
Corrosion Rate of A36 Plate with Zinc Anode and Combination of
Zinc Anode with Continuous Direct Current
Sri Endah Susilowati
a
and Didit Sumardiyanto
b
Universitas 17 Agustus 1945, Jakarta, Indonesia
Faculty of Engineering and Informatics, Universitas 17 Agustus 1945, Jakarta, Indonesia
Keywords: Corrosion Rate, Zinc Anode, DC Current, ASTM A36 Plate.
Abstract:
Corrosion, in general is a process through which refined metals are converted into more stable compounds
such as metal oxides, metal sulfides, or metal hydroxides. Likewise, the rusting of iron involves the formation
of iron oxides via the action of atmospheric moisture and oxygen. Iron is a metal that rusts easily. Iron rust is
a substance produced in corrosion events, which is in the form of a reddish-brown solid substance that is
brittle and porous. The chemical formula of iron rust is Fe2O3 x H2O. The impact of corrosion events is
detrimental. This corrosion rate research uses ASTM A36 plate (steel with low carbon content max 0.17 %C)
for venture floating dock deck with three kinds of treatment: plate without corrosion protection (A), with zinc
anode protection (B) and combined protection from zinc anode and DC electric (C). The corrosion rate is
calculated using the weight loss method. The results showed the magnitude of corrosion rates in treatment A,
B and C respectively: 0.66 mpy, 0.22 mpy and 0.17 mpy. Treatment with zinc anode protection and DC
current produces the smallest corrosion rate value among others. Hardness values tested using Brinnnel with
the values: 136.3 BHN, 205.2 BHN and 202.9 BHN respectively. The highest hardness value on the plate
with zinc anode protection treatment.
1
INTRODUCTION
In recent years, weathering steel has been widely used
in marine engineering structures, such as railway
vehicles, bridges and berths (Yang, F. et al., 2022).
Recently, a lot of efforts have been put into studying
the corrosion process of steel. These include studies
on metal corrosion in various indoor and outdoor
environments and the influence of temperature,
humidity, and corrosion ions on the corrosion rate and
products (Di Sarno et al., 2021), (Ma, Y. et al., 2009),
(Fan, Y. et al., 2020). Indoor environmental
experiments are used to control the experimental
variables and decouple the effects of different factors
on corrosion behaviour (Wu, H. et al., 2019), (Irshad,
H. M. et al., 2022). The marine atmosphere can
significantly accelerate the corrosion rate of carbon
steel. Various studies have discussed the relationship
between chloride ions, water, oxygen, corrosion
products, and corrosion rate in marine atmosphere
through indoor accelerated corrosion experiments
a
https://orcid.org/0000-0003-2284-9235
b
https://orcid.org/0000-0002-0837-8011
(Alcántara, J. et al., 2017), (Zhang, X. et al., 2019),
(Zhang, Z. et al., 2023). Ma et al. (Zhang, B. et al,
2020) proposed that in an atmospheric environment,
chloride ions can penetrate through the rust layer and
accelerate corrosion rate, and that different chloride
ion contents have different effects on the generation
of corrosion products. Fan et al. (Ohtsuka, T. and
Tanaka, S., 2015) analysed the rust layer structure and
proposed that the delamination of the rust layer has
different effects on the penetration of corrosive ions.
The dense rust layer can effectively slow the
corrosion rate, and the density of the inner rust layer
increases with increasing corrosion time. Wang et al.
(Kamimura, T. et al., 2006) suggested that there are
many types of FeOOH in corrosion products and the
proportion of different components changes with the
corrosion duration, and γ-FeOOH gradually changes
into α-FeOOH under the influence of corrosive ions
and oxygen, which causes the rust layer to become
dense and slows down the corrosion rate. Although
various studies on the corrosion of carbon steel in a
432
Susilowati, S. and Sumardiyanto, D.
Corrosion Rate of A36 Plate with Zinc Anode and Combination of Zinc Anode with Continuous Direct Current.
