
An Epistemological Approach to Risk Assessment in
Pharmacovigilance and Mitigation Through Artificial Intelligence
A. D. Skali
Healthcare Innovation Consultant, CEO TheTowerBrand, ex-COO Luci Health, Head of Innovation Forum Barcelona
Branch, Future Business Centre King's Hedges Road, Cambridge, U.K.
Keywords: Pharmacovigilance, Risk Assessment, Drug Safety, Risk Management, Investment Practices, Bibliographic
Research, Benefit-Risk Evaluation, Artificial Intelligence, Epistemology.
Abstract: Pharmacovigilance, which focuses on risk assessment and management in drug safety, offers a robust
foundation for addressing inherent risks in new drug discovery. This bibliographic research article explores
innovative perspectives by drawing parallels between pharmacovigilance and investment practices, as
inspiration to establish a new, in the field of pharmacovigilance, epistemological framework for the
understanding of the main risk inducing elements in pharmacovigilance and the steps and technology we can
adopt to assess and mitigate them.
1 BACKGROUND AND
CONTEXT
Risk assessment and prevention is essential in the
field of healthcare. The judicious application of risk
assessment methodologies serves as a sentinel,
discerning latent hazards embedded within clinical
processes. This discernment facilitates the creation of
a secure healthcare milieu, diminishing the incidence
of adverse events and fortifying the sanctity of patient
well-being.
As we transition to the specific domain of
pharmacovigilance, the importance of risk
management becomes even more pronounced. In the
pharmaceutical landscape, where the stakes are
inherently high, risk assessment plays a pivotal role
in ensuring the safety and well-being of patients. The
intricate web of risks in this context includes not only
the potential side effects of medications but also
regulatory compliance and the complexities of a
globally interconnected pharmaceutical market.
Pharmacovigilance, as a subset of risk
management in healthcare, involves the systematic
monitoring and evaluation of the safety and efficacy
of pharmaceutical products post-market approval.
The application of rigorous risk assessment
frameworks within pharmacovigilance becomes a
linchpin for identifying potential risks associated with
medication use. This includes adverse drug reactions,
unexpected side effects, and any other safety
concerns that may arise during the course of patient
treatment.
In the field of pharmacovigilance, the challenges
and limitations of current risk assessment
methodologies are multifaceted and underscore the
evolving nature of risks associated with
pharmaceutical products.
One prominent challenge lies in the dynamic
nature of risks. Traditional risk assessment
methodologies often struggle to keep pace with the
rapidly changing landscape of pharmaceuticals,
where new drugs are continually introduced, and their
effects may only become apparent after widespread
use. The inherent complexity of biological systems
and the variability in patient responses contribute to
the dynamic nature of risks, necessitating a more
adaptive and responsive approach to risk assessment.
Moreover, the inadequacy of traditional
approaches becomes apparent when addressing
emerging threats. Conventional risk assessment
models may not adequately account for novel and
unforeseen risks that can emerge as a result of
evolving scientific knowledge, changes in patient
demographics, or the introduction of innovative
therapeutic modalities. These emerging threats may
include previously unknown side effects, drug
interactions, or unexpected patient populations
susceptible to adverse reactions.
Additionally, the global interconnectedness of the
Skali, A.
An Epistemological Approach to Risk Assessment in Pharmacovigilance and Mitigation Through Artificial Intelligence.
DOI: 10.5220/0012869800003854
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st Inter national e-Health Forum (IeHF 2023), pages 29-36
ISBN: 978-989-758-711-5
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
29

pharmaceutical market poses challenges to traditional
risk assessment methodologies. The widespread
distribution of pharmaceutical products across
diverse populations and regulatory environments
requires a more comprehensive and globally aligned
approach to risk assessment. Traditional models may
struggle to capture the nuanced variations in risk
profiles across different regions and demographic
groups, potentially leading to incomplete risk
assessments.
