
Technology Support System and Review Process for a Decentralized
Clinical Trial: Trials@Home, RADIAL DCT as Case Study
Sten Hanke
1 a
, Dimitrios Giannikopoulos
3
, Hannes Hilberger
1,2 b
, Theresa Weitlaner
1 c
and Bernhard Neumayer
1 d
1
Institute of eHealth, University of Applied Sciences - FH Joanneum, Alte Poststraße 149, Graz, Austria
2
GSRC, Division of Medical Physics and Biophysics, Medical University of Graz, Graz, Austria
3
BAYER AG, Wuppertal, Germany
Keywords:
Helpdesk, Technology Support System, Knowledge Base, Decentralised Clinical Trials.
Abstract:
Decentralized Clinical Trials (DCTs) revolutionize clinical research by leveraging digital technologies to de-
centralize various aspects inherent in the traditional clinical trial process like the need for patients’ physical
presence. DCTs integrate virtual and remote elements for assessments, data collection, and monitoring, pri-
oritizing convenience. However, the integration of diverse stakeholders and technologies poses challenges in
delivering timely and effective solutions across all trial sites. Addressing this requires the establishment of a
robust technology support system tailored to meet the unique demands of decentralization. This paper out-
lines the requirements for such a system and shares initial insights gained through the learning process. This
system combines a Wiki-like knowledge base with a ticketing system for handling support requests, enabling
the creation of topic-specific tickets and ensuring that queries are directed to the appropriate support agents
swiftly. The implemented helpdesk system in the RADIAL study exemplifies how combining a comprehen-
sive information resource with a responsive ticketing system not only streamlines supporting processes but
also significantly enhances response efficiency and the overall user experience in DCTs. This integrated ap-
proach is pivotal in managing the complexities and dynamic nature of DCTs, ensuring that both patients and
stakeholders benefit from the efficiency and adaptability of decentralized trials.
1 INTRODUCTION
Decentralized clinical trials (DCT) aim to elimi-
nate the need for study patients to be physically
present at the study site for investigations, contribut-
ing to increased efficiency, accessibility, and patient-
friendliness in clinical research. As technology ad-
vances, decentralized approaches are expected to play
a more significant role in the future of clinical trials.
This approach broadens the pool of potential pa-
tients (de Jong et al., 2022), enhances patient adher-
ence (Jain et al., 2022), and reduces the study’s carbon
footprint (Subaiya et al., 2011; Holmner et al., 2014).
While these advancements are promising, the re-
duced on-site presence necessitates a robust support
system. This system should provide a continuously
a
https://orcid.org/0000-0003-3833-4252
b
https://orcid.org/0000-0003-3867-0651
c
https://orcid.org/0009-0005-7076-2929
d
https://orcid.org/0000-0002-4439-4799
accessible knowledge base and direct communica-
tion channels with professionals, ensuring stakehold-
ers are well-informed, up-to-date, and equipped to
handle hardware or other technical issues effectively.
Effective communication and tracking of techni-
cal and process issues are crucial for quality con-
trol. Integrating the technology provider’s support
in a timely and effective manner—from patients to
sites to Clinical Research Associates (CRAs) and ven-
dors—is imperative.
Although similar demands exist in conventional
clinical trials, DCTs introduce additional chal-
lenges due to the integration of different technology
providers and increased reliance on patients for using
technology. This raises expectations for the usability
and technical stability of apps and technologies.
In summary, a technology support system for
DCTs should not only promote seamless interaction
among stakeholders and technologies but also address
the challenge of providing timely solutions to various
sites.
638
Hanke, S., Giannikopoulos, D., Hilberger, H., Weitlaner, T. and Neumayer, B.
Technology Support System and Review Process for a Decentralized Clinical Trial: Trials@Home, RADIAL DCT as Case Study.
DOI: 10.5220/0012433900003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 2, pages 638-645
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

Trials@Home’s technology support system en-
compasses all aspects required for barrier-free partic-
ipation in the RADIAL clinical trial. Given the mul-
titude of stakeholders and roles (patients, site, CRA)
in the study, the support system integrates various ex-
isting solutions provided by project partners, ensuring
an optimized workflow.
