
The Antidiabetic Effect of Black Glutinous Tapai Beverage in
Metabolic Syndrome Rats
Rahmi Khoirunnisa
1
, Wachid Putranto
2
and Ida Nurwati
3
1
Department of Nutrition Sciences, School of Postgraduate, Sebelas Maret University, Surakarta, Indonesia
2
Department of Internal Medicine, Faculty of Medicine, Sebelas Maret University, Surakarta, Indonesia
3
Department of Biochemistry, Faculty of Medicine, Sebelas Maret University, Surakarta, Indonesia
Keywords: Black Glutinous Tapai Beverage, Antidiabetic, Blood Glucose, Metabolic Syndrome.
Abstract: Metabolic syndrome (MetS) known as insulin resistance syndrome, is recognized as a risk factor for type 2
diabetes mellitus. Management of MetS involves a combination of lifestyle changes and pharmacological
interventions. Black glutinous tapai beverage (BGTB) as local food contains Lactobacillus spp., which can
be a potential source of probiotics to improve hyperglycemia. This study aimed to determine the efficacy of
BGTB as an antidiabetic in rat models of MetS. This study used 30 male Sprague Dawley rats which were
divided into five groups, one as a normal group (N) given a standard diet, and the other four groups were
given a high-fat diet for 2 weeks and induced by Streptozotocin (STZ) and Nicotinamide (NA), i.e. negative
control (KN) was given a standard diet, positive control (KP) was given metformin 9 mg, treatment 1 (P1)
and treatment 2 (P2) were given BGTB 0,9 ml 200 gr-1 rat BW and 1,8 ml 200 gr-1 rat BW, respectively for
four weeks. The result showed a significant difference (p=0.000) in blood glucose and HbA1c after giving
BGTB. This study showed that BGTB has the potential to be developed as a functional beverage and source
of probiotics for people with MetS.
1 INTRODUCTION
Metabolic syndrome (MetS), known as insulin
resistance syndrome, is recognized as one of the risk
factors for cardiovascular disease (CVD) and type 2
diabetes mellitus (T2DM). Obesity and insulin
resistance are considered significant factors in the
development of MetS (Limanan & Prijanti, 2013;
Rochlani et al., 2017; Srikanthan et al., 2016). The
prevalence of MetS in Indonesia is 28% in men and
46.2% in women. In Indonesia, hyperglycemia (51%)
is the second component of MetS after hypertension
(61,0%) (Sigit et al., 2020).
MetS management involves a dual approach,
combining lifestyle changes and pharmacological
interventions. Emphasis on environmental and
lifestyle factors such as excessive calorie
consumption, lack of fiber intake, and low physical
activity as major contributors to MetS (PERKENI,
2021; Rochlani et al., 2017). There is no single
treatment for MetS, requiring several types of drugs
and a long period to improve each component. The
development of functional and nutraceutical foods
can be used to limit drug use and minimize side
effects (Ayivi et al., 2020; Rochlani et al., 2017).
Functional foods are intended to reduce the risk
of, slow down, or prevent certain diseases and
improve immunity, and are not medicines or dietary
supplements (Goetzke & Spiller, 2014). Black
glutinous tapai is known as a functional food
obtained from the fermentation process of black
glutinous rice. Black glutinous rice contains high
fiber, anthocyanins, phenols, and antioxidant
activity which can be an alternative healthy snack
for people with dyslipidemia, MetS, and
constipation. Consumption of black glutinous tapai
>11.5 grams per day, has a protective benefit against
MetS by 16 times compared to individuals who
consume <11.5 grams (Fauziyah, 2018; Yulianto,
2022).
The International Scientific Association for
Probiotics and Prebiotics (ISAPP) explained that
some types of fermented foods contain probiotics
that can benefit health (Marco et al., 2021).
Probiotics are microorganisms in the form of
bacteria that are given in sufficient doses to provide
health benefits to their hosts (Scavuzzi et al., 2014).
Khoirunnisa, R., Putranto, W. and Nurwati, I.
The Antidiabetic Effect of Black Glutinous Tapai Beverage in Metabolic Syndrome Rats.
DOI: 10.5220/0012900900004564
In Proceedings of the 5th International Conference on Social Determinants of Health (ICSDH 2023), pages 75-81
ISBN: 978-989-758-727-6; ISSN: 2975-8297
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
75

Black glutinous tapai, in previous studies, was
studied to have several lactic acid bacteria, such as
Lactobacillus fermentum 1 BK 2-5 which have the
potential to become probiotic candidates in black
glutinous tapai (Panjaitan, 2018).
