
Balancing Patient Privacy and Health Data Security: The Role of
Compliance in Protected Health Information (PHI) Sharing
Md Al Amin
a
, Hemanth Tummala
b
, Rushabh Shah
c
and Indrajit Ray
d
Computer Science Department, Colorado State University, Fort Collins, Colorado, U.S.A.
Keywords:
Consent, Patient Privacy, Data Security, PHI Sharing, Provenance, Compliance, Blockchain, Smart Contract.
Abstract:
Protected Health Information (PHI) sharing significantly enhances patient care quality and coordination, con-
tributing to more accurate diagnoses, efficient treatment plans, and a comprehensive understanding of patient
history. Compliance with strict privacy and security policies, such as those required by laws like HIPAA, is
critical to protect PHI. Blockchain technology, which offers a decentralized and tamper-evident ledger system,
hold promise in policy compliance. This system ensures the authenticity and integrity of PHI while facilitating
patient consent management. In this work, we propose a blockchain technology that integrates smart contracts
to partially automate consent-related processes and ensuring that PHI access and sharing follow patient pref-
erences and legal requirements.
1 INTRODUCTION
Acquiring patient consent for healthcare information
sharing is paramount for adhering to policy com-
pliance, particularly concerning regulations like the
Health Insurance Portability and Accountability Act
(HIPAA) in the U.S. and the General Data Protec-
tion Regulation (GDPR) in the E.U (Hutchings et al.,
2021). These regulatory frameworks emphasize pro-
tecting health information and upholding the patient’s
right to privacy. Patient consent is a cornerstone of
these regulations, ensuring individuals have control
over their health data and its dissemination. Under
HIPAA, healthcare entities must obtain explicit con-
sent before sharing healthcare data for purposes be-
yond treatment, payment, or healthcare operations.
Similarly, GDPR enforces strict guidelines on data
consent, processing, and privacy, offering individuals
the ’right to be forgotten’ and the autonomy to de-
cide how their data is used and shared. From a policy
compliance perspective, proper patient consent acqui-
sition is a legal requirement and a trust-building mea-
sure, reinforcing the patient-provider relationship. It
ensures transparency in data handling and builds pa-
tient confidence, knowing their sensitive information
is shared respectfully and responsibly. As healthcare
a
https://orcid.org/0000-0003-1700-7201
b
https://orcid.org/0009-0007-7778-5845
c
https://orcid.org/0009-0005-5658-0950
d
https://orcid.org/0000-0002-3612-7738
continues to integrate with various technologies, up-
holding these consent protocols is crucial for main-
taining the security and privacy of patient data and
adhering to global data protection standards.
Unauthorized health data access and disclosure
are common events in healthcare industries that in-
crease security and privacy concerns. Table 1 shows
the number of compliance complaints received by
the U.S. Department of Health and Human Services
(HHS) Office for Civil Rights (OCR) (Rights (OCR),
2008). The primary reasons for the complaints are (i)
impermissible uses and disclosures of PHI, (ii) lack of
safeguards of PHI, (iii) lack of patient access to their
PHI, (iv) lack of administrative safeguards of elec-
tronic PHI, and (v) use or disclosure of more than the
minimum necessary PHI. These issues can be mini-
mized by enforcing patients’ consent for data access
and sharing decisions and employing proper data pro-
tection mechanisms like encryption and anonymity.
Consent lets patients control their healthcare journey,
enabling them to make choices that align with their
best interests and well-being (Timmermans, 2020).
Enhanced security and privacy technologies are
essential for protecting patient data from being com-
promised, misused, or disclosed. However, substan-
tial evidence indicates that the root of many unau-
thorized EHR access and sharing lies in inadequate
policy adoption, implementation, and enforcement
(Lopez Martinez et al., 2023; Aljabri et al., 2022).
Often, users are granted access privileges inappropri-
Al Amin, M., Tummala, H., Shah, R. and Ray, I.
Balancing Patient Privacy and Health Data Security: The Role of Compliance in Protected Health Information (PHI) Sharing.
DOI: 10.5220/0012767400003767
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 21st International Conference on Security and Cryptography (SECRYPT 2024), pages 211-223
ISBN: 978-989-758-709-2; ISSN: 2184-7711
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
211

Table 1: OCR HHS- Compliance Complaints.
Year Complains Compliance Reviews Technical Assistance Total
2018 25089 438 7243 32770
2019 29853 338 9060 39251
2020 26530 566 5193 32289
2021 26420 573 4244 31237
ately, whether intentionally or not. Policy compliance
frequently falls short, and access control measures are
not rigorously monitored or executed on time. A com-
mon oversight is the blanket assignment of identical
roles and privileges to all employees, neglecting the
nuances of individual patient-level policies. More-
over, auditing and monitoring practices are typically
reactive, triggered only by serious complaints or le-
gal mandates, rather than proactive and consistent.
These policy specification and enforcement flaws sig-
nificantly impact informed consent policies, under-
scoring the need for a more accurate and systematic
approach to effectively protecting patient healthcare
data and preserving privacy.
It is essential to address the following concerns to
guarantee compliance with the applicable privacy and
security policies, industry best practices, and contrac-
tual obligations for sharing PHI: (i) Patient-level poli-
cies or consents are often not properly or timely en-
forced in healthcare data sharing. (ii) Patients lack
assurance that consent for access or sharing purposes
is carried out strictly by designated users, and only if
the stipulated conditions are met are all other requests
rejected. (iii) Data sharing over email or other medi-
ums is insecure due to the absence of encryption or
the use of inadequate and weak encryption algorithms
and key sizes. (iv) The centralized hospital system
serves as a singular source of truth and a potential
single point of failure for managing audit trails. (v)
The absence of a verifiable, unaltered record for con-
sent execution and sharing PHI highlights the need for
comprehensive consent provenance. (vi) Compliance
assessments and audits are not conducted accurately
and timely to check compliance status.
To address the aforementioned challenges and re-
quirements, this paper proposes a framework based
on blockchain and smart contracts for managing and
enforcing informed consent when sharing PHI with
entities outside the treatment team. The approach en-
sures that PHI sharing occurs only when the sender
has obtained the necessary consent from the patient
and the sharing aligns with specific, predefined pur-
poses. In addition to enforcing patient consent, this
approach integrates other relevant security policies
and industry best practices to ensure data protection.
