to Pediatric Septic Shock.
American Journal of Respiratory and Critical Care
Medicine. https://doi.org/10.1164/rccm.201410-
1864OC
AI4IoT 2023 - First International Conference on Artificial Intelligence for Internet of things (AI4IOT): Accelerating Innovation in Industry
and Consumer Electronics
600
