
Objective Evaluation of Sleep Disturbances in Older Adults with
Cognitive Impairment Using a Bed Sensor System and
Self-Organizing Map Analysis
Tomoko Kamimura
1a
, Risa Otsuka
1
, Asaka Domoto
1
, Hikofumi Suzuki
2
and Mamino Tokita
3
1
Department of Health Sciences, Graduate School of Medicine, Shinshu University, 3-1-1 Asahi, Matsumoto, Japan
2
Department of Cyber Science Infrastructure Development, National Institute of Informatics, Tokyo, Japan
3
Department of Global Centre for Advanced Research on Logic and Sensibility, Keio University Tokyo, Japan
Keywords: Sleep Disturbance, Total Sleep Time, Cognitive Impairment, Alzheimer's Disease, Self-Organizing Map.
Abstract: Bed sensor systems are useful for measuring sleep states in cognitively impaired older adults because they
can measure unrestrained individuals. However, there are no criteria for identifying sleep abnormalities using
them. We developed a method to determine sleep abnormalities by analysing data collected by a bed sensor
system using a self-organizing map (SOM). In this study, the sleep states were measured in two cognitively
impaired care-facility residents. These recordings were used to calculate total nocturnal sleep time, wake time
after sleep onset, frequency of leaving the bed, and frequency of awakening in the bed for each day. The data
from these four variables were used to draw an SOM for each individual’s sleep state to identify normal or
abnormal sleep days. We visually determined whether a main cluster was formed in the SOM. If a main cluster
was formed, the days included in the main cluster were defined as the individual's normal days, while other
days were defined as the individual's abnormal days. The above parameters were independently compared
between the two groups, as determined by the SOM. The characteristics of abnormal sleep days identified by
SOM could be explained using these four variables, suggesting the effectiveness of identifying abnormal days
by SOM.
1 INTRODUCTION
Sleep disturbances in older adults with cognitive
impairment adversely affect health status and
increase the burden on caregivers (Webster 2020a;
Okuda 2019; Shi 2018). In addition, it has been noted
that sleep disturbances may exacerbate brain damage
(Irwin 2019; Nedergaard 2020). Therefore, early
detection and management of sleep disturbances are
important for not only maintaining the quality of life
(QOL) of older adults and their caregivers but also for
maintaining the health care system.
Sleep disturbances in older adults with cognitive
impairment have been estimated to range from 20%
to 70% (Guarnieri 2012; Wilfling 2019; Webster
2020b), depending on the assessment method. A
meta-analysis of studies using validated proxy
questionnaires found a pooled prevalence of sleep
disturbance of 20% (95% confidence interval [CI
a
https://orcid.org/0000-0003-2973-2064
16%-24%) among cognitively impaired individuals
living in care homes.
Sleep assessment methods other than proxy
questionnaires include validated self-assessment
questionnaires, polysomnography, and wearable
actigraphy. However, these methods are often
inadequate when individuals are very old or
cognitively impaired because the results are
unreliable or burdensome to the individuals.
To solve this problem, the sleep state of
cognitively impaired older individuals has been
measured using a bed sensor system that measures
sleep state without restraining the individual, and
abnormalities, such as frequency of leaving the bed
and prolonged or shortened total sleep time, have
been identified (Higami 2018). However, the
characteristics of these sleep states vary depending on
the measurement index, and the lack of criteria for
comprehensively determining abnormalities within
Kamimura, T., Otsuka, R., Domoto, A., Suzuki, H. and Tokita, M.
Objective Evaluation of Sleep Disturbances in Older Adults with Cognitive Impairment Using a Bed Sensor System and Self-Organizing Map Analysis.
DOI: 10.5220/0012715000003756
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 13th International Conference on Data Science, Technology and Applications (DATA 2024), pages 253-258
ISBN: 978-989-758-707-8; ISSN: 2184-285X
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
253

and between individuals remains a challenge.
To tackle this issue, we developed a method to
detect abnormalities by analysing data measured by a
bed sensor system with a self-organizing map (SOM),
which is an unsupervised learning clustering method
of artificial intelligence (AI). The SOM is a two-
dimensional plot of data; the shorter the distance
between the data, the higher the similarity of the data.
The reason for using this method is that it is suitable
for comparing the similarity of data with
multidimensional features in an exploratory manner.
In this study, to obtain basic data for the
development of a method for the detection of sleep
abnormalities in cognitively impaired older adults
using a bed sensor system and SOM analysis, we
detected abnormalities based on the similarity of
sleep states within individuals using SOM to extract
their characteristics and confirm whether these
abnormalities can be expressed using conventional
sleep indicators.