DOI: 10.5220/0012584300003821
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 4th International Seminar and Call for Paper (ISCP UTA ’45 JAKARTA 2023), pages 432-436
ISBN: 978-989-758-691-0; ISSN: 2828-853X
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

marine atmosphere exist, some unique environments
need to be considered, such as the influence of strong
electric field (Cheng, X. et al., 2017).
The unique environmental characteristics of
different marine zones are responsible for the corrosion
behavior distinctions. In the atmosphere, the
environment corrosivity is determined by the
temperature, relative humidity, chloride deposition
rate, and pollutants (Jeffrey, R., & Melchers, R. E.,
2009), (Feng, X. et al, 2020), resulting in the corrosion
occurrence under the adsorbed thin electrolyte layer
(Bhandari, J. et al, 2015). In the splash zone, the typical
characteristics are the intermittent wave splashing and
the sufficient oxygen (Xu, Y. et al, 2022), (Yusoff, N.
H. N. et al, 2013). The tidal zone also experiences fluid
erosion because of the seawater scouring (Wang, Y. et
al, 2023). Besides, it also includes the full immersion
stage during a single tidal cycle (Gao, F. et al, 2023).
Moreover, the wet-dry cycles are the common
characteristics of the above three zones, while the
wetting time, frequency, and electrolyte thickness are
different. The distinct feature of the full immersion
zone is the difficulty in oxygen availability. In addition,
the sediment and accumulation of the organisms
contribute to the corrosion process underneath them
(Situmeang, I. D. R., and Heltina, D., 2020).
The use of steel plates on the deck floating dock
of ships is a common thing used as a shipbuilding
material because it is quite adequate. But iron and
steel are so reactive that they have a tendency to
corrode in corrosive areas, namely seawater.
Corrosion is a natural symptom that commonly
occurs in ship plates as a result of interaction with the
surrounding environment so that it undergoes mass
changes in corrosive environments. This study tested
the corrosion rate on the ship's floating dock deck
plate with Zinc Anode and DC electric current. The
determination of the exact protection on the ship's
floating dock deck plate was tested using the
experimental method of seawater solution and Zinc
Anode with electric current. The addition of DC
electric current to the Zinc Anode will increase higher
protection on steel plates so that the risk of damage is
lower and can be used longer.
This study aims to analyze corrosion rate by
calculating weight loss and testing hardness on A36
plate for deck floating dock venture 3 with zinc anode
protection and DC current and zinc anode without
using DC current.
2
METHODS
The steps carried out in this experiment are as
follows:
1. Prepare tools, equipment that will be used to make
corrosion rate test experiments, starting from
preparing plastic measuring cups, sea water,
plates, USB Port cables, and Zinc Anode
2. The first experiment is a corrosion rate
experiment with a plate and seawater put into a
measuring cup and then calculated the corrosion
rate every 168 hours (7 days), 336 hours (14 days),
504 hours (21 days), up to 720 hours (30 days).
3. The second trial was with the plate method
protected with Zinc Anode from 168 hours (7
days), 336 hours (14 days), 504 hours (21 days),
to 720 hours (30 days).
4. The third trial is that the plate is protected with
DC Electric Current and Zinc Anode along 168
hours (7 days), 336 hours (14 days), 504 hours (21
days), up to 720 hours (30 days).
5. After 720 hours (30 days) the results were
calculated using the corrosion rate formula with
the weight loss method.
Figure 1: A36 Plate Material with treatment: A Without
Corrosion Protection, B. With Zinc Anode Protection, C.
With Zinc Anode Protection and DC Current, D A36 Plate
Experimental Results.