It was mentioned that the water content formed
from the fermentation of black sticky rice reached
55.18% on the 3rd day of fermentation (Fauziyah,
2018). This water content is referred to as black
glutinous tapai beverage. Research on utilizing
black glutinous rice juice against MetS conditions
currently does not exist.
In previous studies, BGTB contained 3x10
9
Lactobacillus spp.,. Some types of Lactobacillus can
suppress fasting and postprandial blood glucose. In
addition, it can help activate insulin signaling for
glucose absorption (Bobga et al., 2022; Choi et al.,
2020; Yadav et al., 2018). Black glutinous rice is a
good probiotic carrier because it does not need to go
through another processing process that can reduce
the amount of probiotics before consumption
(Nuraida, 2015). In addition, the deep purple color of
BGTB indicates the presence of anthocyanins have
function as a prebiotic. Black sticky rice contains 257
ppm of anthocyanins (Fauziyah & Pardina, 2020).
Anthocyanins can function as a prebiotic which can
significantly increase the number of Lactobacillus
spp., (Wang et al., 2020; Zhu, 2018).
This study aims to see if black glutinous tapai
beverage can be an alternative functional food for
antidiabetics and patients with MetS.
2 MATERIAL AND METHOD
2.1 Production of BGTB
Making BGTB begins with separating black
glutinous rice from foreign objects and washing it.
Black glutinous rice is then soaked overnight or for
approximately 8 hours. Subsequently, black glutinous
rice is steamed for 1 hour. After that, prepare hot
water that has just been brought to a boil in a ratio of
1:1.2, then put the steamed black glutinous rice into
the boiling water and stir again until evenly
distributed. Cover the pot, and let stand for 30
minutes. Prepare the steaming pot again, then steam
the black sticky rice for ± 1 hour until cooked.
Remove and cool the black glutinous rice. After
cooling, mix the sticky rice with the mashed yeast,
then stir until smooth. Store the tapai in a closed
container and let it sit for ± 3 days. After standing for
3 days at room temperature, the black glutinous rice
is squeezed and the black glutinous rice liquid is
taken, which we call BGTB, and stored in a sterilized
glass bottle.
2.2 Induction of Test Animal
Thirty male Sprague Dawley rats were weighed with
an average weight of 200 grams. For 2 weeks, rats
were given HFD and distilled water ad libitum.
After that, rats will be induced using STZ + NA. The
STZ dose was dissolved in citrate buffer (pH 4.5)
and nicotinamide was dissolved in normal
physiological saline. Rats were induced with
nicotinamide (110 mg/kg bw) in the intraperitoneal
section and 15 minutes later induced with STZ (45
mg/kg bw).
Samples that met the criteria for metabolic
syndrome based on NCEP-ATP III (FBG (>250
mg/dL), triglyceride (≥150 mg/dL), and HDL (<40
mg/dL) levels) were considered to have metabolic
syndrome and were used as experimental animals
(Aydin et al., 2014). This research has received
ethical clearance approval from KEPK FK UNS
(61/UN27.06.11/KEP/EC/2023).
2.3 Testing the Antidiabetic Activity of
Black Glutinous Tapai Beverage
This study was a laboratory experimental study,
using a randomized pretest and posttest-controlled
group design. Divided into five groups of 6 rats, one
group as a normal group and four MetS groups:
● KN: Normal (without treatment)
● K-: MetS (without treatment)
● K+: Administration of metformin 9 mg/kg BW
● P1: Administration of 0.9 mL BGTB
● P2: Administration of 1.8 mL BGTB
2.4 Measurement of Blood Glucose
Levels by GOD-PAP
Blood glucose measurements (FBG and PBG) were
conducted before and after treatment for 28 days.
Qualitative measurements used the Enzymatic
calorimetric Test GOD-PAP method. Glucose in the
sample was oxidized to form gluconic acid and
hydrogen peroxide. Hydrogen peroxide 4-
Aminoatypirene with phenol indicator was
catalyzed with POD to form quinonimine and water.
Serum (sample) was obtained from blood
centrifuged at 3000 rpm for 10 minutes, 10 µl of
serum was taken and mixed with 1000 µl of standard
sugar, then incubated at 37 C or 10 minutes.