The HIPAA Security Rule mandates the requirements
for transmission security are outlined under 45 CFR
§ 164.312(e)(1) Technical Safeguards (Chung et al.,
2006). However, the proposed approach does not di-
rectly guarantee security mechanisms like encryption
for data protection. Instead, it leverages an honest
broker who acts as a blind and secure entity to evalu-
ate the intended PHI and certify its status as required
protection mechanisms are satisfied or not (Alarcon
et al., 2021) . The broker’s attestation is then recorded
in blockchain-based audit trails with other relevant
activity data to support future compliance evalua-
tions and validation. It supports using audit trails or
provenance mechanisms based on blockchain, which
is essential for keeping track of PHI-sharing activi-
ties. Moreover, the proposed framework provides a
compliance-checking mechanism in data-sharing ac-
tivities, ensuring adherence to applicable policies.
Smart contracts, (Buterin et al., 2014), offer an au-
tomated, transparent system that upholds the integrity
and accountability of the consent for sharing PHI.
Through this smart contract-based approach, the pro-
posed framework not only automates processes but
also guarantees the accurate execution of informed
consent, thereby enhancing the security and reliabil-
ity of PHI sharing. Blockchain technology ensures
the immutability of submitted records, safeguarding
the integrity of the audit trail and enabling the detec-
tion of any unauthorized alterations. Blockchain se-
curity features, including non-repudiation, ensure that
participants cannot deny their actions (Le and Hsu,
2021).
This work is the first to capture patients’ informed
consent for PHI sharing to ensure policy compliance
through preserving provenance and conducting com-
pliance checking. It also considers and enforces other
applicable security policies and industry best prac-
tices mandated by the various laws, regulations, stan-
dards, and contractual obligations to meet the compli-
ance requirements. Significant contributions include
(i) implementing a mechanism to capture patients’
consent for sharing healthcare data beyond the treat-
ment team members. (ii) Storing obtained consents in
decentralized and distributed networks (blockchain)
to overcome a single point of truth sources and fail-
ure. (iii) Considering applicable security and pri-
vacy policies, regulatory requirements, and contrac-
tual obligations to ensure compliance-based sharing.
(iv) Enforcing informed consent and applicable poli-
cies while making authorization decisions to share
health records. (v) Equipping blockchain-based au-
dit trail mechanisms to guarantee data provenance.
(vi) Incorporating compliance assessment methods to
identify compliance and non-compliance PHI shar-
ing. (vii) Offering consent services to provide precise
and comprehensive insights into the consent granted
and the extent of its execution.
SECRYPT 2024 - 21st International Conference on Security and Cryptography
212

2 RELATED WORK
Blockchain technology has increasingly been adopted
in healthcare for various services, particularly for
sharing protected health information among health-
care providers, patients, and other stakeholders.
Blockchain facilitates a more efficient, transparent,
and patient-centered delivery of healthcare services,
making it an essential component in modern health-
care infrastructure. Fan et al., (Fan et al., 2018), pro-
posed a blockchain-based secure system, MedBlock,
to share electronic medical records among authorized
users. It provides security and privacy with access
control protocols and encryption technology while
sharing patient healthcare data.
Shah et al., (Shah et al., 2019), proposed a med-
ical data management framework to facilitate data
sharing. It gives patients full control over access
to their medical data. It also ensures that patients
know who can access their data and how it is used.
Zhuang et al., (Zhuang et al., 2020), addressed a
blockchain-based patient-centric health information-
sharing mechanism protecting data security and pri-
vacy, ensuring data provenance, and providing pa-
tients full control over their health data. However,
consent structure and compliance requirements are
not addressed, which are very important to give pa-
tients confidence in how their consent is executed and
how data is protected.
Alhajri et al., (Alhajri et al., 2022), explored the
criticality of implementing legal frameworks to safe-
guard privacy within fitness apps. By examining how
various fitness apps handle consent and privacy poli-
cies, their research highlighted the crucial role of con-
sent as outlined in the GDPR. The authors proposed
the adoption of blockchain technology as a means
to govern user consent for sharing, collecting, and
processing fitness data, ensuring a process centered
around human needs and compliant with legal stan-
dards. Nonetheless, the study failed to present a tech-
nical architecture for their blockchain-based proposal.
Amofa et al. approached a blockchain-based per-
sonal health data sharing framework with an underly-
ing mechanism to monitor and enforce acceptable use
policies attached to patient data (Amofa et al., 2018).
Generated policies are consulted with smart contracts
to make decisions on when the intended data can be
shared or otherwise. All entities cooperate to protect
patient health records from unauthorized access and
computations. Balistri et al., (Balistri et al., 2021),
designed the BlockHealth solution for sharing health
data with tamper-proofing and protection guarantees.
They store the patient’s healthcare data in a private
database, and the hash of the healthcare data is stored
in the blockchain to ensure data integrity.
The above-mentioned papers summarized the ap-
plication and benefits of using blockchain for health-
care data sharing and essential services. However,
they failed to address the security and privacy re-
quirements mandated by various laws and regula-
tory agencies, such as HIPAA and GDPR. The ma-
jor requirements demand patient consent and proper
protection, such as encryption, while sharing health
records. In addition, it is crucial to maintain audit logs
and check that those activities did not violate any poli-
cies. This paper proposes sharing informed consent as
the smart contract for authorization with provenance
and compliance-checking mechanisms.
3 PROPOSED APPROACH
The main objective is to ensure compliance with ap-
plicable security and privacy policy for PHI shar-
ing. To ensure compliance, we need proper pol-
icy enforcement, including maintaining provenance
and performing compliance status checks promptly
and properly. For enforcement, this paper considers
patient-informed consent, where the sender has per-
mission from the patient to share the intended PHI
with the receiver for specific purposes. Also, proper
data protection mechanisms are considered. How-
ever, instead of ensuring data protection directly, this
work leverages an honest broker to verify and certify
the data protection mechanism. PHI-sharing activities
are recorded as audit trails to provide provenance and
reconstruct events in a manner that reflects their ac-
tual occurrence. A private blockchain-based approach
is proposed (Section 4). Finally, a blockchain con-
sensus mechanism called Proof of Compliance (PoC)
is approached, Section 5, for performing auditing.
This audit rigorously examines the enforcement ac-
tions against the policy standards and informed con-
sent, using the provenance data to verify and certify
the policy’s compliance status while sharing health
records. The seamless connection between policy
enforcement, provenance, and the auditing process
forms the backbone of a secure and compliant system.