3
RESULTS AND DISCUSSION
3.1 Corrosion Rate
Figure 1 shows a comparison of corrosion rate of
protected versus unprotected carbon steel A36. The
result of corrosion rate experiments with plates
without using protection resulted in the greatest
corrosion rate of 0.66 mpy. Corrosion protection the
best is to use protection with zinc anode fed by DC
current. The corrosion rate on this plate is 0.17 mpy
(the lowest). The results of the immersion test without
the protection tested on sodium chloride solution for
30 days showed the greatest weight loss and the
Corrosion Rate of A36 Plate with Zinc Anode and Combination of Zinc Anode with Continuous Direct Current
433
corrosion rate was 0.66 mpy. This is due to the high
dissolved salts causing an increase in the conductivity
of the salt solution (Irshad, H. M. et al., 2022), the
steel undergoes corrosion due to the presence of the
Cl- ion where the Cl- ion will break the passive layer
on carbon steel or prevent the formation of a passive
layer on carbon steel (Situmeang, I. D. R., and
Heltina, D., 2020). On protected carbon steel, less
weight loss was observed than the unprotected carbon
steel. It can be seen from Figure 1 that the rate of
corrosion of steel protected by an anode of zinc
sacrificed and DC current was slower compared to the
rate of corrosion of unprotected carbon steel plate A
36. The results of the calculation of corrosion rates
with time and treatment variations are presented in
Table 1 and Figure 2 below:
Table 1: Calculation of Corrosion Rate of A36 plate.
Figure 2: Corrosion Rate on Various Treatments.
A zinc anode's protective properties result from a
strongly negative reduction potential, which is more
negative than the metal it is protecting. Oxidants,
which corrode metals, will oxidize the zinc anode
rather than the protected metal structure, thus
preventing the structure from being corroded.
3.2 Hardness Test
The results of the hardness test calculation can be
seen in Table 2 and Figure 3 below:
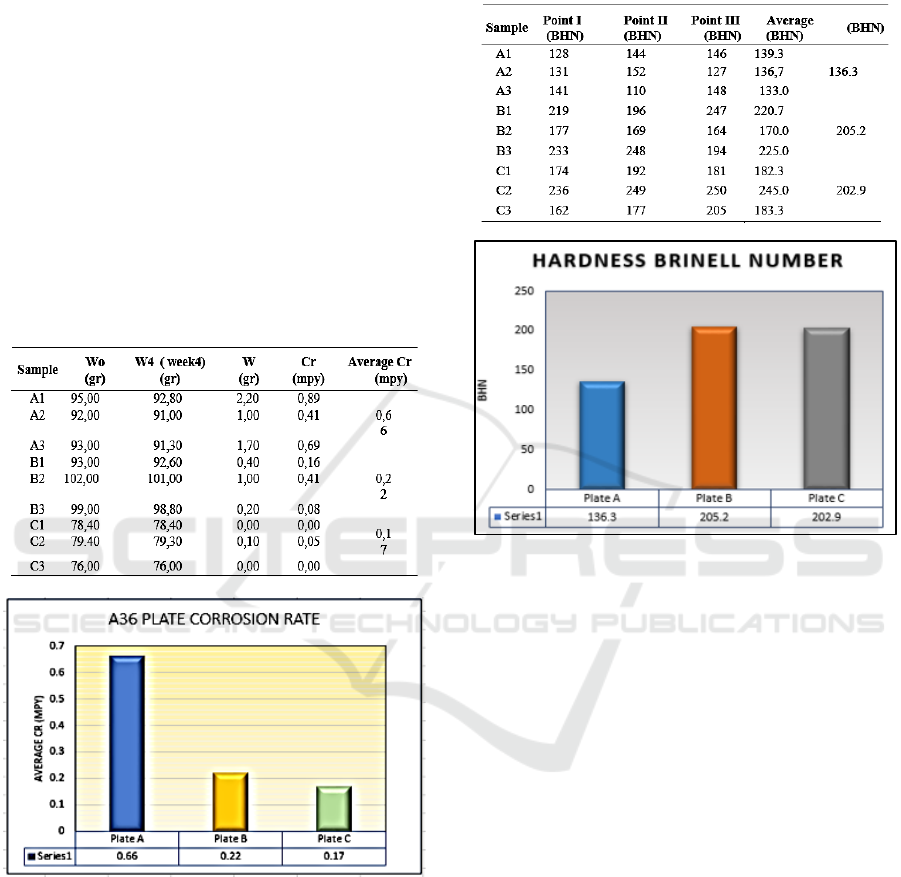
Table 2: The Hardness Brinnel Number.
Figure 3: A36 plate hardness test on various treatments.