Absorbance was measured by comparing the results
ICSDH 2023 - The International Conference on Social Determinants of Health
76
of the test solution with standard glucose levels at a
wavelength of 500 nm with a UV-Vis
spectrophotometer (Subiyono et al., 2016). Blood
glucose measurements (FBG and PBG) were
conducted before and after treatment for 28 days.
Qualitative measurements used the Enzymatic
calorimetric Test GOD-PAP method. Glucose in the
sample was oxidized to form gluconic acid and
hydrogen peroxide. Hydrogen peroxide 4-
Aminoatypirene with phenol indicator was
catalyzed with POD to form quinonimine and water.
Serum (sample) was obtained from blood
centrifuged at 3000 rpm for 10 minutes, 10 µl of
serum was taken and mixed with 1000 µl of standard
sugar, then incubated at 37
°
C or 10 minutes.
Absorbance was measured by comparing the results
of the test solution with standard glucose levels at a
wavelength of 500 nm with a UV-Vis
spectrophotometer (Subiyono et al., 2016).
2.5 Measurement of HbA1c by Elisa
Method
Measurement of HbA1c levels was carried out after
28 days of treatment, using the sandwich enzyme
immunoassay technique. First, the plate was washed
with wash buffer 2 times. Then 100 μl of standard,
sample, and control were added into each well,
sealed, and incubated for 90 minutes at 37oC. The
plate was washed with wash buffer 2 times without
soaking. 100 μl biotin solution was added into each
well and incubated for 60 minutes at 37oC. Washed
the plate with wash buffer 3 times soaking for 1
minute each time. Then, 100 μl of SABC solution was
added into the wells and incubated for 30 minutes at
370. After that, wash the plate again by using wash
buffer 5 times and soak it for 1 minute for each wash.
add 90 μl TMB substrate in each well and incubate
for 10-20 minutes at 37
o
C. Added 50 μl stop solution
in each well. Read the absorption with a microplate
reader at a wavelength of 450 nm and calculated the
levels (Triyono, 2016).
2.6 Data Analyze
Data were analyzed using the One-way ANOVA test
and continued using the Post Hoc Tukey HSD test.
This test is used to determine the impact of giving
black glutinous rice juice on FBG, PBG, and HbA1c
levels. If the data is not normally distributed, it will
be continued with the Kruskal Wallis non-parametric
test and then continued using the Games-Howell test.
3 RESULT
As the result shown in Table 1, both FBG and PBG in
the K+, P1, and P2 groups decreased, with the highest
decrease in the P2 group. Based on the
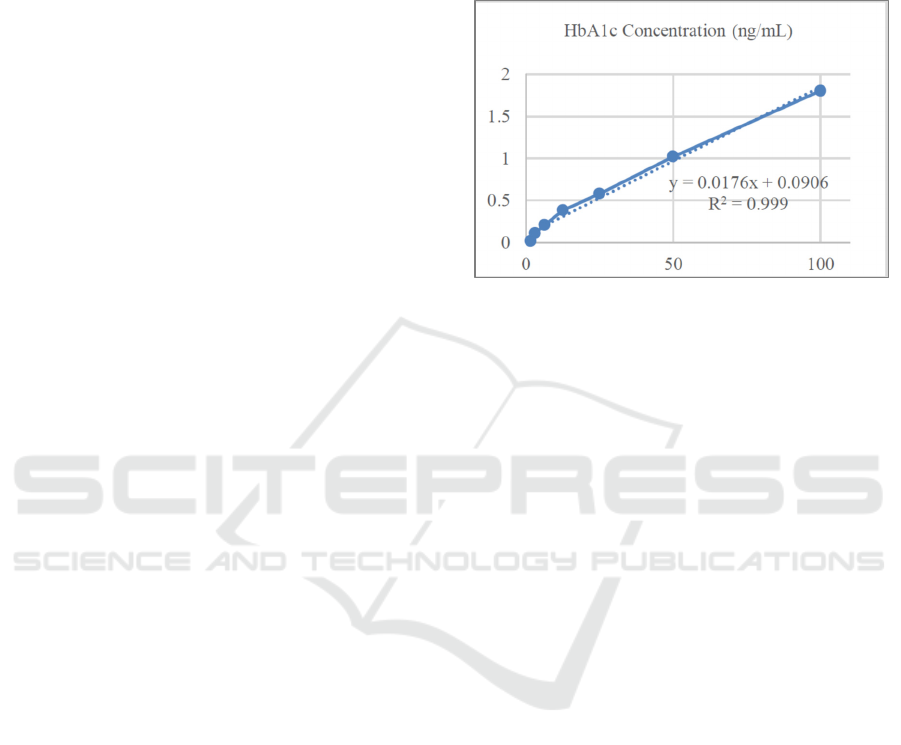
Figure 1: Standard Curve HbA1c.