3.1 Patient-Provider Agreement (PPA)
The patient-provider agreement, or PPA, aims to de-
termine who is responsible for what in treatment. A
PPA is formed when a patient visits a hospital and is
properly documented to deliver healthcare services. It
differs from organization to organization. Healthcare
organizations adjust what they need from patients and
what they expect from them to match those needs,
Balancing Patient Privacy and Health Data Security: The Role of Compliance in Protected Health Information (PHI) Sharing
213

treatments, and responsibilities. This is done based on
the nature and needs of treatment and services. Also,
the components and representation of the PPA depend
on the hospital or clinic. Figure 1 shows the struc-
ture of a PPA, and Algorithm 1 illustrates the gradual
processes for creating a PPA with the required com-
ponents. The main concept of PPA is adopted from
(Al Amin et al., 2023). The authors focused on con-
sent management for medical treatment and diagno-
sis purposes, mainly for the treatment team members.
They did not include patient consent and other re-
quirements for health data sharing beyond the treat-
ment team. This paper extends the PPA structure to
analyze the requirements and formalize the consent
components for PHI sharing. A PPA is formally com-
posed of five tuples:
PPA = (PC, PrC, T IC, SIC, ROC)
satisfying the following requirements:
(A) PC is a finite set of patient components contain-
ing the patient’s personal information, contact in-
formation, mailing information, pharmacy infor-
mation, billing and insurance information, emer-
gency contact, and others. The patient is respon-
sible for providing and maintaining these compo-
nents’ valid, accurate, and updated information.
(B) PrC is a finite set of provider components, includ-
ing the treatment team, prescription, and others.
The provider is responsible for creating an effec-
tive team to provide appropriate care. Everything
from treatment to insurance coverage and billing
is considered during the patient treatment period.
(C) T IC is a finite set of treatment informed con-
sent components. It denotes that the patient has
permitted the designated treatment team to ac-
cess medical records. Treatment team members
include doctors, nurses, support staff, lab tech-
nicians, billing officers, emergency contact per-
sons, and others assigned by the authority. Some
outsider members are insurance agents, phar-
macists/pharmacy technicians, doctors/lab techni-
cians from another hospital, etc.
(D) SIC is a finite set of sharing informed consent
components. It denotes the patient’s consent
to sharing medical data for a specific purpose.
Both the sender and the receiver must have con-
sent. The primary purpose of this work is SIC,
including (i) identifying, capturing, and storing
consent components, (ii) enforcing consents with
other applicable security policies and industry
best practices to ensure policy compliance while
making PHI-sharing decisions, (iii) defining and
capturing provenance information with the en-
Figure 1: Patient-Provider Agreement (PPA) Components.
forced consents to maintain audit trails, (iv) per-
forming compliance checking using consensus
mechanisms; (v) providing services for both given
and executed consents, etc. It does not consider
other components: PC, PrC, T IC, and ROC.
(E) ROC is a finite set of regulatory and other com-
ponents. It has applicable security and privacy
policies to comply with the requirements of lo-
cal government, state government, federal govern-
ment, foreign government, and regulatory agen-
cies (HIPAA, GDPR) if necessary. It also includes
contractual obligations in some cases.
3.2 Sharing Informed Consent (SIC)
Before approving, patients need to know clearly about
the sharing informed consent, particularly who can
share which PHI with whom for what purposes—and
also the protection mechanism while sharing PHI dur-
ing transmission over the network. Figure 2 shows
the SIC conceptual framework structure. Sharing in-
formed consent is formally composed of four tuples:
SIC = (S, R, PHI, P)
satisfying the following requirements:
(a) S is a finite set of authorized senders denoted as
{S
1
, S
2
, S
3
, ......S
s
} for s number of senders. The
sender can share certain healthcare data with the
receiver, who has permission from the patient.
The sender may be a member of the patient treat-
ment team or anyone from the provider.
SECRYPT 2024 - 21st International Conference on Security and Cryptography
214

Algorithm 1: Patient-Provider Agreement (PPA) Formation.
Input : (i) PC, (ii) PrC, (iii) T IC, (iv) SIC, (v) ROC, (vi) R
PPA
,
(vii) BN
SC
1 /* R
PPA
: secured PPA repository, BN
SC
:
blockchain network smart contract */
Result: A formal PPA
2 Input Parameters Initialization
PPA
i
← {PC
i
, PrC
i
, T IC
i
, SIC
i
, ROC
i
} where i is patient identity
(i) PC ← {PC
1
, PC
2
, PC
3
, PC
4
, PC
5
, PC
6
......................PC
M
}
3 (ii) PrC ← {PrC
1
, PrC
2
, PrC
3
, PrC
4
, PrC
5
, PrC
6
..........PrC
N
}
4 (iii) T IC ← {T IC
1
, T IC
2
, T IC
3
, T IC
4
, T IC
5
, T IC
6
........T IC
T
}
5 (iv) SIC ← {SIC
1
, SIC
2
, SIC
3
, SIC
4
, SIC
5
, SIC
6
..............SIC
S
}
6 (v) ROC ← {ROC
1
, ROC
2
, ROC
3
, ROC
4
, ROC
5
, ROC
6
...ROC
R
}
7 PPA Components Integrity Calculation /* H(∂)
calculates hash of ∂ */
8 (a) H
PC
← H(PC
1
, PC
2
, PC
3
, PC
4
, PC
5
, PC
6
....................PC
M
)
9 (b) H
PrC
← H(PrC
1
, PrC
2
, PrC
3
, PrC
4
, PrC
5
, PrC
6
.........PrC
N
)
10 (c) H
T IC
← H(T IC
1
, T IC
2
, T IC
3
, T IC
4
, T IC
5
, T IC
6
........T IC
T
)
11 (d) H
SIC
← H(SIC
1
, SIC
2
, SIC
3
, SIC
4
, SIC
5
, SIC
6
.............SIC
S
)
12 (e) H
ROC
← H(ROC
1
, ROC
2
, ROC
3
, ROC
4
, ROC
5
, ROC
6
..ROC
R
)
13 (f) H
PPA
i
← H(H
PC
, H
PrC
, H
T IC
, H
SIC
, H
ROC
)
14 PPA Finalization if PPA
i
is complete then
15 /* presence of PC, PrC, T IC, SIC, ROC */
16 if (R
PPA
+ PPA
i
) contains no conflicts then
17 (i) do R
PPA
← (R
PPA
+ PPA
i
)
18 (ii) add ID
PPA
i
to patient profile, P
i
19 (iii) call BN
SC
(ID
PPA
i
, H
PPA
i
)
20 /* PPA integrity verification reference */
21 Return: Success (PPA
i
added to R
PPA
)
22 else
23 Error: (R
PPA
+ PPA
i
) contains conflicts
24 /* PPA
i
revision required to add */
25 end if
26 else
27 Error: PPA
i
cannot be created (incomplete PPA)
28 end if
(b) R is a finite set of authorized users who re-
ceive protected health information from autho-
rized senders. A finite set of r number authorized
receivers denoted as {R
1
, R
2
, R
3
, ......R
r
}. The re-
ceiver may be from other hospitals, labs, medi-
cal research institutes, pharmaceutical companies,
marketing departments, government officials, etc.