Using the RADIAL study as an example, this pa-
per presents an exemplary approach to consolidate
available tools into one helpdesk system. The pa-
per details the system’s requirements, the RADIAL
implementation, the achievement of quality control
through automated Key Performance Indicator (KPI)
exports, and an issue-tracking process. It also sum-
marizes initial experiences from the study.
2 BACKGROUND
In contemporary clinical trial technology, the inte-
gration of Electronic Data Capture (EDC) and Clin-
ical Trial Management Systems (CTMS) has become
commonplace. Recent studies, such as the investiga-
tion conducted by Zhang et al. (Zhang et al., 2013),
underscore a significant rise in electronic data collec-
tion facilitated by various self-developed, commer-
cially available, or open-source software solutions.
These technological advancements have prompted a
reevaluation of the interpretation of ICH E6 and the
Good Clinical Practice guidelines by sponsors (Bhatt,
2023).
ICH E6(R2) was amended in 2023 to encourage
the implementation of technological advances to en-
hance the efficiency of clinical trial conduct. The sub-
sequent update, E6(R3), has elevated the guidelines
for incorporating state-of-the-art technologies in man-
aging clinical trials (Bhatt, 2023).
The guidelines recommend adapting the use of
technology in clinical trial conduct to fit patient’s at-
tributes and trial design specifics (Council, 2023).
The emphasis on technology within these guidelines
has broad implications for all aspects of trial conduct,
including quality, ethics, and stakeholder responsibil-
ities (Bhatt, 2023).
The relevance of these guidelines becomes partic-
ularly evident with the emergence of DCTs. While
the guidelines advocate technology use for various
processes, spanning from data acquisition to patient
interaction and consent processes, they do not specif-
ically address technology support for issue tracking
and helpdesk support involving different stakeholders
in DCTs.
In clinical trials, particularly in DCTs, such a sup-
port system can play a pivotal role in managing and
addressing the varied needs of patients and the in-
volved technologies. DCTs, often spanning different
countries and languages, encounter unique challenges
like language barriers as well as varying levels of IT
proficiency among patients and site users. To address
these, a comprehensive support system is essential.
One critical component is a ticketing system. This
system allows patients to raise queries, which are then
addressed by a dedicated support team. The unique-
ness of this system lies in its dual function. Besides
resolving individual queries, the support team also
contributes to a centralized knowledge base. When
a query is resolved, the team develops articles or con-
tent related to that issue, enhancing the knowledge
base. This proactive approach ensures that frequently
asked questions are readily available to all patients,
thereby reducing repetitive queries and streamlining
the support process.
Quality Assurance (QA) teams should monitor
these tickets to identify and mitigate any risks to pa-
tient safety or data integrity. This comprehensive
oversight ensures that the support system not only ad-
dresses current issues but also continuously evolves to
enhance the efficiency and safety of the clinical trial
process.
Quality assurance (QA) emerges as a pivotal pro-
cess for effecting quality improvements in the con-
text of technological enhancements. Zhang et al. in-
troduced the QA Issue Tracking System (QAIT), a
centralized platform for systematic information col-
lection and management to identify and correct QA
errors effectively (Zhang et al., 2013).
A streamlined process for tracking issues and fa-
cilitating communication, along with a comprehen-
sive knowledge repository accessible to various stake-
holders in DCTs, further enhances risk management.
A strategic evolution in clinical trial monitoring is
evident with the adoption of Risk-Based Monitoring
(RBM), as reported by Barnes et al. (Barnes et al.,
2021). Capitalizing on increased connectivity and
data analytics advances, RBM represents a targeted
approach to error detection. In complex trial work-
flows, such as those encountered in DCTs, RBM faces
distinct challenges, especially when integrating new
technologies and coordinating with multiple stake-
holders.
For DCTs, which rely on diverse systems and
processes, the imperative, extending beyond conven-
tional clinical trials, is to find mechanisms to reduce
costs and establish robust quality control and issue
tracking processes.
Agrafiotis et al. demonstrated that centralized
monitoring activities have the potential to identify a
substantial proportion (95%) of the findings revealed
Technology Support System and Review Process for a Decentralized Clinical Trial: Trials@Home, RADIAL DCT as Case Study
639

by on-site monitoring visits (Agrafiotis et al., 2018).
This underscores the importance of tracking issues
and documentation, particularly in mHealth and home
environment technologies, where diverse technolo-
gies and vendors are integrated into a singular setup.