Kruskall-Walis statistical test, there were differences
in each group (p < 0.05). Followed by Games-Howell
post hoc test shows no difference in influence
between groups K+ and P2.
The HbA1C level was calculated by substituting
the absorbance value (y) of the sample at 450 nm
wavelength into the logarithm regression equation y
= (equals to) ax + b, which was obtained from the
standard HbA1C curve so that the HbA1C
concentration value (x) was obtained. Based on the
Kruskal Walis test, black glutinous rice juice had a
significant effect on HbA1c levels between groups.
Post Hoc Tukey HSD test showed a significant
difference (P < 0.05) between doses of intervention
groups. From the difference in comparison with the
K- group in the intervention group, the P2 group had
a better effect of 55.28%.
4 DISCUSSION
The high-fat diet (HFD) formula adds a fat
component of up to 40%-50% of total calories for 4-
8 weeks (Gao & Zheng, 2014). The HFD is made by
the modern human diet which is currently found to be
higher in fat than the recommended diet (20%-25%)
(KEMENKES RI, 2019). The characteristics
obtained from HFD are obesity, impaired glucose
tolerance, and insulin resistance (Husna et al., 2019).
High levels of fat in the blood affect the ability of
insulin receptors and cause the expression of glucose
transporter type 4 (GLUT 4) to decrease. The
The Antidiabetic Effect of Black Glutinous Tapai Beverage in Metabolic Syndrome Rats
77

decrease in GLUT 4 expression causes glucose
transportation into the cell membrane to be disrupted,
decreasing glucose transport activity. Therefore,
HFD can cause high blood glucose levels (Rahmawati
et al., 2017).
Blood glucose levels after intervention in KN and
K-groups increased by 1.89% and 0.4%, respectively.
The increase in FBG in the normal group can be
caused by the age of the rats entering early adulthood
(Nurmawati, 2017), which was 14 weeks old at the
end of the intervention. Physiologically, increasing
age causes a decrease in the function of pancreatic β-
cells (Setiyorini & Wulandari, 2017). Along with age,
the ability of pancreatic β-cells will weaken until they
are damaged, which triggers hyperglycemia
(Rahmawati et al., 2017).
The increase in FBG in the K- group may be due
to the administration of HFD for two weeks and
STZ+NA induction without BGTB administration.
Furthermore, the results of this study showed that the
average FBG levels in K+, P1, and P2 experienced a
significant decrease after the administration of BGTB
(p<0.05). For the FBG pre-test and post-test from the
analysis results using paired t-test, treatment groups
K +, P1, and P2 showed the effect of BGTB
administration. Similar results of research on rice
bran fermented using Lactobacillus fermentum
MF423, it is known to improve hyperglycemia
conditions in vitro and in vivo and increase the
antioxidant capacity of diabetic rats (Ai et al., 2021).
In addition, it was found that L. rhamnosus BSL and
L. rhamnosus R23 have potential as probiotic foods
and are promising agents for managing T2DM (Eko
Farida et al., 2020). The Lactobacillus bacteria in
BGTB can help maintain the gut microbiota. This
effect inhibits the transfer of bacterial endotoxins into
the bloodstream and reduces circulating
lipopolysaccharides and proinflammatory cytokines,
which in turn decreases inflammation resulting in
decreased insulin resistance and controlled blood
glucose levels (Hindri et al., 2020).
Some LAB strains were reported to inhibit the
enzyme α-glucosidase and have antioxidant activity.
Inhibition of this enzyme will reduce glucose
absorption and thus reduce blood glucose levels. α-
glucosidase is an enzyme in the filamentous
peripheral membrane that catalyzes the process of
carbohydrate digestion, and inhibiting the activity of
α-glucosidase has been demonstrated to decrease
glucose absorption and reduce blood glucose levels
(E. Farida & Jennie, 2019). Inhibition of the enzyme
α-glucosidase is one of the therapeutic approaches to
reduce PBG levels because by inhibiting the enzyme
α-glucosidase, it can delay the breakdown of
oligosaccharides and disaccharides into
monosaccharides so that compounds that can inhibit
the work of the enzyme α-glucosidase can be used as
oral drugs for patients with type 2 diabetes (Febrinda
et al., 2013).