2 METHODS
2.1 Participants
Participants of this study were residents of a geriatric
healthcare facility in Japan. The inclusion criteria
were (1) a cognitive function score validated by the
Japanese Ministry of Health, Labor and Welfare
(Tago 2021) of II b (i.e., symptoms, behaviours, and
communication difficulties that interfere with daily
life are sometimes observed at home, but the patient
can be independent if someone pays attention to
them) or worse; (2) the need to monitor sleep and
leave the bed during the night and use a bed sensor
system; and (3) sleep data had been collected for at
least 21 consecutive days.
2.2 Equipment
The bed sensor system used in this study was a Nemuri
Scan (Paramount Bed Corporation), which was
installed under the bed mattress. Equipped with a
highly sensitive pressure sensor, the system detects the
body movements of the examinee on the bed through a
mattress and calculates an activity score every minute
that reflects the intensity and frequency of body move-
ments, excluding movements caused by respiration and
heartbeats. Nemuri Scan uses a proprietary algorithm
to detect one of three states per minute: the examinee
leaves the bed, awakens in the bed, or sleeps in the bed.
These results are directly output as comma
separated value (CSV) files, as well as daily sleep
indices, such as total sleep time and time awake after
falling asleep, calculated from the data. The validity
of these indicators has already been verified (Kogure
2011). Only the CSV data were used in this study.
2.3 Data Analysis
An SOM was created using CSV data from 6:00 p.m.
to 8:00 a.m. during the measurement period for each
individual. Days with missing CSV values during the
measurement period were excluded from the analysis.
Four variables (see Table) were calculated daily using
the CSV data: total nocturnal sleep time, wake time
four values were used to determine the SOM as the
sleep state of the day.
In the SOM, we visually determined whether the
main cluster, which accounts for the majority of the
data, was formed, and defined days included in the
main cluster as the individual's normal sleep days and
days not included in the main cluster as the
individual's abnormal sleep days. R studio ver.
1.3.1093. was used to create the SOM.
To confirm whether the abnormalities detected by
the SOM can be expressed using conventional sleep
indicators, the values of each of the above four
variables were compared between the two groups of
normal and abnormal sleep days in each case, as
determined by the SOM. Comparisons between the
two groups were made using the Mann–Whitney U
test, and a p-value <0.05 was considered statistically
significant using SPSS ver. 26.
Table 1: Four variables were calculated daily using the CSV data.
DATA 2024 - 13th International Conference on Data Science, Technology and Applications
254
2.4 Ethical Consideration
This study was approved by the Medical Ethics
Committee of Shinshu University School of
Medicine.
3 RESULTS
Data were collected from a 97-year-old woman for 28
days (case A) and a 91-year-old man for 51 days (case
B). There was 1 day of missing data in each case
during the measurement period, and the data of that
day were excluded from the analysis. The purpose of
using the Nemuri scan was as follows. Case A was at
risk of falling due to anaemia, cognitive decline, and
a history of hip fracture, and required monitoring the
transfer of a portable toilet at night. Case B was at risk
of falling or getting lost when going to the toilet at
night due to Alzheimer's disease and a history of
lumbar spinal stenosis and needed to be monitored
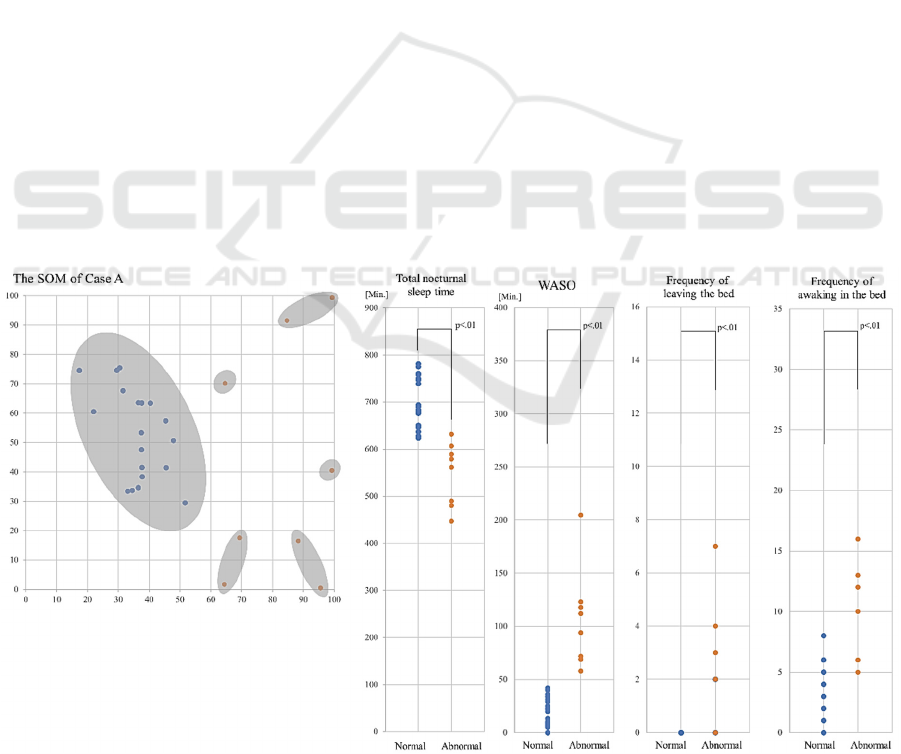
when going to the toilet at night. The main cluster was
formed in the SOM for case A (Figure 1). This cluster
consisted of data from 20 normal sleep days. Eight
abnormal sleep days fell outside the cluster.