This complexity heightens the importance of provid-
ing comprehensive helpdesk support and knowledge
bases for CRAs, sites, and other stakeholders.
DCTs, being relatively novel, present distinct
challenges necessitating specialized helpdesk sup-
port. The work of de Jong et al., involving interviews
with European regulators and assessors of clinical tri-
als, has shed light on challenges related to DCTs,
including the use of technical devices and measure-
ments in at-home situations (de Jong et al., 2022). In
response, the Trials@Home project has established
a knowledge base and a helpdesk technology sup-
port system. This system serves the dual purpose of
assisting CRAs and sites in navigating technology-
supported DCTs and providing a robust tool for qual-
ity control, issue tracking, and compliance monitor-
ing.
3 RADIAL CASE STUDY
3.1 The Trials@Home Project
Clinical trials have long been a cornerstone of
evidence-based medicine, yet the traditional model
poses challenges in terms of patients burden, ge-
ographical constraints, and data reliability. The
Trials@Home project emerges as an innovative re-
sponse, seeking to transform this landscape by intro-
ducing a decentralized approach to clinical research.
At its core, Trials@Home leverages state-of-the-
art digital technologies, mobile medical devices and a
Mobile App to facilitate remote data collection. Pa-
tients are empowered to contribute to research en-
deavors from their own residences, alleviating the lo-
gistical and time-related burdens associated with on-
site visits. The integration of mobile medical devices
enables continuous, real-time monitoring, ensuring
a comprehensive dataset while bolstering patient en-
gagement and compliance.
The patient-centric ethos underpinning the project
not only fosters inclusivity but also addresses dispar-
ities in access to healthcare resources. By harness-
ing the capabilities of mobile medical devices, Tri-
als@Home offers a dynamic platform for researchers
to gather high-fidelity, ecologically valid data.
The project further aims to establish a framework
for seamless collaboration between clinical investiga-
tors, technologists, and patients, ensuring a cohesive
research experience.
Through the Trials@Home project, we anticipate
not only an evolution in the execution of clinical trials
but also a paradigm shift in how we approach patient
engagement and data collection in medical research.
This endeavor holds the potential to democratize ac-
cess to research opportunities, drive efficiency, and
ultimately, accelerate the development of innovative
healthcare solutions.
3.2 RADIAL Design
The RADIAL study, as part of the IMI’s Tri-
als@Home initiative, exemplifies the innovation in
DCTs. Distinguished by its ”bring-your-own-device”
(BYOD) methodology, the study integrates conven-
tional, hybrid, and entirely remote trial formats, cater-
ing to diverse technological needs. Study patients use
their own mobile phones to install the Clinpal® Mo-
bile App to access the study’s interfaces. Addition-
ally, patients use mobile medical devices in the form
of a Mallya smart cap for insulin injection
1
, an Ac-
coCheck glucometer to control blood sugar
2
, and a
blood pressure meter to measure blood pressure val-
ues. The study is designed to test three discrete clini-
cal trial approaches in two different study parts:
• Part A:
– Arm 1: Adopts a traditional model with exclu-
sively onsite patient interactions.
– Arm 2: A hybrid model, blending onsite inter-
actions with remote engagements.
• Part B:
– Arm 3: A fully remote, decentralized arm,
facilitating all patient interactions from their
homes, exemplifying the full potential of DCTs
in clinical research.
3.3 RADIAL Technology
The scope of RADIAL is facilitated by a range of
DCT technologies and devices. Table 1 presents
the vendors, DCT technologies utilized, installation
types, and their relevance to specific study parts.
3.4 Requirements
In the search for an effective technology support sys-
tem for a large-scale trial like RADIAL, several key
requirements have been identified. The requirements
1
https://biocorpsys.com/en/our-products/connected-d
evices/mallya/
2
https://www.accu-chek.at/
HEALTHINF 2024 - 17th International Conference on Health Informatics
640

Table 1: Vendors and DCT technologies applied within RADIAL.