Table 1: Blood Glucose on rats with different treatments.
Blood
Glucose
Treatment Group
KN
K
- K+ P1 P2
Fasting (mg/dL)
Pre-test
69.08±1.77
270.66
±2.18
269.70
±2.37
269.76
±4.11
267.39
±2.16
Post-test
70.39±1.52
271.79
±2.39
96.82
±4.04
126.37
±5.13
88.46
±2.97
Δ Mean±SD*
1.31±-0.25
a
1.13±0.21
a
-172.88
±1.67
b
-143.39
±1.02
c
-178.93
±0.81
b
p
pre-
p
ost** 0.000 0.001 0.262 0.782 0.462
Postprandial (mg/dL)
Pre-test
88.23±1.37
285.30
±1.98
284.06
±2.54
284.06
±3.49
283.84
±2.72
Post-test
92.49±2.12
293.65
±2.43
117.15
±3.85
142.41
±5.13
117.27
±13.02
Δ Mean±SD*
4.26±0.75
a
8.35±0,45
b
-166.9
1±1.31
c
-141.65
±1,64
d
-166,57
±10,3
c
p
p
re-
p
ost** 0.000 0.028 0.028 0.000 0.028
*Delta value (different mean between pre and post-test): positive
number indicated an increase and negative indicated a decrease.
**p<0.05 in each column indicated a significant difference
between pre and post-test.
a,b,c,d
)Numbers followed by the same letter indicate no significant
difference
Probiotics produce short-chain fatty acid (SCFA),
namely propionic acid, which can inhibit
gluconeogenesis in the liver to suppress glucose
production and reduce insulin resistance (Bishehsari
et al., 2018). Probiotics can act as inhibitors of the
enzyme α-glucosidase found in intestinal microfili so
that they can cause a decrease in blood glucose levels
(Gomes et al., 2014). A meta-analysis showed that
consuming probiotics can improve glucose
metabolism at a modest level (Zhang et al., 2016).
From the results of Hindri's research, it is stated that
the administration of Lactobacillus fermentum can
reduce blood glucose levels, and HbA1c (Hartini,
2016).
HbA1c levels were compared in each group, from
the results of Kruskal Walis there were differences in
each treatment group (p<0.05). Followed by the
Howell games test, there was an effect of BGTB
administration on HbA1c. In both FBG and PBG,
there was no difference in the K+ and P2 groups,
where 1.8 ml BGTB had similarities with metformin
in reducing blood glucose levels. The improvement
of MetS conditions by metformin is associated with
increased GLP-1 expression, AMPK activation, and
improved gut microbiota composition so it will have
an impact on increasing insulin sensitivity and
decreasing the process of lipolysis (Wang et al.,
2020).
ICSDH 2023 - The International Conference on Social Determinants of Health
78

The administration of BGTB was more effective
in reducing blood glucose levels presumably due to
the role of anthocyanins as antioxidants. Antioxidants
capture free radicals and reduce inflammation by
suppressing TNF-α production. Decreased TNF-α
production can improve insulin sensitivity through
increased autophosphorylation of insulin receptors,
increased tyrosine kinase activity on insulin
receptors, and increased GLUT-4 expression.
Increased GLUT-4 expression causes the
transportation of blood glucose to the cell membrane
to increase and blood glucose levels to decrease.
Anthocyanins as antidiabetics are also able to activate
AMPK in adipose tissue, muscle, and liver. Tasked
with maintaining energy balance in cells, AMPK will
suppress glyconeogenesis and lipolysis by
phosphorylating acetyl-CoA carboxylase and
AcetylCoA Oxidase, thus preventing glucose
formation in the liver and FFA formation and
increasing insulin sensitivity (Herrera-balandrano et
al., 2021; Takikawa et al., 2010). In addition, it can
also increase GLUT-4 (Glucose Transporter type 4),
so that glucose uptake into cells will increase (Ifadah
et al., 2021).
5 CONCLUSION
Black glutinous tapai beverage contains
Lactobacillus sp., and anthocyanins, which had been
demonstrated to have an antidiabetic effect. Our study
showed that BGTB could improve blood glucose
(FBG and PBG) and HbA1c of metabolic syndrome
rats. These effects might be beneficial to further
extend the application of BGTB as a potential
probiotic and prebiotic for metabolic syndrome.