The reliance on spontaneous reporting systems for
adverse drug reactions is another limitation. Such
systems heavily depend on healthcare professionals
and patients voluntarily reporting adverse events,
leading to underreporting and a potential lag in
identifying risks. This limitation hampers the real-
time assessment of risks associated with
pharmaceutical products.
In the context of pharmacovigilance, the existing
literature on risk assessment and mitigation provides
valuable insights into the challenges and
advancements in ensuring drug safety. However,
there are noticeable gaps and areas where
epistemological perspectives are underexplored.
The current literature predominantly focuses on
the technical and methodological aspects of risk
assessment, such as signal detection, data mining, and
statistical modeling. While these approaches are
crucial, there is a paucity of literature delving into the
underlying epistemological foundations that shape
our understanding of risk in pharmacovigilance.
One evident gap lies in the exploration of the
ontological and epistemological assumptions inherent
in risk assessment methodologies. Understanding the
nature of knowledge and reality as it pertains to drug
safety is crucial for refining risk assessment models.
For instance, the ontological status of adverse events,
whether they are discrete entities or part of a complex
network of interconnected factors, remains a topic
that warrants deeper philosophical exploration.
Furthermore, there is limited literature on the
epistemic uncertainties associated with
pharmacovigilance data. Epistemological
perspectives can shed light on the inherent
uncertainties in observational data, the reliability of
different sources, and the interpretative challenges in
discerning causality. Addressing these epistemic
uncertainties is pivotal for improving the accuracy
and reliability of risk assessments.
The potential contributions of integrating
epistemology into risk management practices are
substantial. Epistemological perspectives can inform
the development of more robust risk models by
providing a foundation for understanding what counts
as evidence, how causality is established, and the
nature of knowledge production in pharmacovigilance.
This integration can enhance the transparency and
accountability of risk assessment processes, as it
encourages a critical examination of the assumptions
and values that underpin decision-making.
Moreover, incorporating epistemological
considerations can foster interdisciplinary
collaboration between experts in pharmacovigilance,
philosophy, and other relevant fields. This
collaboration can lead to a more comprehensive and
holistic approach to risk assessment, considering not
only the technical aspects but also the epistemological
underpinnings that shape our understanding of drug
safety.
In the context of pharmacovigilance, the
integration of effective risk management strategies
aligns with the broader goal of fostering a culture of
patient safety. By systematically identifying,
assessing, and mitigating risks associated with
pharmaceutical products, the healthcare industry can
uphold the highest standards of patient care and well-
being. This interconnected approach underscores the
symbiotic relationship between robust risk
management practices, patient safety, and the
integrity of the pharmaceutical industry.
This paper embarks on the ambitious journey of
unraveling the intricate relationship between
epistemology and risk assessment in
pharmacovigilance. Its overarching goal is to
contribute a nuanced understanding that enriches the
current discourse on drug safety by delving into the
philosophical underpinnings and epistemic
dimensions inherent in the field. The primary focus of
this exploration is on meticulously examining the
epistemological facets of risk assessment in
pharmacovigilance.
This endeavor involves unraveling the intricacies
of how information regarding drug safety is
perceived, interpreted, and validated. Within this
focus, particular emphasis will be placed on
elucidating both the ontological essence of adverse
events and the epistemic processes that govern
knowledge production in the realm of
pharmacovigilance.
The scope extends beyond the superficial layers,
aiming for an in-depth examination of the
philosophical foundations that shape current risk
assessment methodologies. This includes a critical
analysis of how philosophical perspectives influence
our conceptualization of adverse events. Within this
extended examination, the exploration encompasses a
comprehensive analysis of the nature of knowledge
production in pharmacovigilance. This sheds light on
IeHF 2023 - International e-Health Forum
30

how epistemological assumptions contribute to the
construction of narratives surrounding drug safety.