The median (interquartile range) of total nocturnal
sleep time, WASO, frequency of leaving the bed, and
frequency of awakening in the bed on the abnormal
days in case A were 570.0 (482.5-602.5) minutes,
103.0 (69.8-121.8) min, 2.0 (0.5-3.8) times, and 11.0
(6.0-12.8) times, respectively. The median of each of
the four variables on abnormal days was lower than
that on normal days (p<0.01, Figure 1).
The main cluster was also formed in the SOM of
Case B (Figure 2). This cluster consisted of data from
35 normal sleep days. There were 16 abnormal sleep
days outside this cluster.
The median (interquartile range) of total nocturnal
sleep time, WASO, frequency of leaving the bed, and
frequency of awakenings in the bed on the abnormal
days of case B were 432.0 (379.3-515.0) minutes,
196.5 (156.0-258.5) minutes, 8.5 (6.0-11.8) times,
and 14.0 (7.8-24.8) times, respectively. The median
total nocturnal sleep time, WASO, and frequency of
leaving the bed on abnormal days were significantly
worse than those on normal days (p<0.05, p<0.01,
p<0.01, respectively). respectively, Figure 2).
Since several clusters were found in the abnormal
days group in case B, we performed a supplementary
analysis comparing the status of the four variables in
the three subgroups (B1, B2, and B3; Figure 3).
All three subgroups had longer WASO than the
normal group, but the days belonging to B1 were
characterized by the longest WASO, with relatively
more frequent leaving the bed and awakening in the
bed. The days belonging to B2 were characterized by
the most frequent leaving the bed and less frequent
awakening in the bed, and the days belonging to B3
were characterized by less frequent leaving the bed
and most frequent awakening in the bed.
Figure 1.
Objective Evaluation of Sleep Disturbances in Older Adults with Cognitive Impairment Using a Bed Sensor System and Self-Organizing
Map Analysis
255
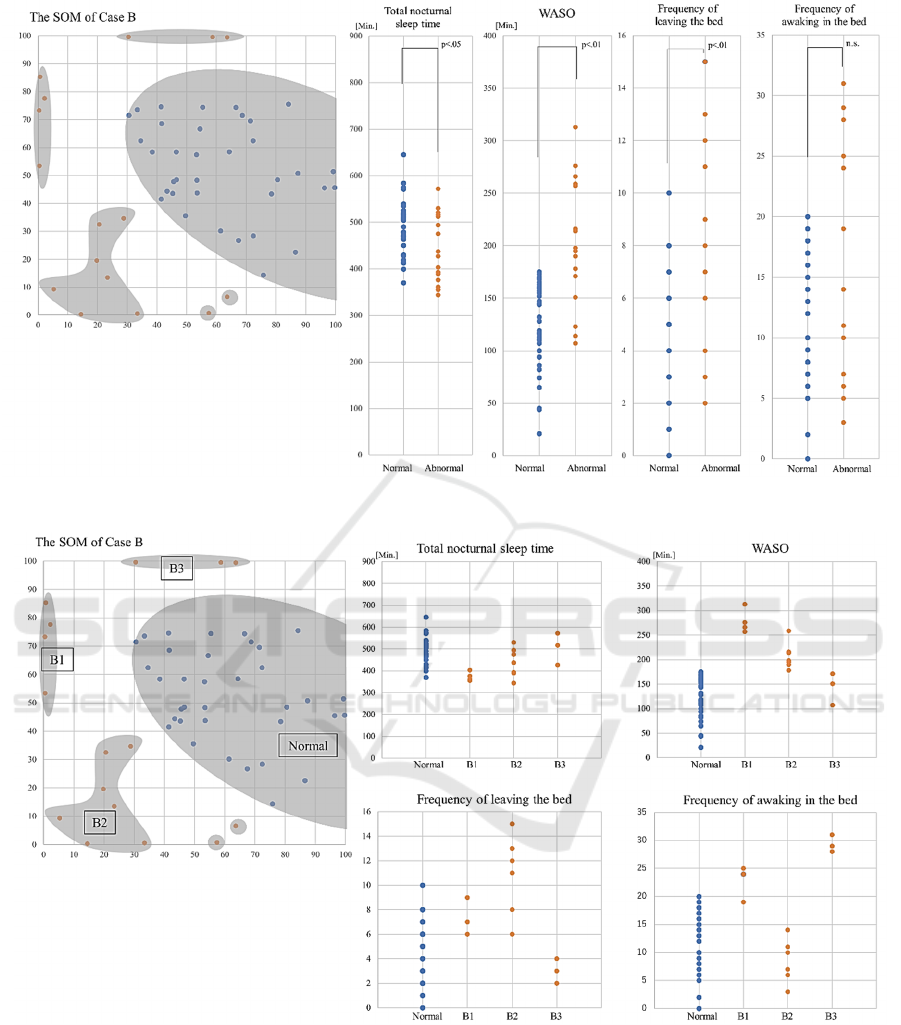
Figure 2.
Figure 3.
4 DISCUSSIONS
This study suggests that it is possible to detect
abnormal sleep days in older adults with cognitive
impairment by measuring using a bed sensor system
and performing SOM analysis of the data. We
determined that it is possible to simultaneously detect
abnormalities in individuals with multiple patterns of
abnormalities, such as in case B in this study.
Furthermore, the abnormalities discriminated by
SOM analysis could be explained by comparing
several conventional sleep measures, suggesting the
validity of the SOM analysis results.
DATA 2024 - 13th International Conference on Data Science, Technology and Applications