ACKNOWLEDGEMENTS
The authors would like to thank everyone who
accompanied the steps in writing this article. Also,
they highly appreciate the Department of Nutrition
Science at Sebelas Maret University and the UGM
PSPG Laboratory staff for assisting the authors in
completing this research.
REFERENCES
Ai, X., Wu, C., Yin, T., Zhur, O., Liu, C., Yan, X., Yi, C.
P., Liu, D., Xiao, L., Li, W., Xie, B., & He, H. (2021).
Antidiabetic Function of Lactobacillus fermentum
MF423-Fermented Rice Bran and Its Effect on Gut
Microbiota Structure in Type 2 Diabetic Mice.
Frontiers in Microbiology, 12(June), 1–14.
https://doi.org/10.3389/fmicb.2021.682290
Ayivi, R. D., Gyawali, R., Krastanov, A., Aljaloud, S. O.,
Worku, M., Tahergorabi, R., Claro, R., & Ibrahim, S.
A. (2020). Lactic Acid Bacteria : Food Safety and
Human Health Applications. 202–232.
Bishehsari, F., Engen, P. A., Preite, N. Z., E.Tuncil, Y.,
Naqib, A., Shaikh, M., Rossi, M., Wilber, S., J.Green,
S., Hamaker, B. R., Khazaie, K., F, T. M. V. C. B., &
Keshavaezian, A. (2018). Dietary Fiber Treatment
Corrects the Composition of Gut Microbiota, Promotes
SCFA Production, and Suppresses Colon
Carcinogenesis. Mdpi.
https://doi.org/10.3390/genes9020102
Bobga, P. T., Fossi, B. T., Taiwe, G. S., Nkanpira, K. T.,
Ebote, N., Ngwa, F. A., Laure, L., Tatsinkou, T.,
Yuwong, W. B., & Ndip, L. M. (2022). Evaluation of
the Anti-Diabetic Potential of Probiotic Lactobacillus
fermentum ( PRI 29 ) Isolated from Cameroonian
Fermented Cow Milk in Alloxan Induced Diabetes
Type-1 Mice Model. 3362, 381–393.
https://doi.org/10.36348/sjpm.2022.v07i10.001
Choi, J., Lee, S., & Park, Y. (2020). Anti-diabetic Effect of
Mulberry Leaf Extract Fermented with Lactobacillus
Plantarum. 52(2).
Farida, E., & Jennie, B. (2019). Aktivitas Antioksidan Dan
Penghambatan Α-Glukosidase Oleh Ekstrak Etanol
Bakteri Asam Laktat Indigenus. Jurnal Teknologi Dan
Industri Pangan, 30(1), 56–63.
https://doi.org/10.6066/jtip.2019.30.1.56
Farida, Eko, Nuraida, L., Giriwono, P. E., & Jenie, B. S. L.
(2020). Lactobacillus rhamnosus Reduces Blood
Glucose Level through Downregulation of
Gluconeogenesis Gene Expression in Streptozotocin-
Induced Diabetic Rats. International Journal of Food
Science, 2020. https://doi.org/10.1155/2020/6108575
Fauziyah, N. (2018). Makanan Fungsional Tape Ketan
Hitam Mencegah Sindroma Metabolik (G. P. E.
Mulyono & Surmita (eds.); Pertama). Poltekkes
Kemenkes Bandung.
Fauziyah, N., & Pardina, S. F. (2020). Makanan Fungsional
Tape Ketan Hitam Efektif Menurunkan Kolesterol Total
(G. P. E. Mulyo & Surmita (eds.); Pertama). Poltekkes
Kemenkes Bandung.
Febrinda, A. E., Astawan, M., Wresdiyati, T., & Dewi
Yuliana, N. (2013). Kapasitas Antioksidan Dan
Inhibitor Alfa Glukosidase Ekstrak Umbi Bawang
Dayak. Jurnal Teknologi Dan Industri Pangan, 24(2),
161–167. https://doi.org/10.6066/jtip.2013.24.2.161
Gao, F., & Zheng, Z. M. (2014). Animal Models of Diabetic
Neuropathic Pain. 100–106.
Goetzke, B. I., & Spiller, A. (2014). Health-improving
Lifestyles of Organic and Functional Food Consumers.