The paper actively advocates for interdisciplinary
collaboration between experts in pharmacovigilance,
philosophy, and related fields. While promoting such
collaboration, it acknowledges inherent limitations in
providing exhaustive analyses of the technical
intricacies within the pharmacovigilance domain.
The proposed framework for integrating
epistemology into risk management practices will be
thoroughly discussed within the specific context of
pharmacovigilance. This includes practical insights
into how epistemological considerations can enhance
transparency, accountability, and accuracy in the
assessment of drug safety. By navigating through
these interconnected realms, the paper aims not only
to shed light on the philosophical dimensions of risk
assessment in pharmacovigilance but also to advocate
for a collaborative and informed approach towards
ensuring drug safety.
2 A SIMPLIFIED FRAMEWORK
FOR DECISION MAKING
In contemplating the seemingly disparate realms of
investing and pharmacovigilance, one might readily
dismiss any potential correlation based on their
ostensible divergent objectives. However, upon
closer examination of their core essence and
methodological approaches, an unexpected similarity
emerges.
Pharmacovigilance, in its essence, constitutes the
scientific discipline and set of actions dedicated to the
vigilant monitoring of medicine safety and the
proactive management of any issues that may arise in
this domain. The World Health Organization
encapsulates this concept as encompassing activities
directed at detecting, assessing, understanding, and
preventing adverse reactions to medicines and other
medicine-related problems. In essence,
pharmacovigilance is a continuous process of
monitoring medicine safety, with the overarching aim
of reducing risks and optimizing benefits.
Conversely, the foundational objective of
investing is rooted in the strategic utilization of
available information and analytical tools to make
judicious decisions. These decisions are not solely
oriented towards maximizing profit; they necessitate
a comprehensive evaluation of associated risks and
the implementation of strategic measures to mitigate
these risks effectively.
While the goals of investing and
pharmacovigilance may appear divergent at first
glance, a closer examination reveals a shared pursuit
– the reduction of risks and the enhancement of
positive outcomes. Both disciplines, albeit operating
in distinct domains, converge on the fundamental
principle of informed decision-making to achieve
outcomes that are not only focused on obtaining
benefits but also resilient in the face of inherent
uncertainties.
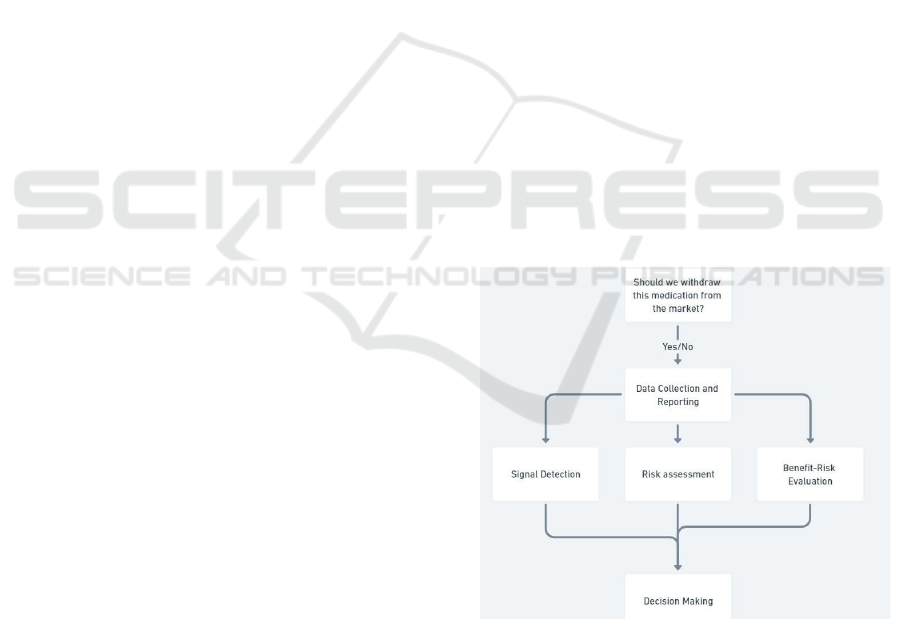
In the realm of pharmacovigilance, a succinct
framework guiding the evaluation of whether a drug
should remain on the market involves several key
steps:
Data Collection and Reporting Signal Detection
Risk Assessment Benefit-Risk Evaluation
Risk Minimization Strategies
What unites these procedural steps is a
fundamental principle shared with diverse domains
such as investing, marketing, and management.