(c) PHI is a finite set, d number, of health data de-
noted by {PHI
1
, PHI
2
, PHI
3
, ......PHI
d
}. It is an
electronic version of a patient’s medical data that
healthcare providers keep over time. They are
protected health information and contain sensitive
patient information. PHI must be protected from
any kind of unauthorized access, disclosure, and
sharing. Table 2 shows ten (10) types of PHI,
considered for each patient, with PHI ID, name,
description, and potential creators.
(d) P is a finite set of purposes. It indicates the ob-
jective of the PHI sharing by the senders with the
receivers. Receivers must use the received PHI for
the intended purposes. A finite set of purposes, a
Figure 2: Sharing Informed Consent (SIC) Structure.
p number, can be denoted as {P
1
, P
2
, P
3
, ......P
p
}.
The objective of sharing protected health informa-
tion outlines the specific reasons for its sharing. The
recipient must utilize the shared PHI exclusively for
its designated purpose. The potential reasons for shar-
ing PHI in this study include, but are not limited to:
(i) Treatment: Providers or patients need to share
PHI with other providers from external hospitals
to provide better treatment. Also, patients must
move to different regions, like states or countries,
due to family movement, job transfers, or new
jobs. Patients need to share or transfer healthcare
data from the previous providers to the current.
(ii) Diagnosis: Present providers sometimes need
more skilled human resources, appropriate ma-
chinery, instruments, or sophisticated technology
to diagnose disease. But it is urgently required
to do that to give proper treatment and services
to save patients’ lives or minimize damages. Pa-
tients’ health data must be transferred or shared
with other providers or labs to complete diagnosis
and make proper treatment plans for the patients.
(iii) Marketing: Healthcare data sharing for marketing
purposes involves using patient data to promote
healthcare services, products, or initiatives. This
can help healthcare providers tailor their services
to patient needs, inform patients about new treat-
ments or products, and improve patient engage-
ment. Only the receiver entity can use the shared
data as intended and should not share it with other
associates for extended business purposes.
(iv) Research: Sharing PHI for medical research pur-
poses holds significant potential for advancing
Balancing Patient Privacy and Health Data Security: The Role of Compliance in Protected Health Information (PHI) Sharing
215
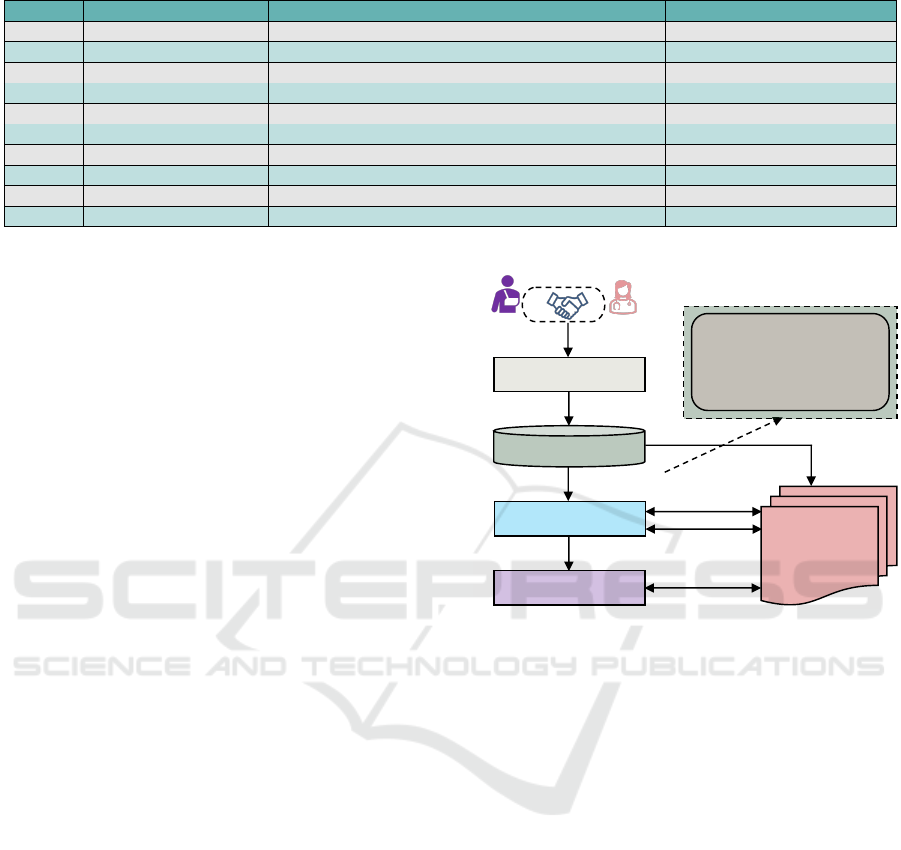
Table 2: Sample Patient Protected Health Information (PHI) Structure.
PHI ID PHI Name PHI Description PHI Creator
PHI-1001 Demographic Information Basic personal information like name, date of birth, gender, contact Patient, Support Staff
PHI-1002 Previous Medical History Old medical records from another hospitals and providers Patient, Support Staff
PHI-1003 Immunizations, Vaccinations Immunization records that are administered over time Patient, Pathology Lab Technician
PHI-1004 Allergies Various allergies sources, triggering condition, remediation Patient, Support Staff, Path Lab Tech
PHI-1005 Visit Notes Physiological data, advises, follow-up, visit details Doctor, Nurse
PHI-1006 Medications, Prescription Pharmacy information, prescribed medications like name, dosage Doctor
PHI-1007 Pathology Lab Works Biological samples analysis like blood, tissue, other substances Pathology Lab Technician
PHI-1008 Radiology Lab Works Imaging results such as X-rays, CT, MRI, Ultrasound, PET scans Radiology Lab Technician
PHI-1009 Billing, Insurance Bank account, credit/debit card, and insurance policy information Patient, Support Staff, Billing Officer
PHI-1010 Payer Transactions Bills of doctor visit, lab works, and medications Billing Officers, Insurance Agent
medical knowledge, leading to breakthroughs in
understanding diseases, improving and develop-
ing new treatments, improving healthcare systems
and services, and enhancing patient outcomes.
Patients’ privacy and rights must be respected.