The result of hardness testing without protection
is 136.3 BHN, while with Zinc Anode protection is
205 BHN and on a combined treatment between Zinc
Anode and DC Current is 202.9 BHN (Fig. 3). This
result is above the minimum limit of marine plate
hardness. Cathodic protection is an effective way to
prevent stress corrosion cracking (cracking due to
corrosion), by restoring the direction of the corrosion
current to restore electrons that decompose from
certain metals, which are immune or immune so that
the corrosion process on metals can be reduced or
eliminated.
The principle of cathodic protection is to provide
electrons for the metal structure to be protected. The
underlying theory is that if current flows from the
positive pole to the negative pole (conventional
electrical theory) the structure will be protected if
current enters from the electrodes. Conversely, the
corrosion rate will increase if the current enters
through the metal to the electrode. In the process of
corrosion in case of scratches or peeling of the
coating. The presence of water vapor, CO2 gas in the
air and other particles, a mini voltaic cell occurs with
Zn as the anode and Fe as the cathode. Zn will
oxidize first because its Eo value is smaller than Fe,
ISCP UTA ’45 JAKARTA 2023 - THE INTERNATIONAL SEMINAR AND CALL FOR PAPER (ISCP) UTA ’45 JAKARTA
434

so electrolytic corrosion (an electrochemical reaction
that oxidizes metals) does not occur.
From the calculation of the corrosion rate of
plates using protection, better results are obtained
compared to no protection. This happens because of
the potential difference, then the electron current will
flow from the installed anode and will resist the
electron current from the nearby metal, so that the
metal turns into a cathode region. This is what slows
down the plate experiencing the corrosion rate while
on the contrary the plate without protection will
release electrons causing damage to the plate so that
it is easy to corrode.
4 CONCLUSION
1. From the results of the three experiments,
results were obtained that showed that
corrosion occurred which was characterized by
a decrease in the weight of objects.
2. After calculating the corrosion rate value using
the weight loss, the result is a slower corrosion
rate when using zinc anode protection and DC
current, followed by zinc anode protection and
finally without using protection.
3. From the three experiments, it was obtained that
the value of corrosion rate with protection with
zinc anode and DC current was 0.17 mpy or
corrosion rate was 17%, then with zinc anode
protection with a value of 0.22 mpy or corrosion
rate was 22% and the last one was without
protection the value obtained was 0.66 mpy or
corrosion rate was 66%.
4. With this research, it has become the initial stage
to be applied directly, so that the use of this
method can save routine repair costs in the
shipping industry.
REFERENCES
Yang, F., Yuan, M. M., Qiao, W. J., Li, N. N., & Du, B.
(2022). Mechanical degradation of Q345 weathering
steel and Q345 carbon steel under acid corrosion.
Advances in Materials Science and Engineering, 2022.
Di Sarno, L., Majidian, A., & Karagiannakis, G. (2021).
The effect of atmospheric corrosion on steel structures:
A state-of-the-art and case-study. Buildings, 11(12), 571.
Ma, Y., Li, Y., & Wang, F. (2009). Corrosion of low carbon
steel in atmospheric environments of different chloride
content. Corrosion science, 51(5), 997-1006.
Fan, Y., Liu, W., Li, S., Chowwanonthapunya, T.,
Wongpat, B., Zhao, Y., ... & Li, X. (2020). Evolution of
rust layers on carbon steel and weathering steel in high
humidity and heat marine atmospheric corrosion.
Journal of Materials Science & Technology, 39, 190-199.
Wu, H., Lei, H., Chen, Y. F., & Qiao, J. (2019). Comparison
on corrosion behaviour and mechanical properties of
structural steel exposed between urban industrial
atmosphere and laboratory simulated environment.
Construction and Building Materials, 211, 228-243.
Irshad, H. M., Toor, I. U., Badr, H. M., & Samad, M. A.
(2022). Evaluating the Flow Accelerated Corrosion and
Erosion–Corrosion Behavior of a Pipeline Grade
Carbon Steel (AISI 1030) for Sustainable Operations.
Sustainability, 14(8), 4819.