Despite apparent differences in decisions, they
collectively adhere to a basic yet robust structure—
the problem-solving structure—which forms the
bedrock of the scientific method.
At the core of these decision-making processes
lies a simple and universal structure, akin to the
scientific method. When embarking on drug usage,
individuals are essentially testing a basic hypothesis:
"Is this drug sufficiently beneficial to justify potential
associated risks?"
"Do we possess comprehensive information to ensure
the accuracy of our decisions?"
Figure 1: Basic framework for the scientific method.
In its essence, this structured decision-making
process maintains a constant framework, its iterations
adapting to our evolving comprehension of the
situation or the issues at hand. This universal
approach extends beyond specific domains,
encompassing diverse fields such as marketing,
investing, management, sports, and, as asserted in this
context, pharmacovigilance. At the core of effective
problem-solving lies the validation or affirmation of
a hypothesis grounded in experimental data (Figure
1). This involves exploring correlations or causations
among various elements to make informed
predictions and actively working to minimize the
An Epistemological Approach to Risk Assessment in Pharmacovigilance and Mitigation Through Artificial Intelligence
31
likelihood of recurring errors.
The debate surrounding the applicability of this
streamlined decision-making model to
pharmacovigilance may require further discusiont.
However, the valuable perspective gained by
examining the field through this lens provides clarity,
fostering a nuanced understanding of its intricacies.
3 THE FRAMEWORK AND
PHARMACOVIGILANCE
In the context of pharmacovigilance and the
delineated steps, applying this straightforward model
could be represented as follows:
DAE, AE, or SAE → Information Gathering →
Judgment
Where Adverse Drug Effects (ADEs) constitute a
comprehensive category encompassing any harmful
or unintended effect resulting from drug usage,
Adverse Drug Reactions (ADRs) constitute a subset
of ADEs, specifically referring to unwanted and
harmful effects caused by a medication when taken at
normal doses during the regular course of treatment,
and Spontaneous Adverse Effects (SAE) denote
unintended, harmful reactions to a drug occurring
without any apparent cause or known pattern.
And the element that we always have to take into
account when approaching risk assessment is the
distinction arises between the "real risk" and the
"assumed risk." During the process of risk
assessment, there's a common inclination to believe
that the available information is comprehensive
enough to ensure accurate decision-making.
A compelling analogy that encapsulates this idea
is the iceberg metaphor. What's visible above the
waterline represents only a fraction of the entire
structure, with a substantial portion hidden beneath
the surface. This concept is reminiscent of the
challenges faced by ships navigating near glaciers.
Initially, ships assumed that wooden hulls were
sufficient to navigate these icy terrains, leading to
numerous sinkings. The realization that the actual
risk, the unknown factors, outweighed the assumed
risk prompted strategic measures. Ships began
reinforcing their hulls with metal, establishing
specific routes and timings to navigate through
glaciers. While they couldn't precisely determine the
size of each glacier, they made educated guesses
about potential risks, enabling them to accomplish
their goals despite incomplete knowledge.
Three essential elements emerge for informed
decision-making across various domains:
understanding the known, which involves delving into
the philosophy of knowledge (epistemology);
identifying main risks by discerning primary risks
based on existing knowledge and formulating initial
technological solutions; and establishing a feedback
loop, creating a continuous learning mechanism for
iterative improvements over time, fostering
adaptability and enhanced decision-making
capabilities.