Vendor Technology Component Installation Relevant Part(s)
AARDEX MEMS Adherence Software
(MEMS AS®)
Custom A (arm 2), B
AARDEX MEMS® Mobile App Custom A (arm 2), B
eClinical Health Radial Study App Custom All
eClinical Health Clinpal® Platform (branded as RA-
DIAL Study Portal)
Configured with custom
components
All
Investis Digital RADIAL Study Website Custom B
Signant Health Smart Signals Telemedicine® (pre-
viously ‘Virtrial Telemedicine’)
Configured with custom
components
B
Signant Health SmartSignals RTSM® Configured with custom
components
All
have partly been derived from findings in the liter-
ature (see Chapter 2) and the requirements needed
to serve the RADIAL study design. Several tools
have been tested based on this. The final selection,
the UVdesk open-source helpdesk solution, fulfills
most of the requirements, providing a comprehen-
sive ticketing system and knowledge base. Further-
more, it offers the flexibility to adapt the solution
to our specific requirements. The final requirements
are that the system shall offer a centralized reposi-
tory of information, including answers to frequently
asked questions (FAQs), and provide an adaptable
and extensible content management system (CMS).
Moreover, system users should be able to contact per-
sons with detailed knowledge on specific topics of
the study in a customer support and service-oriented
manner. Different roles with varying access permis-
sions to the helpdesk system, such as content cre-
ator, ticket agent, or administrator shall be possible
to be defined. The requirements specifically identi-
fied for RADIAL include consolidating information
and knowledge from various channels into a single
helpdesk system, exchanging training materials and
expertise through eLearning, and imposing restric-
tions on access to learning resources by requiring a
password for the website to prevent general availabil-
ity.
4 SELECTION OF SUPPORT
SYSTEM PLATFORM
RADIAL support system is built on UVdesk, an open-
source helpdesk solution
3
. UVdesk provides a fully
functional project package, which can be configured
to the specific needs of the trial:
• A customizable, user-friendly dashboard for easy
access and navigation.
3
https://www.uvdesk.com/en/opensource/
• A helpdesk knowledge base (KB) that can be de-
signed to RADIAL’s needs i.e., to support Clinical
Research Associates (CRAs) and site staff.
• Backend access for modifying and expanding the
knowledge base, using a Content Management
System (CMS).
• A dashboard for ticket agents to manage and re-
spond to support requests efficiently and effec-
tively.
Additionally, UVdesk’s database structure facilitates
the extraction of key performance indicators (KPIs)
such as request count, response frequency, and aver-
age resolution time, through SQL queries. These met-
rics can be compiled into a report thereby providing
valuable insights into the system’s performance and
efficiency.
4.1 Customizing the Support System for
RADIAL
Figure 1 shows the landing page of the implemented
RADIAL helpdesk system after login. Users can
search for information and will be suggested related
articles containing the search key words in their meta-
data.
Figure 1 also outlines the structure designed for
RADIAL to provide an intuitive navigation, with the
first level presenting topics as tiles for easy access.
The layout encourages self-service browsing through
various folders:
• Frequently Asked Questions (FAQs): Contains
categorized answers to common queries from
study sites and teams.
• Vendors: Hosts technical and specific documents
from organizations providing products or services
for RADIAL.
• Site/CRA Training: Includes materials specifi-
cally for sites and CRAs, such as Site Initiation
Technology Support System and Review Process for a Decentralized Clinical Trial: Trials@Home, RADIAL DCT as Case Study
641

Visit (SIV) documents and technology manuals.
• Patient Training: Offers training resources for pa-
tients in different study arms, covering topics like
app usage and medical event reporting.
• Other Categories: Organizes information akin to
Site/CRA and Patient training in a topic-based
layout, including categories like Informed Con-
sent and Product & Logistics.
In addition, offers a quick search text box as well as
quick links to navigate directly to vendor, study, glos-
sary, and to the investigator meeting information.
The Tiles contain multiple categories, which in
turn include various articles, linkable across different
folders for relevance. Regular discussions in helpdesk
scrums also focus on enhancing the Knowledge Base,
especially the FAQs, based on emerging ticket issues.
4.2 Support System Roles
To streamline operations, we have established five
distinct user roles. Each subsequent role encompasses
the permissions of the preceding ones, plus additional
capabilities:
• Content Creators: Typically, CRAs and tech-
nical support staff, they organize and add to the
knowledge base. Published content appears on the
front-end (Figure 1), while drafts are for internal
use.
• Ticket Creators: CRAs and clinical site support
staff can create and edit tickets, with various types
like process or app-related issues. Ticket type, site
ID and patient ID are required for additional con-
text
• Ticket Agents: In addition to creating tickets,
they can respond, reassign, or modify ticket sta-
tus. They enjoy unrestricted ticket management.