Other purposes might exist depending on the na-
ture and requirements of the treatment, patient condi-
tions, provider business policy, etc. This study con-
siders only the four purposes mentioned above. After
receiving shared data, the receiver performs specified
operations to complete the job. It is assumed that the
receiver cannot share data with other users who do not
have permission from the patients. More specifically,
the receiver’s healthcare system does not allow the
sharing of PHI by any means, like printouts, email, or
screenshots. However, this paper doesn’t provide de-
tailed mechanisms or techniques for preventing data
sharing without patients’ consent at the receiver end.
3.3 SIC Smart Contract Deployment
Once a Patient-Provider Agreement, or PPA, is cre-
ated and stored in the repository, all sharing informed
consent components are deployed to the blockchain
network. For each patient, there is one smart contract
that contains all consents for that particular patient.
If there isn’t a smart contract, the authority deploys
one, transfers ownership to the patient, and updates
the contract address to the patient’s profile and hospi-
tal systems. The contract address is an identifier for a
smart contract in the blockchain network. This smart
contract-based approach provides an automated sys-
tem and guarantees the integrity and accountability of
the deployed consents. Once consents are deployed
or added to the smart contract, they cannot be altered.
The authorization module needs to access these smart
contracts to make decisions considering the sender,
receiver, purpose attributes, environmental factors, or-
ganizational policies, regulatory frameworks, etc.
Upon finalizing the PPA, it transforms and secures
storage in a PPA repository. Subsequently, an in-
tegrity marker, such as a hash (H
PPA
i
) generated by
Patient Profile
Hospital System
Patient
Provider
1 Agreement
Sharing Informed Consent (SIC)
§ Sharing Informed Consent 1
§ Sharing Informed Consent 2
§ Sharing Informed Consent 3
……………………....
§ Sharing Informed Consent N
Secured PPA Repository
Smart Contract Deployment
Unit (SCDU)
2 Format
Translation
4 SIC Components
Patient Provider Agreement
(PPA)
5 SIC Integrity Check
6 SIC Smart Contract
3 PPA Integrity
7 Update SIC
Information
8 Consent Query
Response
Public Blockchain
Network
Figure 3: SIC Smart Contract Deployment Process.
the Algorithm 1, is stored on the blockchain alongside
the PPA ID for later modification detection. These
are depicted in Steps 2 and 3 in Figure 3. The Smart
Contract Deployment Unit (SCDU) then gathers all
components of the informed consent from the PPA
(Step 4). It verifies their integrity to ensure no de-
liberate or accidental alterations have occurred (Step
5). As a secure entity, the SCDU does not alter con-
sent components, noting that any modification inval-
idates the consent. If the consents remain unmodi-
fied, the SCDU creates and deploys the correspond-
ing smart contracts on the blockchain network (Step
6) and then updates the patient’s profile and the hospi-
tal system (Step 7). Users can make queries with the
required credentials regarding informed consent and
get responses in Step 8 from the blockchain network.
3.4 Honest Broker, Applicable Policies
and Industry Best Practices
Alongside patient consent, the proposed approach in-
corporates relevant security policies and industry best
practices before sharing protected health information.
For instance, a security policy might require a data
SECRYPT 2024 - 21st International Conference on Security and Cryptography
216

protection mechanism during data transfer between
systems. For treatment and diagnosis purposes, en-
cryption is a recommended protection method.
Similarly, anonymity is a recommended protec-
tion method for marketing and research purposes,
where patient identifiers must be removed before
sharing. The targeted PHI must be anonymous using
proper techniques and tools before sending the data
from the host healthcare system to the receiver. The
host system indicates where patients’ PHI is created
or presently stored. Healthcare organizations deploy
appropriate encryption and anonymity mechanisms.
This study does not directly ensure PHI encryption
and anonymity. Instead, this approach leverages an
honest broker, a trusted entity that evaluates the en-
cryption algorithm, key size, and data anonymity sta-
tus (Alarcon et al., 2021). After checking, the hon-
est broker certifies or attests to the status, which is
recorded in audit trails as proof for policy compliance
verification, along with other components like sharing
informed consents, timestamps, etc.
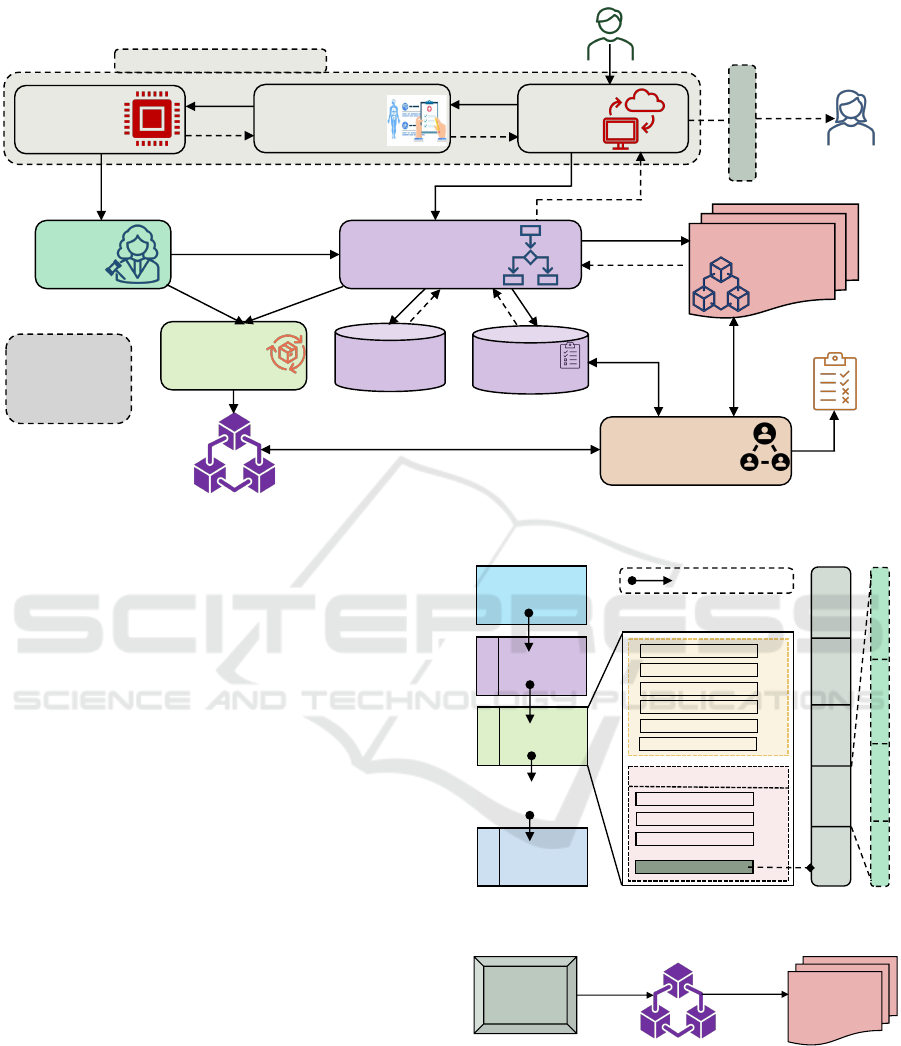
3.5 PHI Sharing Authorization Process
Consent enforcement ensures that related consents are
executed while making decisions for the PHI shar-
ing requests. All consents are stored on the public
blockchain network as smart contracts and cannot be
enforced until they are called. The authorization mod-
ule (AM) considers sharing informed consent with
applicable policy and required attributes while mak-
ing decisions. The attributes may be subject, object,
operation, and environmental attributes. The sender
must provide the necessary credentials for identifica-
tion and authentication. Figure 4 shows the informed
consent enforcement for PHI-sharing authorization.