3.1 Reducing the Gap Between Real
and Perceived Risk
All risk reduction strategies are rooted in two
fundamental principles: augmenting our
understanding of causal/correlation
relationships and mitigating errors associated with
human judgment. The empirical evidence from the
notable investor Ray Dalio and his hedge fund,
Bridgewater Associates, underscores the
transformative impact of enhancing these two
principles on decision-making. The initial imperative
is to amass more pertinent information while ensuring
its accuracy and relevance. In the realm of
pharmacovigilance, diverse avenues exist to gather
information on potential adverse effects, adverse drug
effects, and spontaneous adverse effects.
Figure 2: Application of the epistemological framework to
pharmacovigilance.
However, the underlying questions remain
consistent. Pertinent inquiries include assessing the
severity, frequency, reversibility, and likelihood of
potential adverse drug reactions (ADRs). These
questions bear substantial weight, influencing critical
IeHF 2023 - International e-Health Forum
32

decisions such as whether to proceed with drug
commercialization or delay it. The pivotal factor lies
in the reliability and trustworthiness of the amassed
information. Consequently, a refined framework for
pharmacovigilance can be articulated (Figure 2).
In addressing these inquiries, additional
considerations come to the forefront:
Determining the specific information essential for
making informed decisions is a foundational step.
Evaluating the reliability of the information received
is paramount, questioning its accuracy and relevance.
Scrutinizing the trustworthiness of the
information source, whether it be a program or an
individual, becomes crucial. Assessing the
believability of the person or the reliability of the tool
involves questioning when it was last validated and
calibrated in a similar context. Assessing the
credibility of the person providing information and
the trustworthiness of the tools used to obtain
information is imperative. Interrogating the methods
employed and the tools utilized in information
acquisition is essential for ensuring their reliability.
Gauging the truthfulness of the information and
its compatibility with a usable format forms a critical
component of the evaluation.
The significance and prioritization of these
questions vary depending on the context. The
applicability of these inquiries differs substantially,
whether in the domain of pharmacovigilance during
the clinical trial phase or the post-marketing phase.
Due to constraints of brevity, this discussion
primarily aims to provide an overarching perspective
on these concepts and elucidate how digital tools can
contribute to enhancing the safety of our endeavors.
4 MAIN CAUSES FOR ERRORS
IN THE DATA COLLECTION
AND REPORTING STAGE
During the data collection phase, various challenges
can significantly impact the reliability and
comprehensiveness of gathered information:
Underreporting is a prominent issue involving the
failure of healthcare professionals, patients, and
pharmaceutical companies to report adverse events
related to medications. This leads to incomplete
safety profiles and hinders the identification of risks.
Conversely, overreporting can occur, attributing
unrelated events to medication use, requiring
meticulous sorting to discern genuine concerns.
Factors such as ignorance, lethargy, complacency,
diffidence, insecurity, and the absence of feedback
contribute to underreporting.
Delays in reporting adverse events pose a serious
challenge, hindering the ability to take timely
corrective actions and assess the overall impact on
patient safety. Inconsistent and non-standardized
reporting practices across healthcare institutions,
regions, or countries complicate data collection and
analysis, impeding the identification of trends and
patterns in adverse events.
Patient selection bias arises when participants in
pharmacovigilance studies are not representative of
the general population taking the drug, potentially
skewing results. For instance, studies may include
only patients with specific medical conditions or
those taking the drug at high doses.
Poor data quality, including inaccuracies,
duplications, or missing data, undermines the
reliability of pharmacovigilance databases, leading to
erroneous conclusions. Incomplete information and
confounding factors, such as multiple medications or
underlying health conditions, complicate data
interpretation.
Effective communication between regulatory
agencies, pharmaceutical companies,
pharmacovigilance teams, healthcare providers, and
patients is crucial. Gaps in communication hinder the
timely exchange of safety information and
collaborative efforts to mitigate risks. Addressing
these challenges is essential for enhancing the
accuracy and utility of pharmacovigilance data.