256

Previous studies have shown that sleep
disturbances in older adults with dementia are
characterized by frequent leaving the bed (Higami
2018), prolonged or shortened total sleep time
(Higami 2018), decreased sleep efficiency (Cote
2021), and greater inter-daily circadian variability
(Cote 2021). Another study reported the
characteristics of sleep disturbances that vary
according to the cause of cognitive impairment
(Fukuda 2022), dementia severity (Blytt 2021), and
the presence or absence of complications such as pain
and depression (Blytt 2021). However, to the best of
our knowledge, this is the first study to detect
abnormal days based on intra-individual sleep
variability in an older adult with cognitive
impairment who had sleep problems and showed that
there are different types of abnormalities from day to
day.
In the future, we would like to investigate the
relationship between the disease and sleep
disturbances, such as whether the characteristics of
nocturnal sleep shown in case B of Alzheimer's
disease in this study indicate variability other than the
diurnal variability of the circadian rhythm, which is
one of the characteristics of this disease.
One limitation of this study was the small number
of individuals in whom abnormal sleep was detected.
Therefore, it is necessary to verify the reliability of
our findings by including a greater number of older
adults with cognitive impairments in subsequent
studies.
Another limitation is that the state of normal sleep
is not necessarily generally normal since the study
focused on the detection of abnormalities.
An important limitation of our measurement is
that it is unable to detect sleep disturbances in
individuals immediately after the start of data
collection because a certain amount of data
accumulation is required to detect abnormal sleep. To
address this issue, we are currently investigating the
possibility of extracting standard sleep patterns by
accumulating data from multiple cases, including
older adults without cognitive impairment, and
conducting an SOM analysis. If this standard pattern
can be extracted, it may lead to the early detection of
abnormalities in each individual by comparison with
the standard pattern.
5 CONCLUSIONS
The characteristics of abnormal sleep days identified
by SOM could be explained using these four variables
i.e. total nocturnal sleep time, wake time after sleep
onset, frequency of leaving the bed, and frequency of
awakening in the bed for each day suggesting the
effectiveness of identifying abnormal days by SOM.