A sender submits a data sharing request to the PHI
sharing unit in Step 1. Sharing unit forwards request
to authorization module for decision in Step 2. It
also requests that the PHI storage unit send the in-
tended PHI to the protection mechanism unit in Steps
2a and 2b. The honest broker receives encrypted
or anonymized data in Step 3. After analyzing, it
sends a report to AM in Step 4. The AM queries the
blockchain network through the corresponding smart
contract to get sharing informed consent information
for the sharing request in Step 3a and 4a. It also
makes queries for requests related to applicable poli-
cies and required attributes in Steps 3b and 3c. It re-
ceives the policy and attributes in Steps 4b and 4c.
After evaluating, it makes an authorization decision
and sends it to the sharing unit in Step 5. If the re-
quest is approved, the sharing unit gets encrypted or
anonymized data based on the purpose in Steps 7a and
7b. Then, it delivers the intended PHI through email
or protocol to the receiver in Step 8.
The audit trail recording unit collects logs from
AM in Step 6a and from the honest broker in Step
6b. It combines logs and stores as an audit trail in
Step 6c in Private Audit Blockchain. Section 4 dis-
cusses block structure and others. The compliance
status checking is done in Steps 9a, 9b, and 9c by the
Proof of Compliance consensus mechanism. Compli-
ance status reports are produced in Step 10. Section 5
discusses the required mechanism. For this study, it is
considered that the authorization module is not com-
promised or tampered with. It is the reference moni-
tor for making access decisions and must be tamper-
proof (Mulamba and Ray, 2017). Also, the commu-
nication channel between AU and the smart contract
access points or apps is secured from malicious users.
4 PHI SHARING PROVENANCE
Enforcing an applicable set of policies is crucial,
but preserving data provenance to show adherence to
these policies is also essential. Nevertheless, policy
compliance cannot be quantified or confirmed in iso-
lation. An independent auditor conducts a thorough
policy audit to verify compliance with the policy, uti-
lizing the available provenance data to ascertain and
certify the policy’s compliance status. For an accurate
policy compliance assessment, two critical elements
must be diligently maintained: (i) consent and policy
lineage and (ii) PHI sharing activity audit trails. This
section contains the detailed provenance mechanisms
dedicated to preserving the policy lineage’s integrity
and ensuring the audit trails’ authenticity.
4.1 Consent and Policy Lineage
Policy lineage involves a comprehensive record of all
policies that guide the authorization module’s deci-
sions. It’s a transparent and traceable record of the
policy history and its application in decision-making
processes. For this study, sharing informed consent
is mainly considered for decision-making. Since all
consents are deployed as smart contracts, blockchain
networks can create policy lineages. However, this
paper does not consider other HIPAA-related poli-
cies, such as physical security, provider training, etc
(Chung et al., 2006).
4.2 PHI Sharing Activity Audit Trails
Integrity in policy enforcement ensures that events are
documented faithfully, reflecting the sequence and na-
Balancing Patient Privacy and Health Data Security: The Role of Compliance in Protected Health Information (PHI) Sharing
217
1 PHI Sharing Request
Private
Audit
Blockchain
Protected Health
Information (PHI)
Storage
Authorization
Module (AM)
Honest
Broker
Receiver
Sender
2b PHI
2 Decision
Request
5 Decision
Response
3 EN/AN PHI
Audit Trails
Recording Unit
3a Consent Query
4a Consent Return
EN: Encrypted
AN: Anonymous
3b: Policy Query
3c: Attribute Query
4b: Policy Return
4c: Attribute Return
4 PHI EN/AN Status Report
Protocol/Email
8
EN/AN PHI
6c Audit Trails
7a
EN/AN PHI
Encryption
Anonymity
Mechanism
Provider Healthcare System
Proof of Compliance
(PoC)
Compliance Checking
PHI
Sharing
Unit
7b
EN/AN PHI
Compliance Status
2a PHI
6b Honest Broker Logs
6a AM Logs
9a Audit Trails
9b Policy
9c Informed
Consents
10 Report
Public Blockchain
Network
Policy
Repository
Attribute
Repository
3c 4c 4b 3b
Figure 4: Compliance-based PHI Sharing Authorization Process.
ture of actions taken. This authenticity is crucial for
transparency and accountability. Provenance plays a
key role by offering a detailed and unalterable his-
tory of policy enforcement actions as they are carried
out, safeguarding against any tampering of records.
The alteration of audit trails or unauthorized access to
healthcare data is strictly prohibited to maintain the
sanctity of the process. Maintaining the integrity of
the audit trail is essential for policy compliance as-
surance. If integrity is compromised, checking com-
pliance status to find compliance and non-compliance
cases is questionable. The blockchain provides these
requirements as ledger properties. This work adopts
private blockchain as an audit trail storage system.