British Food Journal, 116(3), 510–526.
https://doi.org/10.1108/BFJ-03-2012-0073
Gomes, A. C., Bueno, A. A., De Souza, R. G. M. H., &
Mota, J. F. (2014). Gut microbiota, probiotics and
The Antidiabetic Effect of Black Glutinous Tapai Beverage in Metabolic Syndrome Rats
79

diabetes. Nutrition Journal, 13(1).
https://doi.org/10.1186/1475-2891-13-60
Hartini, S. (2016). Hubungan HBA1c Terhadap Kadar
Glukosa Darah Pada Penderita Diabetes Mellitus Di
RSUD. Abdul Wahab Syahranie Samarinda Tahun
2016. Jurnal Husada Mahakam, IV(3), 171–180.
Herrera-balandrano, D. D., Chai, Z., Hutabarat, R. P., Beta,
T., Feng, J., Ma, K., Li, D., & Huang, W. (2021). Redox
Biology Hypoglycemic and hypolipidemic effects of
blueberry anthocyanins by AMPK activation : In vitro
and in vivo studies. Redox Biology, 46, 102100.
https://doi.org/10.1016/j.redox.2021.102100
Hindri, S., Jansen, S., Urip, H., & D, S. (2020). Antidiabetic
Activity of Lactobacillus fermentum Bacteria from
Dengke Naniura Goldfish (Cyprinuscarpio) in
Nicotinamide- Streptozotosin Induced Rats. Asian
Journal of Pharmaceutical Research and Development,
8(6), 77–80.
Husna, F., Suyatna, F. D., Arozal, W., & Purwaningsih, E.
H. (2019). Model Hewan Coba pada Penelitian
Diabetes Animal Model in Diabetes Research. 6(3),
131–141.
Ifadah, R. A., Rizkia, P., Wiratara, W., & Anam, C. (2021).
Ulasan Ilmiah : Antosianin dan Manfaatnya untuk
Kesehatan. 3(2), 11–21.
Limanan, D., & Prijanti, A. R. (2013). Hantaran Sinyal
Leptin dan Obesitas: Hubungannya dengan Penyakit
Kardiovaskuler. EJournal Kedokteran Indonesia, 1(2).
https://doi.org/10.23886/ejki.1.2063.144-155
Marco, M. L., Sanders, M. E., & Gänzle, M. (2021). The
International Scientific Association for Probiotics and
Prebiotics (ISAPP) consensus statement on fermented
foods. Nature Reviews Gastroenterology &
Hepatology, 18(March).
https://doi.org/10.1038/s41575-020-00390-5
Nuraida, L. (2015). A review: Health promoting lactic acid
bacteria in traditional Indonesian fermented foods.
Food Science and Human Wellness, 4(2), 47–55.
https://doi.org/10.1016/j.fshw.2015.06.001
Nurmawati, T. (2017). Studi Respon Fisiologis dan Kadar
Gula Darah pada Tikus Putih (Rattus Norvegicus) yang
Terpapar Streptozotocin (STZ). Jurnal Ners Dan
Kebidanan (Journal of Ners and Midwifery), 4(3), 244–
247. https://doi.org/10.26699/jnk.v4i3.art.p244-247
Panjaitan, R. et al. (2018). Seleksi Isolat Bakteri Asam
Laktat Asal Tempe Dan Tape Sebagai Kandidat
Probiotik. Jurnal Teknologi Dan Industri Pangan,
29(2), 175–184.
https://doi.org/10.6066/jtip.2018.29.2.175
PERKENI. (2021). Pedoman: Pengelolaan dan
Pencegahan Diabetes Melitus Tipe 2 Dewasa di
Indonesia (2021st ed.). PB PERKENI.
Rahmawati, F. C., Djamiatun, K., & Suci, N. (2017).
Pengaruh Yogurt Sinbiotik Pisang Terhadap Kadar
Glukosa dan Insulin Tikus Sindrom Metabolik. Jurnal
Gizi Klinik Indonesia, 14(1), 10.
https://doi.org/10.22146/ijcn.19379
RI, K. (2019). Profil Kesehatan Indonesia 2018.
https://doi.org/10.1136/jcp.40.5.591-b
Rochlani, Y., Pothineni, N. V., Kovelamudi, S., & Metha,
J. L. (2017). Metabolic syndrome : pathophysiology ,
management , and modulation by natural compounds.