• Administrators: Responsible for user manage-
ment, including adding, removing, and modifying
roles and permissions. This role is primarily held
by FHJ’s technical support staff.
• Account Owner: The initial system user, usually
an FHJ developer. This role has administrative
rights but uniquely, cannot be removed from the
system.
4.3 Workflow Integration
In conventional, hybrid, and decentralized clinical tri-
als, the support chain typically operates as follows:
1. Study patients approach clinical support staff or
Clinical Research Associates (CRAs) with in-
quiries.
2. Site personnel respond or direct their questions to
CRAs.
3. If an immediate answer isn’t available, both site
users and CRAs can utilize the Knowledge Base
(KB), either by browsing topic folders or using the
free text search field, as illustrated in Figure 1.
4. While the KB encompasses comprehensive re-
sources like manuals, training materials, videos,
and guidelines, some queries may remain unre-
solved. In such instances, clinical site support
staff or CRAs initiate a ticket through the RA-
DIAL helpdesk.
5. Ticket agents then take responsibility for man-
aging these requests via the helpdesk’s ticketing
dashboard.
Ticket agents in the RADIAL trial’s support system,
adhering to the coverage plan, manage tickets through
the ticketing system efficiently. They either assign
tickets to themselves or to another more experienced
agent to address the specific issue. Upon assignment,
automated email notifications are sent to the respec-
tive agents.
Familiarity with the knowledge base content en-
ables agents to direct users to relevant informa-
tion or offer additional support. In scenarios where
the knowledge base lacks the required information,
agents have two options:
• discuss the issue in the weekly helpdesk scrum
and RADIAL core study team meeting, or
• consult with study team members involved in
protocol design, technology setup, or User Ac-
ceptance Testing (UAT). If necessary, they may
also seek assistance from the technology provider,
who will handle the issue in their ticketing system.
Upon resolution, the third-party vendor informs
the RADIAL helpdesk, ensuring proper documen-
tation and closure of the ticket.
Ticket agents have the capability to include listed con-
tacts as collaborators on a ticket. When agents re-
spond to a query, their response is automatically dis-
patched to both the user’s and collaborators’ emails.
Users or collaborators can conveniently respond back
directly through their email. Each response triggers
an automatic email notification to the agent, ensuring
they are promptly informed. The ticketing dashboard
facilitates seamless communication, allowing agents
to view and reply to user responses. This interac-
tive process, featuring email notifications to all par-
ties, continues until the ticket creator’s inquiries are
fully resolved.
HEALTHINF 2024 - 17th International Conference on Health Informatics
642

Figure 1: Start page of the RADIAL helpdesk knowledge base.
4.4 Help Desk Tickets and Issues
Review and Classification
To effectively manage help desk tickets in the RA-
DIAL study, a comprehensive approach is adopted.
All help desk tickets, regardless of their status, are
exported into a global issues tracker. This tracker
undergoes tier review by the technology governance
team, overseeing the technological aspects of RA-
DIAL, and the Quality Assurance team, focusing
on patient and data risk management. Operational
teams also contribute to this process, thereby ensur-
ing all technology-related issues are centralized in this
tracker. The Governance team classifies issues into
distinct categories, as outlined in Table 2. The classi-
fication determines the subsequent handling process.
”Bugs and Errors” are given high priority, potentially
triggering immediate or scheduled software updates
based on impact and risk analysis. ”Process-related”
tickets are important and are communicated to clin-
ical operations. ”Technical support” issues may en-
hance the Knowledge Base and other published ma-
terials. ”User Access and Permissions” span a range
from straightforward issues, addressed with prepared
responses, to complex ones necessitating detailed re-
view or vendor escalation. Lastly, ”General Inquiries
and Information” cover non-technical questions and
those not fitting into other categories, ensuring a com-
prehensive and structured approach to ticket manage-
ment.
5 OVERSIGHT
5.1 KPIs and Reports
As articulated in the work of (Bertram et al., 2010),
issue trackers within the helpdesk context exhibit var-
ied perspectives among stakeholders. Notably, project
managers tend to focus on high-level summaries,
while the quality assurance group categorises cases
by project area or type. Enhancing existing knowl-
edge assets necessitates the establishment of key per-
formance indicators (KPIs). A survey conducted by
(Rastogi et al., 2013) examined the significance of
those indicators, soliciting ratings from two compa-
nies and distinguishing between the expectations of
different roles, such as bug reporters and bug owners.