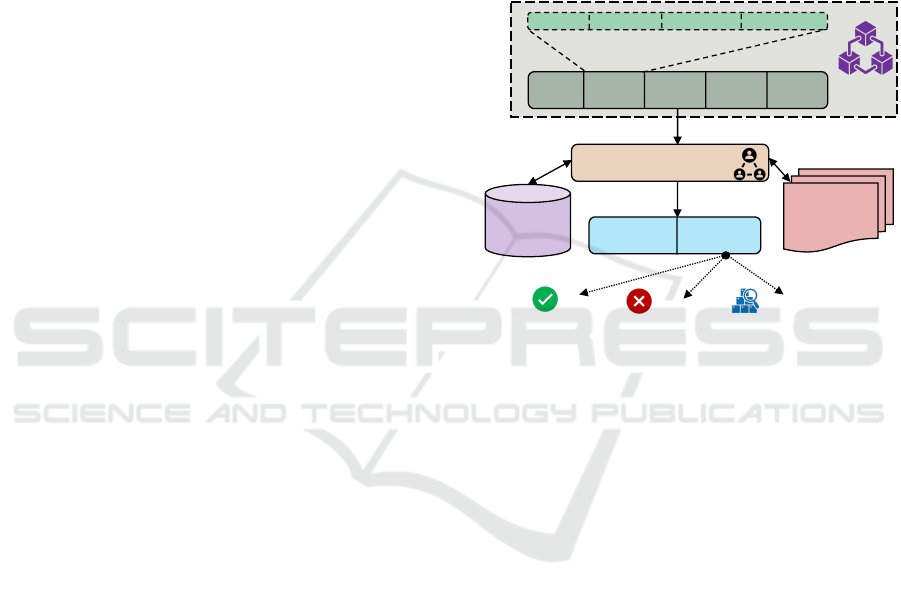
Figure 5 illustrates the private audit blockchain’s
block components and structure. Each block has a
block header part that contains block metadata and
a data part that stores the audit trail data. Each au-
dit trail has five components: (i) audit trail ID; (ii)
informed consent ID or SIC ID; (iii) honest broker
ID; (iv) honest broker report; and (v) timestamp data.
The audit trail ID provides unique identifiers; the in-
formed consent ID, or SIC ID, indicates the consent
that is executed to share the intended PHI. From SIC
ID, it is possible to get the components: sender, re-
ceiver, PHI, and purpose. The honest broker ID indi-
cates which broker certifies or attests to the intended
PHI’s protection status (encryption or anonymity). Fi-
nally, the timestamp means the time when the sharing
authorization is done. Steps 6a, 6b, and 6c in Figure
Genesis Block
…
.
1
st
Block
2
nd
Block
3
rd
Block
N
th
Block
Block Header
Previous Block Hash
Hash Difficulty Target
ATT Unit Hash
Block Hash
Block Nonce
Block Timestamp
Audit Trail Data
Audit Trail - 1
Audit Trail - 2
Audit Trail - 3
Audit Trail - M
Audit Trail Transaction (ATT) Unit
…
Bloc Hash
Block Data
Block Hash
Block Data
Block Hash
Block Data
Previous Block Hash
Audit Trail
ID
Informed
Consent
ID
Honest
Broker
ID
Honest
Broker
Report
Timestamp
Data
Sender
Receiver
PHI
Purpose
Figure 5: Audit Blockchain Block Structure.
Public
Blockchain
Network
Policy
Enforcement
Unit
Private Audit Blockchain
Audit
Trai ls
Audit Block
ID and Hash
Figure 6: Storing Audit Blockchain Block ID and Hash.
4 show the process of capturing audit trails from the
authorization module and honest broker.
Enforcement activity data is collected and stored
in a private blockchain known as an audit blockchain
as immutable records to ensure consent provenance
and maintain compliance. The private blockchain
network is managed and maintained by an author-
SECRYPT 2024 - 21st International Conference on Security and Cryptography
218
ity, which means reading and writing permissions are
given to limited participants or users. In this case, the
trust and transparency of the private blockchain are
questionable. It doesn’t provide a public eye to main-
tain trust and transparency. Storing audit trails on the
public blockchain gives trust and transparency, which
is another issue to consider. Firstly, audit trails con-
tain sensitive information like user activities, and stor-
ing them on a public blockchain creates security and
privacy concerns. Secondly, audit trails produce enor-
mous amounts of data, which requires a lot of money
to store on the public blockchain. This is not feasible
from a business perspective, as it increases business
operation and treatment costs and service charges.
To overcome the aforementioned issues, this re-
search stores audit trail data on a private blockchain
called the private audit blockchain. Then, it stores
the private audit blockchain block ID and block hash
as integrity on the public blockchain. Storing block
ID and integrity requires a small cost and provides
trust and transparency. Any modifications to private
audit blockchain data can be detected by comparing
the block’s current and stored hashes with those on
the public blockchain. Figure 6 shows the private and
public blockchain relationship for storing audit block
ID and integrity in a public blockchain like Ethereum.
We have configured a private blockchain that is based
on the Ethereum client (Samuel et al., 2021) with the
necessary smart contracts and API for capturing and
storing audit trail data in the audit blockchain.
5 COMPLIANCE VERIFICATION
Enforcing applicable policies and maintaining au-
dit trails are not enough to ensure policy compli-
ance. There must be some mechanism to check
compliance status using deployed and enforced poli-
cies with audit trails. The compliance checker must
be an independent and separate entity from the pol-
icy enforcer and audit trail unit. This paper pro-
poses a blockchain consensus mechanism to perform
compliance-checking operations on the audit trails us-
ing deployed sharing informed consents (SIC) and
other applicable policies. The consensus mechanism,
called Proof of Compliance (PoC), is governed by a
set of independent, distributed, and decentralized au-
ditor nodes. Section 3 discusses the sharing informed
consent structure and deployment process as the smart
contract in the public blockchain. Section 4 gives the
audit trail capturing and storing mechanism.