4.1 Main Causes for Error in the Case
of Signal Detection in
Pharmacovigilance
Pharmacovigilance systems encounter various
challenges that can impede their effectiveness. One
crucial aspect is sensitivity, representing the proportion
of actual adverse drug reactions (ADRs) reported to the
system. A low sensitivity raises concerns, indicating
that a considerable number of ADRs may go
unreported. This limitation compromises the system's
ability to comprehensively capture and address
potential risks associated with medications.
Another significant challenge is the Low Positive
Predictive Value (PPV), which denotes the proportion
of reported ADRs truly caused by the drug. A low
PPV introduces noise and potential confusion into the
pharmacovigilance system, as a significant portion of
reported ADRs may not be directly attributable to the
drug in question.
The difficulty in detecting rare ADRs is also a
noteworthy challenge. Due to their infrequent
occurrence in a limited population, rare ADRs are
An Epistemological Approach to Risk Assessment in Pharmacovigilance and Mitigation Through Artificial Intelligence
33

often underreported, making it challenging for the
pharmacovigilance system to identify and address
these less common but potentially severe adverse
events.
Long-term ADRs pose a distinct challenge, as
their manifestations may be delayed for months or
even years. This delayed onset makes it difficult to
associate these ADRs with the medication, leading to
underreporting and hindering the timely
identification of such adverse events.
Cultural influences play a significant role in ADR
reporting practices. In Japan, a cultural norm
discourages complaining, extending to the reporting
of ADRs. This cultural inclination results in
reluctance among both patients and doctors to report
ADRs, contributing to underreporting in the country.
Similarly, traditional Chinese medicine reflects a
belief that side effects are inherent and necessary for
the medicine to be effective. This belief may
discourage patients from reporting ADRs, viewing
them as integral to the healing process. Cultural
variations also impact the types of ADRs reported,
with a preference for reporting skin-related ADRs in
Asian countries compared to liver-related ADRs in
Western countries.
These challenges underscore the intricate nature
of pharmacovigilance and emphasize the need for
nuanced strategies to effectively address them. A
comprehensive approach that considers cultural
factors, enhances sensitivity and PPV, and tackles the
difficulties in detecting rare and long-term ADRs is
essential for ensuring the robustness of
pharmacovigilance systems.
5 NEW TECHNOLOGY AND AI
TO HELP GET REDUCE THE
BIASES AND ERRORS
In addressing the challenges of measuring real risk in
decision-making, strategies include specific training
and hiring experts in risk assessment and
epistemology. This discussion focuses on using
digital solutions, emphasizing AI/ML's role in data
ingestion, including duplicate detection and anomaly
identification. Machine learning aids in detecting
ADRs, performing safety surveillance, and managing
signal detection, such as automating the classification
of first-person reports of ADRs in social media. It
offers advantages in detecting ADRs not captured by
medical professionals, processing data quickly, and
utilizing personal information in social media posts
related to ADRs.
Machine learning is also employed to classify
ADRs, determining the seriousness of patient cases
through different algorithms based on precision,
recall, and accuracy. Clinical trials, crucial for drug
approval, face structural limitations, and post-
marketing monitoring through AE reports in
pharmacovigilance is not error-proof due to biases
like underreporting, especially for rare events and
drug-drug interactions. Machine learning aids in
streamlining adverse event reports, comparing rule-
based queries and semi-supervised machine learning
against a reference standard.
In pharmacoepidemiology, ML predicts adverse
events, facilitating early quality assurance measures.
Its use in signal detection and analysis automates
processes, adapting to patients presenting with
multiple disease states, medications, and ADRs.
Institutions like Connecticut Children’s Medical
Center leverage machine learning to streamline
adverse event reports. Additionally, ML in
pharmacoepidemiology studies drug interactions in
real-life conditions, predicting adverse events
promptly for patient safety.