Currently, foundational metrics such as the number
of high-priority bugs, assigned bugs, or resolved bugs
serve as the basis for RADIAL KPIs. Additionally,
metrics like Priority Weighted Fixed Issues (PWFI),
which not only quantify the number but also consider
the priority of bugs per owner, are under consideration
for analysis pending the availability of more data.
The following KPIs for Issue Tracking are actively
employed in RADIAL:
• Number of Issues per day per month
• Number of Issues by Type
• Number of Issues by Status
• Number of Issues by Priority
• Number of Issues by Replies
Technology Support System and Review Process for a Decentralized Clinical Trial: Trials@Home, RADIAL DCT as Case Study
643
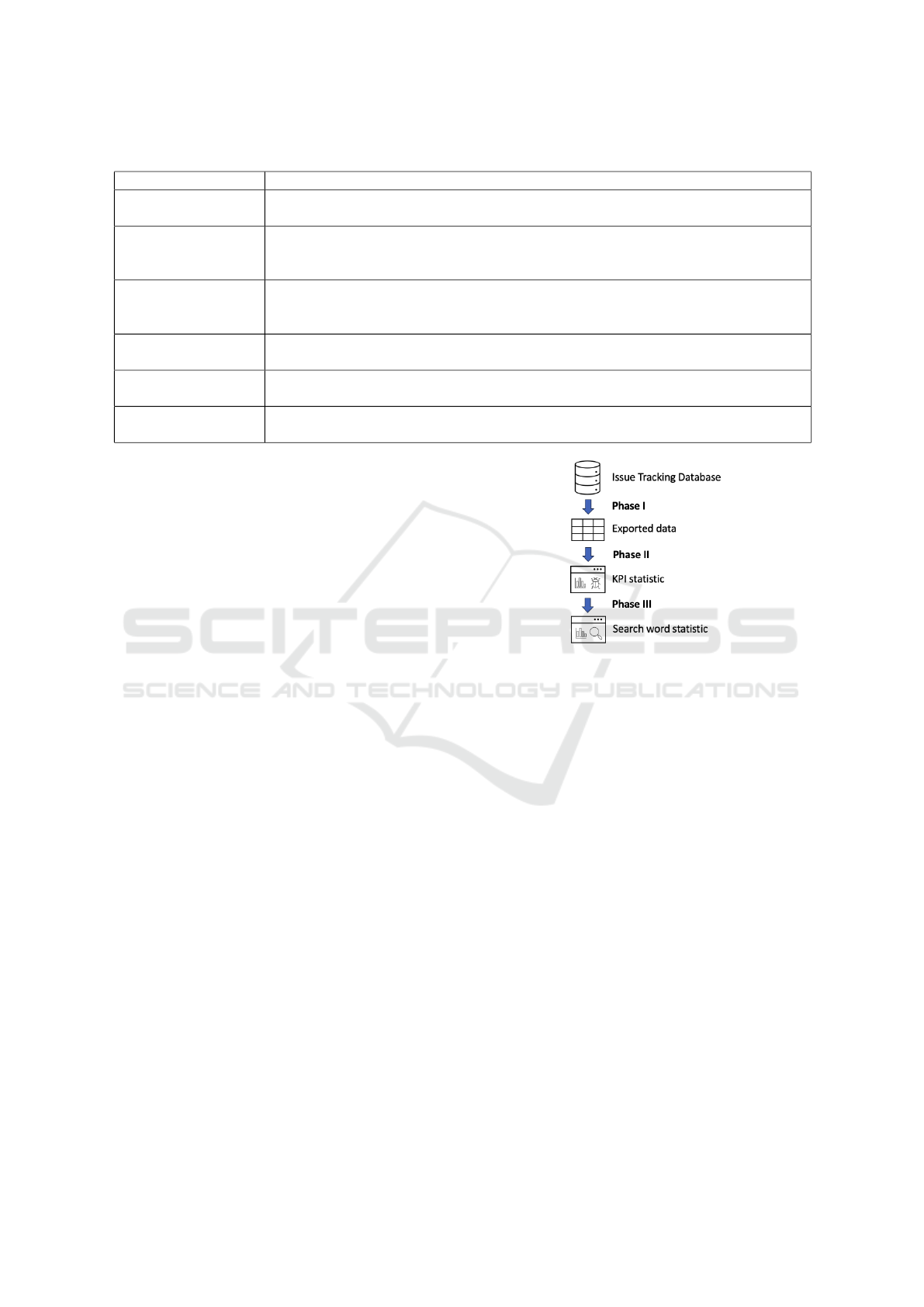
Table 2: Issue classification like performed by Governance team.