Using SOM analysis, we also showed that there are
different abnormalities from day to day in older adults
with cognitive impairment.
REFERENCES
Blytt, K. M., Flo-Groeneboom, E., Erdal, A., Bjorvatn, B.,
& Husebo, B. S. (2021). Sleep and its association with
pain and depression in nursing home patients with
advanced dementia - across-sectional study. Frontiers
in psychology, 12, 633959.
Cote, A. C., Phelps, R. J., Kabiri, N. S., Bhangu, J. S., &
Thomas, K. K. (2021). Evaluation of wearable
technology in dementia: a systematic review and meta
analysis. Frontiers in medicine, 7, 501104.
Fukuda, C., Higami, Y., Shigenobu, K., Kanemoto, H., &
Yamakawa, M. (2022). Using a non-wearable
actigraphy in nursing care for dementia with Lewy
Bodies. American journal of Alzheimer's disease and
other dementias, 37, 15333175221082747.
Guarnieri, B., Adorni, F., Musicco, M., Appollonio, I.,
Bonanni, E., Caffarra, P., Caltagirone, C., Cerroni, G.,
Concari, L., Cosentino, F. I., Ferrara, S., Fermi, S.,
Ferri, R., Gelosa, G., Lombardi, G., Mazzei, D.,
Mearelli, S., Morrone, E., Murri, L., Nobili, F. M.,
Sorbi, S. (2012). Prevalence of sleep disturbances in
mild cognitive impairment and dementing disorders: a
multicenter Italian clinical cross-sectional study on 431
patients. Dementia and geriatric cognitive disorders,
33, 50–58.
Higami, Y., Yamakawa, M., Shigenobu, K., Kamide, K., &
Makimoto, K. (2019). High frequency of getting out of
bed in patients with Alzheimer's disease monitored by
non-wearable actigraphy. Geriatrics & gerontology
international, 19, 130–134.
Irwin, M. R., & Vitiello, M. V. (2019). Implications of sleep
disturbance and inflammation for Alzheimer's disease
dementia. The Lancet. Neurology, 18, 296–306.
Kogure, T., Shirakawa, S., Shimokawa, M., & Hosokawa,
Y. (2011). Automatic sleep/wake scoring from body
motion in bed: validation of a newly developed sensor
placed under a mattress. Journal of physiological
anthropology, 30, 103–109.
Nedergaard, M., & Goldman, S. A. (2020). Glymphatic
failure as a final common pathway to dementia. Science
(New York, N.Y.), 370, 50–56.
Okuda, S., Tetsuka, J., Takahashi, K., Toda, Y., Kubo, T.,
& Tokita, S. (2019). Association between sleep
disturbance in Alzheimer's disease patients and burden
on and health status of their caregivers. Journal of
neurology, 266, 1490–1500.
Shi, L., Chen, S. J., Ma, M. Y., Bao, Y. P., Han, Y., Wang,
Y. M., Shi, J., Vitiello, M. V., & Lu, L. (2018). Sleep
disturbances increase the risk of dementia: a systematic
review and meta-analysis. Sleep medicine reviews, 40,
4–16.
Objective Evaluation of Sleep Disturbances in Older Adults with Cognitive Impairment Using a Bed Sensor System and Self-Organizing
Map Analysis
257

Tago, M., Katsuki, N. E., Yaita, S., Nakatani, E.,
Yamashita, S., Oda, Y., & Yamashita, S. I. (2021). High
inter-rater reliability of Japanese bedriddenness ranks
and cognitive function scores: a hospital-based
prospective observational study. BMC geriatrics, 21,
168.
Webster, L., Powell, K., Costafreda, S. G., & Livingston,
G. (2020a). The impact of sleep disturbances on care
home residents with dementia: the SIESTA qualitative
study. International psychogeriatrics, 32, 839–847.
Webster, L., Costafreda Gonzalez, S., Stringer, A.,
Lineham, A., Budgett, J., Kyle, S., Barber, J., &
Livingston, G. (2020b). Measuring the prevalence of
sleep disturbances in people with dementia living in
care homes: a systematic review and meta-analysis.
Sleep, 43, zsz251.
Wilfling, D., Dichter, M. N., Trutschel, D., & Köpke, S.
(2019). Prevalence of Sleep Disturbances in German
Nursing Home Residents with Dementia: A Multicenter
Cross-Sectional Study. Journal of Alzheimer's disease :
JAD, 69, 227–236.
DATA 2024 - 13th International Conference on Data Science, Technology and Applications
258