The utility of machine learning is underscored in
its application to screen and analyze voluminous
datasets of adverse event reports through
sophisticated algorithms and text mining. Specific
implementations include the development of
algorithms like "AwareDX," exhibiting the capacity
to predict sex-specific risks of adverse drug effects
with a remarkable degree of precision, and the
identification of targeted patient populations
vulnerable to specific toxicities. Machine learning
further aids in predicting drug side effects during
post-marketing surveillance, leveraging knowledge
extracted from literature to enhance the efficacy of
spontaneous reporting system methods.
Artificial intelligence makes significant strides in
integrating prediction uncertainties into patient safety
through the deployment of deep learning-based
computer-aided diagnosis, yielding more dependable
results in cases fraught with ambiguity. However, the
seamless integration of AI into existing
pharmacovigilance systems raises potential
challenges, potentially amplifying workload and
complexity. The judicious implementation of AI/ML
in PV is recommended, specifically when it
streamlines workload, simplifies complexity, or
optimizes budget allocation, enabling more effective
resource deployment for critical aspects ensuring
patient safety.
The integration of AI/ML into pharmacovigilance
encounters legal challenges in both Europe and the
United States, particularly concerning the liability for
IeHF 2023 - International e-Health Forum
34

errors arising from artificial intelligence technology.
Despite these legal impediments, the promising
potential of AI/ML in pharmacovigilance remains
evident, prompting a critical examination of how to
harness these technologies effectively to construct a
future fit for purpose. Establishing a seamlessly
connected system for the flow of inputs and outputs
across diverse data systems emerges as a critical
imperative. Such a system would not only foster an
interactive continual learning solution but also
enhance the understanding of the benefit–risk profiles
of medicines and vaccines. Additionally, it would
empower prescribers, patients, and other stakeholders
to obtain pertinent information and pose inquiries as
needed, thereby contributing to a more informed and
responsive healthcare ecosystem.
REFERENCES
Caster, O. (2018). Benefit-Risk Assessment in
Pharmacovigilance. In A. Bate (Ed.), Evidence-Based
Pharmacovigilance (Methods in Pharmacology and
Toxicology). Humana Press.
https://doi.org/10.1007/978-1-4939-8818-1_13
Coleman, T. S. (2012). Quantitative Risk Management: A
Practical Guide to Financial Risk, (B. Litterman,
Foreword).
Deng, L., & Chang, Y. (2022). Risk Management of
Investment Projects Based on Artificial Neural
Network. Wireless Communications and Mobile
Computing, 2022, Article ID 5606316, 13 pages.
https://doi.org/10.1155/2022/5606316
Huysentruyt, K., Kjoersvik, O., Dobracki, P., Savage, E.,
Mishalov, E., Cherry, M., Leonard, E., Taylor, R.,
Patel, B., & Abatemarco, D. (2021). Validating
Intelligent Automation Systems in Pharmacovigilance:
Insights from Good Manufacturing Practices. Drug
Safety, 44(3), 261-272. https://doi.org/10.1007/s40264-
020-01030-2
Pascarella, G., Rossi, M., Montella, E., Capasso, A., De
Feo, G., Botti, G., Nardone, A., Montuori, P., Triassi,
M., D'Auria, S., & Morabito, A. (2021, July 8). Risk
Analysis in Healthcare Organizations: Methodological
Framework and Critical Variables. Risk Management
and Healthcare Policy, 14, 2897-2911.
https://doi.org/10.2147/RMHP.S309098
Hamid, A. A. A., Rahim, R., & Teo, S. P. (2022).
Pharmacovigilance and Its Importance for Primary
Health Care Professionals. Korean Journal of Family
Medicine, 43(5), 290-295.
https://doi.org/10.4082/kjfm.21.0193
Expert Group on Clinical Trials. (2017, April 25). Risk
proportionate approaches in clinical trials:
Recommendations of the expert group on clinical trials
for the implementation of Regulation (EU) No
536/2014 on clinical trials on medicinal products for
human use.