Issue class Description
Bugs and Errors This category includes all issues related to software or system bugs, glitches, and
errors. It covers problems that result from defects in code or applications.
Process-Related Issues falling under this category are related to business processes, workflow, or pro-
cedures that may need improvement, clarification, or adjustment. These issues are not
necessarily technical but affect how tasks are executed.
Technical Support Technical issues that require help desk assistance or technical support team fall into
this category. It includes hardware, software, network, and other technology-related
problems.
User Access and Per-
missions
This category encompasses issues related to user access, permissions, authentication,
and authorization. It includes requests for access changes and password resets.
General Inquiries
and Information
For non-technical questions or general inquiries that don’t fit into the other categories
Not relevant for RA-
DIAL
Tickets not related to RADIAL
• Number of Issues by Agent
• Average Age of Issue
The process for generating those KPIs can be de-
scribed into three phases which are shown in Figure
2.
Phase 1: Export data from the Issue Tracking
Database. The initial step involves extracting data
from the relational SQL database to create a spread-
sheet, facilitating further processing for reporting pur-
poses. Variables such as ID, status, priority, type,
replies, last reply, issue age, and agent-customer as-
sociations are included.
Phase 2: Development of a Python Web applica-
tion for KPIs. Subsequently, a Python Dash web
application was designed to transform the spreadsheet
into a comprehensible report of KPIs. This phase also
entails additional preprocessing including the calcu-
lation of the average issue age and replies.
Phase 3: Expansion of the Web Application for the
Knowledge Base. In this phase, the existing appli-
cation is expanded to incorporate top search keywords
for the current month as well as for all-time. This aug-
mentation aims to identify prevalent issues and con-
tribute to a more robust understanding of recurring
problems.
6 CONCLUSIONS
DCTs offer several advantages over conventional tri-
als, including reduced patient travel burden, enhanced
study accessibility, and the potential for less biased
Figure 2: Phases for generating statistics of the RADIAL
Issue Tracking System and the Knowledge Base.
and more diverse cohorts by accessing clinical studies
globally. However, designing studies with remote and
virtual components, such as telemedicine and mobile
health devices, poses challenges. Ensuring technolog-
ical stability and user self-sufficiency is crucial. Addi-
tionally, effective communication is required among
stakeholders from different sites and countries, in-
cluding site members and CRAs, while managing var-
ious technology providers with different service level
agreements and helpdesk availabilities.
We introduced a system for the RADIAL study
to address these challenges, comprising a helpdesk
ticketing system for multiple stakeholders and a Wiki-
like knowledge base documenting technology usage,
study procedures, training materials, and FAQs.
The system is currently undergoing testing and
evaluation, with additional results and insights to be
published. While supporting the RADIAL study,
the system facilitates clear process linking between
study personnel and different technology providers.
However, it has been observed that situations de-
manding immediate support, such as direct patient
phone interactions, may encounter challenges due to
HEALTHINF 2024 - 17th International Conference on Health Informatics
644

the extended communication chain involving multiple
stakeholders.
The helpdesk is restricted to a limited number of
users and will be enhanced in the future to increase
its scalability. Caching mechanisms and daily server
snapshots for backup purposes are currently being im-
plemented to speed up the user experience. If the
helpdesk experiences performance issues over time,
vertical scaling can be employed to boost the existing
capabilities (e.g. CPU, RAM) of the server. In the fu-
ture, the helpdesk will evolve by implementing load
balancing strategies across multiple servers. This will
efficiently distribute incoming requests, balancing the
network load and ensuring high availability by utiliz-
ing multiple servers in case of a server failure.
Despite challenges, documenting various issues
during the study for quality control and risk man-
agement is essential. The implemented system al-
lows for the collection and processing of technical
bugs, process-related issues, and facilitates updates
like new software releases.