Alcántara, J., de la Fuente, D., Chico, B., Simancas, J., Díaz,
I., & Morcillo, M. (2017). Marine atmospheric corrosion
of carbon steel: A review. Materials, 10(4), 406.
Zhang, X., Zhou, X., & Nilsson, J. O. (2019). Corrosion
behaviour of AA6082 Al-Mg-Si alloy extrusion: The
influence of quench cooling rate. Corrosion Science,
150, 100-109
Zhang, Z., Zhang, F., Du, G., Li, G., Fan, W., Wang, Y., ...
& Wang, X. (2023). Effect of intermittent and
continuous direct current electric fields on the initial
corrosion of steel in simulated marine environment.
Materials Today Communications, 35, 105629.
Zhang, B., Wei, X. X., Wu, B., Wang, J., Shao, X. H., Yang,
L. X., ... & Ma, X. L. (2019). Chloride attack on the
passive film of duplex alloy. Corrosion Science, 154,
123-128.
Ohtsuka, T., & Tanaka, S. (2015). Monitoring the
development of rust layers on weathering steel using in
situ Raman spectroscopy under wet-and-dry cyclic
conditions. Journal of Solid State Electrochemistry, 19,
3559-3566.
Kamimura, T., Hara, S., Miyuki, H., Yamashita, M., &
Uchida, H. (2006). Composition and protective ability
of rust layer formed on weathering steel exposed to
various environments. Corrosion Science, 48(9), 2799-
2812.
Cheng, X., Jin, Z., Liu, M., & Li, X. (2017). Optimizing the
nickel content in weathering steels to enhance their
corrosion resistance in acidic atmospheres. Corrosion
Science, 115, 135-142.
Jeffrey, R., & Melchers, R. E. (2009). Corrosion of vertical
mild steel strips in seawater. Corrosion Science, 51(10),
2291-2297.
Feng, X., Yan, Q., Lu, X., Wu, T., Zhang, Y., Zuo, Y., &
Wang, J. (2020). Protection performance of the
submerged sacrificial anode on the steel reinforcement
in the conductive carbon fiber mortar column in splash
zones of marine environments. Corrosion Science, 174,
108818.
Bhandari, J., Khan, F., Abbassi, R., Garaniya, V., & Ojeda,
R. (2015). Modelling of pitting corrosion in marine and
offshore steel structures–A technical review. Journal of
Loss Prevention in the Process Industries, 37, 39-62.
Xu, Y., Huang, Y., Cai, F., Lu, D., & Wang, X. (2022).
Study on corrosion behavior and mechanism of AISI
4135 steel in marine environments based on field
exposure experiment. Science of The Total
Environment, 830, 154864.
Corrosion Rate of A36 Plate with Zinc Anode and Combination of Zinc Anode with Continuous Direct Current
435

Yusoff, N. H. N., Ghazali, M. J., Isa, M. C., Daud, A. R., &
Muchtar, A. (2013). Effects of powder size and metallic
bonding layer on corrosion behaviour of plasma-
sprayed Al2O3-13% TiO2 coated mild steel in fresh
tropical seawater. Ceramics International, 39(3), 2527-
2533.
Wang, Y., He, J., Xie, F., Zhang, Y., Wang, G., Sun, D., ...
& Jia, W. (2023). The synergistic effect of parallel
magnetic field and sulfate-reducing bacteria on stress
corrosion cracking of buried X80 pipeline steel.
Engineering Failure Analysis, 154, 107644.
Gao, F., Li, J., Zhou, N., Luo, X., Yang, H., Chai, F., &
Yang, C. (2023). Investigating the corrosion
performance of hull steel with different microstructure
in a tropical marine atmosphere. Journal of Materials
Research and Technology, 27, 2600-2614.
Situmeang, I. D. R., & Heltina, D. (2020, May). Cathodic
protection on stuctures of carbon steel using sacrificial
anode methode for corrosion control. In IOP
Conference Series: Materials Science and Engineering
(Vol. 845, No. 1, p. 012015). IOP Publishing.
ISCP UTA ’45 JAKARTA 2023 - THE INTERNATIONAL SEMINAR AND CALL FOR PAPER (ISCP) UTA ’45 JAKARTA
436