Randeep, V. C., Divya, P., Susmita, A., Sushmitha, P.,
Ramya, Ch., & Chandini, K. (2022, December 12).
Automation in pharmacovigilance: Artificial
intelligence and machine learning for patient safety.
Journal of Innovations in Applied Pharmaceutical
Science (JIAPS), 7(3), 118-122.
https://doi.org/10.37022/jiaps.v7i3.37 Hillson, D.
(2023). The Risk Management Handbook: A Practical
Guide to Managing the Multiple Dimensions of Risk
(6th ed.). Routledge.
Ito, S., & Narukawa, M. (2022). Estimation of the Under-
Reporting of Suspected Serious Adverse Drug
Reactions in Japan Using An Interrupted Time Series
Analysis. Therapeutic Innovation & Regulatory
Science, 56(2), 358–365.
https://doi.org/10.1007/s43441-022-00379-z
García-Abeijon, P., Costa, C., Taracido, M., Herdeiro, M.
T., Torre, C., & Figueiras, A. (2023). Factors
Associated with Underreporting of Adverse Drug
Reactions by Health Care Professionals: A Systematic
Review Update. Drug Safety, 46(7), 625–636.
https://doi.org/10.1007/s40264-023-01302-7
Drukker, K., Chen, W., Gichoya, J., Gruszauskas, N.,
Kalpathy-Cramer, J., Koyejo, S., Myers, K., Sá, R.C.,
Sahiner, B., Whitney, H., Zhang, Z., & Giger, M.
(2023). Toward fairness in artificial intelligence for
medical image analysis: Identification and mitigation of
potential biases in the roadmap from data collection to
model deployment. Journal of Medical Imaging
(Bellingham), 10(6), 061104. doi:
10.1117/1.JMI.10.6.061104.
Sartori, D., Aronson, J. K., & Onakpoya, I. J. (2020).
Signals of adverse drug reactions communicated by
pharmacovigilance stakeholders: Protocol for a scoping
review of the global literature. Systematic Reviews,
9(1), 180. doi: 10.1186/s13643-020-01429-z.
Garcia-Agundez, A., García-Martín, E., & Eickhoff, C.
(2022, May 20). Editorial: The Potential of Machine
Learning in Pharmacogenetics, Pharmacogenomics and
Pharmacoepidemiology. Frontiers in Pharmacology,
13, 928527. https://doi.org/10.3389/fphar.2022.928527
Wewering, S., Pietsch, C., Sumner, M., Markó, K., Lülf-
Averhoff, A. T., & Baehrens, D. (2022, June). Machine
learning approach to identify adverse events in
scientific biomedical literature. Clinical and
Translational Science, 15(6), 1500-1506.
https://doi.org/10.1111/cts.13268
Yang, S., & Kar, S. (2023). Application of artificial
intelligence and machine learning in early detection of
adverse drug reactions (ADRs) and drug-induced
toxicity. Artificial Intelligence Chemistry, 1(2),
100011. https://doi.org/10.1016/j.aichem.2023.100011
Kim, H. R., Sung, M., Park, J. A., Jeong, K., Kim, H. H.,
Lee, S., & Park, Y. R. (2022). Analyzing adverse drug
reaction using statistical and machine learning
methods: A systematic review. Medicine (Baltimore),
101(25), e29387.
https://doi.org/10.1097/MD.0000000000029387
Lee, J. E., Kim, J. H., Bae, J. H., et al. (2022). Detecting
early safety signals of infliximab using machine
An Epistemological Approach to Risk Assessment in Pharmacovigilance and Mitigation Through Artificial Intelligence
35

learning algorithms in the Korea adverse event
reporting system. Scientific Reports, 12, 14869.
https://doi.org/10.1038/s41598-022-18522-z
IeHF 2023 - International e-Health Forum
36