Furthermore, the KB extends beyond the RA-
DIAL study and could potentially benefit similar stud-
ies in the future. Patient access to the KB, currently
unavailable, could empower patients, aligning with
the trend of patient engagement. As Language Model
technologies like ChatGPT emerge, the KB may serve
as a domain-specific knowledge repository, enabling
the training of LLMs for helpdesk chatbots. This ad-
vancement could provide more specific and straight-
forward support, eliminating the need for users to
search diverse documents themselves.
ACKNOWLEDGEMENTS
This work has received support from the EU/EFPA In-
novative Medicines Initiative Joint Undertaking Tri-
als@Home (grant No. 831458). The Innovative
Medicines Initiative (IMI) website can be accessed
through the following link: www.imi.europa.eu.
DISCLAIMER
The research leading to these results was conducted
as part of the Trials@Home consortium. This paper
only reflects the personal view of the stated authors
and neither IMI nor the European Union, EFPIA, or
any Associated Partners are responsible for any use
that may be made of the information contained herein.
REFERENCES
Agrafiotis, D. K., Lobanov, V. S., Farnum, M. A., Yang, E.,
Ciervo, J., Walega, M., Baumgart, A., and Mackey,
A. J. (2018). Risk-based Monitoring of Clinical Tri-
als: An Integrative Approach. Clinical Therapeutics,
40(7):1204–1212.
Barnes, B., Stansbury, N., Brown, D., Garson, L., Gerard,
G., Piccoli, N., Jendrasek, D., May, N., Castillo, V.,
Adelfio, A., Ramirez, N., McSweeney, A., Berlien, R.,
and Butler, P. J. (2021). Risk-Based Monitoring in
Clinical Trials: Past, Present, and Future. Therapeutic
Innovation and Regulatory Science, 55(4):899–906.
Bertram, D., Voida, A., Greenberg, S., and Walker, R.
(2010). Communication, collaboration, and bugs: The
social nature of issue tracking in small, collocated
teams. In Proceedings of the 2010 ACM Conference
on Computer Supported Cooperative Work, CSCW
’10, page 291–300, New York, NY, USA. Association
for Computing Machinery.
Bhatt, A. (2023). The revamped Good Clinical Practice E6
( R3 ) guideline : Profound changes in principles and
practice ! 6:167–171.
de Jong, A. J., van Rijssel, T. I., Zuidgeest, M. G. P.,
van Thiel, G. J. M. W., Askin, S., Fons-Mart
´
ınez,
J., Smedt, T. D., de Boer, A., Santa-Ana-Tellez, Y.,
and and, H. G. (2022). Opportunities and challenges
for decentralized clinical trials: European regulators’
perspective. Clinical Pharmacology & Therapeutics,
112(2):344–352.
Holmner,
˚
A., Ebi, K. L., Lazuardi, L., and Nilsson, M.
(2014). Carbon footprint of telemedicine solutions -
unexplored opportunity for reducing carbon emissions
in the health sector. PLoS ONE, 9(9):e105040.
Jain, B., Bajaj, S. S., and Stanford, F. C. (2022). Random-
ized clinical trials of weight loss: Pragmatic and digi-
tal strategies and innovations. Contemporary Clinical
Trials, 114:106687.
Rastogi, A., Gupta, A., and Sureka, A. (2013). Samiksha:
Mining issue tracking system for contribution and per-
formance assessment. In Proceedings of the 6th In-
dia Software Engineering Conference, ISEC ’13, page
13–22, New York, NY, USA. Association for Comput-
ing Machinery.
Subaiya, S., Hogg, E., and Roberts, I. (2011). Reducing
the environmental impact of trials: a comparison of
the carbon footprint of the CRASH-1 and CRASH-2
clinical trials. Trials, 12(1).
Zhang, Y., Sun, W., Gutchell, E. M., Kvecher, L., Kohr,
J., Bekhash, A., Shriver, C. D., Liebman, M. N., Mu-
ral, R. J., and Hu, H. (2013). QAIT: A quality assur-
ance issue tracking tool to facilitate the improvement
of clinical data quality. Computer Methods and Pro-
grams in Biomedicine, 109(1):86–91.
Technology Support System and Review Process for a Decentralized Clinical Trial: Trials@Home, RADIAL DCT as Case Study
645