SAGE Journals, 11(8), 215–225.
https://doi.org/10.1177/1753944717711379
Scavuzzi, B. M. S., Herrique, F., Miglioranza, L. H.,
Colado, S. A. N., & Dichi, I. (2014). Impact of
Prebiotics , Probiotics and Synbiotics on Components
of the Metabolic Syndrome. Annals of Nutritional
Disorders and Therapy, September.
Setiyorini, E., & Wulandari, N. A. (2017). Hubungan Status
Nutrisi dengan Kualitas Hidup pada Lansia Penderita
Diabetes Mellitus Tipe 2 yang Berobat di Poli Penyakit
dalam RSD Mardi Waluyo Blitar. Jurnal Ners Dan
Kebidanan (Journal of Ners and Midwifery), 4(2), 125–
133. https://doi.org/10.26699/jnk.v4i2.art.p125-133
Sigit, F. S., Tahapary, D. L., Trompet, S., Sartono, E., &
Dijk, K. W. Van. (2020). The prevalence of metabolic
syndrome and its association with body fat distribution
in middle ‑ aged individuals from Indonesia and the
Netherlands : a cross ‑ sectional analysis of two
population ‑ based studies. Diabetology & Metabolic
Syndrome, 1–11. https://doi.org/10.1186/s13098-019-
0503-1
Srikanthan, K., Feyh, A., Visweshwar, H., Shapiro, J. I., &
Sodhi, K. (2016). Systematic Review of Metabolic
Syndrome Biomarkers : A Panel for Early Detection ,
Management , and Risk Stratification in the West
Virginian Population. International Journal of Medical
Science, 13(1), 25–38.
https://doi.org/10.7150/ijms.13800
Subiyono, Martsiningsih, M. A., & Gabrela, D. (2016).
Gambaran Kadar Glukosa Darah Metode GOD-PAP (
Glucose Oxsidase – Peroxidase Aminoantypirin )
Sampel Serum dan Plasma EDTA ( Ethylen Diamin
Terta Acetat ). 5(1), 5–8.
Takikawa, M., Inoue, S., Horio, F., & Tsuda, T. (2010).
Dietary Anthocyanin-Rich Bilberry Extract
Ameliorates Hyperglycemia and Insulin Sensitivity via
Activation of AMP-Activated Protein Kinase in
Diabetic Mice 1 – 3. 527–533.
https://doi.org/10.3945/jn.109.118216.Berry
Triyono. (2016). Aktivitas Ekstrak Etalonik Daun
Kangkung Darat ( Ipomoea reptans Poir.) Terstandar
Sebagai Antihiperglikemia terhadap Kadar HbA1c dan
Kadar ALT pada Tikus Jantan Galur Wistar yang
Diinduksi Streptozotosin.
Wang, P., Deng, X., Zhang, C., & Yuan, H. (2020). Gut
microbiota and metabolic syndrome. 0(7).
https://doi.org/10.1097/CM9.0000000000000696
Yadav, R., Dey, D. K., Vij, R., Meena, S., Kapila, R., &
Kapila, S. (2018). Microbial Pathogenesis Evaluation
of anti-diabetic attributes of Lactobacillus rhamnosus
MTCC : 5957 , Lactobacillus rhamnosus MTCC : 5897
and Lactobacillus fermentum MTCC : 5898 in
streptozotocin induced diabetic rats. Microbial
Pthogenesis, 125(October), 454–462.
https://doi.org/10.1016/j.micpath.2018.10.015
Yulianto, W. A. (2022). The Potential of Glutinous Rice
Tape Added with Lactobacillus plantarum DAD-13 and
ICSDH 2023 - The International Conference on Social Determinants of Health
80

Saccharomyces BoulardII as a Probiotic Food. Journal
of Funtional Food and Nutraceutical, 4(1), 57–66.
https://doi.org/10.33555/jffn.v4i1.96
Zhang, Q., Wu, Y., & Fei, X. (2016). Effect of probiotics
on glucose metabolism in patients with type 2 diabetes
mellitus: A meta-analysis of randomized controlled
trials. Medicina (Lithuania), 52(1), 28–34.
https://doi.org/10.1016/j.medici.2015.11.008
Zhu, Y. (2018). Metabolism and prebiotics activity of
anthocyanins from black rice (Oryza sativa L.) in vitro.
Plos One.
The Antidiabetic Effect of Black Glutinous Tapai Beverage in Metabolic Syndrome Rats
81