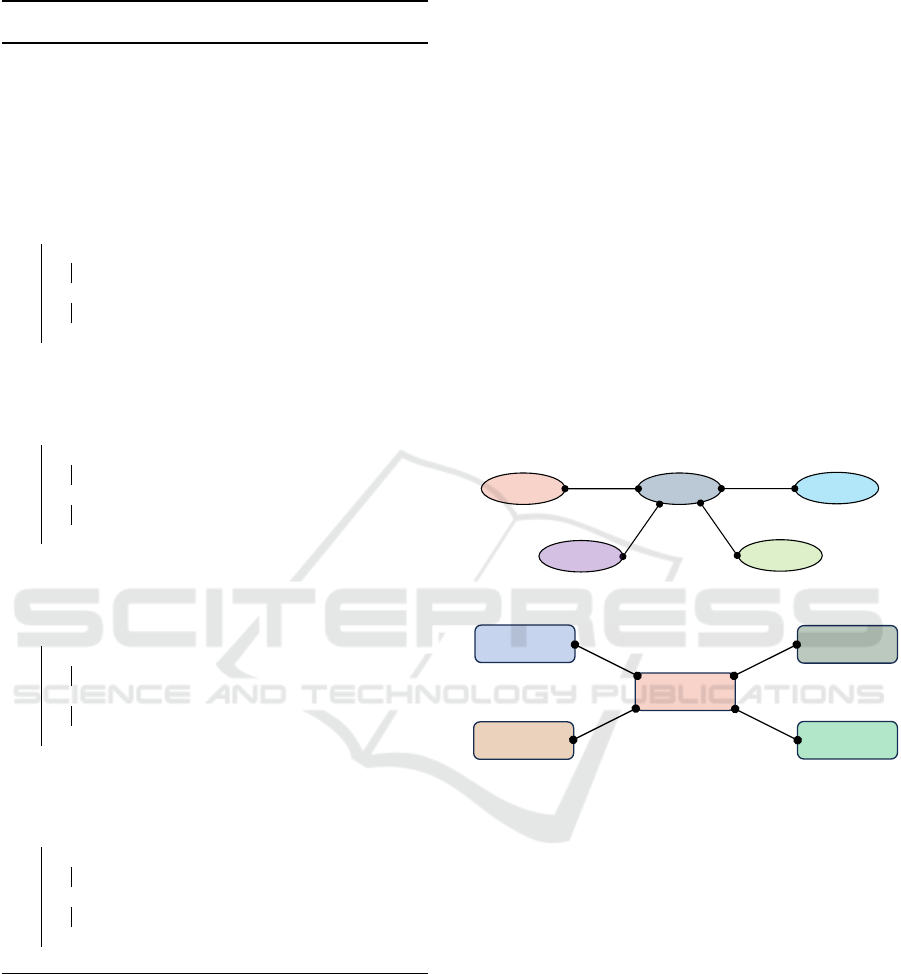
Figure 7 depicts the transaction structure of the
Proof of Compliance consensus mechanism. The PoC
takes input from an audit trail that contains (i) audit
trail ID, (ii) informed consent ID or SIC ID, (iii) hon-
est broker ID, (iv) honest broker report, and (v) times-
tamp data. Applicable policy and sharing informed
consent are retrieved from the policy repository and
public blockchain to check the status of each audit
trail. After verifying, each auditor node determines
the compliance status for each transaction. There are
three compliance statuses: (i) compliant, which indi-
cates there are no security and privacy policy viola-
tions; (ii) non-compliant means there is a policy vio-
lation, and (iii) non-determined defines that required
information is not available to check status.
Audit
Trail ID
Informed
Consent
ID
Honest
Broker
ID
Honest
Broker
Report
Timestamp
Data
Sender
Receiver
PHI
Purpose
Audit Trail ID
Compliance Status
Proof of Compliance (PoC)
Compliant
Non-Compliant
Not-Determined
Audit
Trails
Private Audit
Blockchain
Public
Blockchain
Network
Policy
Repository
Informed Consents
Policy
Figure 7: Proof of Compliance (PoC) Transaction Structure.
The auditor nodes can be hospitals, various gov-
ernments, regulatory agencies, insurance companies,
business associates, and others. They do not store
audit trail data and are responsible for maintaining
compliance status for each transaction. Reports from
all auditor nodes are collected and combined for the
final decision. Algorithm 2 shows the core func-
tionalities of PoC: signature verification and order,
transaction validation, policy compliance verification,
and ledger modification. Due to page constraints,
we do not include detailed protocols, communication
mechanisms, and synchronization techniques. They
are our future research communications with perfor-
mance evaluations for compliance accuracy measure-
ments, data security and privacy, and others.
6 SIC PROVENANCE SERVICES
Patients need to be provided with the specifics of their
given sharing informed consent: who can share what
PHI with whom, and for what purposes? Addition-
ally, patients should understand the execution of their
consent, including the details of who shares which
healthcare data, the timing of these actions, and oth-
ers. They should also know whether those sharing
Balancing Patient Privacy and Health Data Security: The Role of Compliance in Protected Health Information (PHI) Sharing
219
Algorithm 2: Proof of Compliance (PoC) Consensus
Method.
Input : (i) list of transactions (T xns) and (ii) set of policy Plcy
Output: (i) list of accepted/rejected transactions (T xns) and (ii)
list of transactions that are policy compliance
1 Initialization (i) N
Order
: order nodes, (ii) N
Validator
:
validator/endorser nodes, (iii) N
Audit
: audit nodes, and (iv)
N
Committer
: committer nodes
2 Signature Verification and Order
3 T xn
Valid
= [] /* accepted transaction list */
4 T xn
Invalid
= [] /* rejected transaction list */
5 for i ← T xns
Start
to T xns
End
by 1 do
6 if ζ(PK
i
, T nx
i
) == Signed
T nx
i
then
7 T xn
Valid
← Txn
Valid
+ T xn
i
8 else
9 T xn
Invalid
← Txn
Invalid
+ T xn
i
10 end if
11 end for
12 Transaction Validation T xn
Accepted
= [] /* accepted
transaction list */
13 T xn
Re jected
= [] /* rejected transaction list */
14 for i ← T xn
Valid
Start
to T xn
Valid
End
by 1 do
15 if ζ(PK
i
, T nx
i
) == Signed
T nx
i
then
16 T xn
Accepted
← Txn
Accepted
+ T xn
Valid
i
17 else
18 T xn
Re jected
← Txn
Re jected
+ T xn
Valid
i
19 end if
20 end for
21 Policy Compliance Verification
22 T xn
Compliance
= [] /* compliance transactions */
23 T xn
NonCompliance
= [] /* noncompliance transactions */
24 for i ← T xn
Accepted
Start
to T xn
Accepted
End
by 1 do
25 if ζ(PK
i
, T nx
i
) == Signed
T nx
i
then
26 T xn
Compliance
← Txn
Compliance
+ T xn
Accepted
i
27 else
28 T xn
NonCompliance
← Txn
NonCompliance
+ T xn
Accepted
i
29 end if
30 end for
31 Ledger Modification
32 T xn
Compliance
= [] /* compliance transactions */
33 T xn
NonCompliance
= [] /* noncompliance transactions */
34 for i ← T xn
Accepted
Start
to T xn
Accepted
End
by 1 do
35 if ζ(PK
i
, T nx
i
) == Signed
T nx
i
then
36 T xn
Compliance
← Txn
Compliance
+ T xn
Accepted
i
37 else
38 T xn
NonCompliance
← Txn
NonCompliance
+ T xn
Accepted
i
39 end if
40 end for
activities comply with the applicable security and pri-
vacy policies, regulatory requirements, industry best
practices, contractual obligations, etc. This section
outlines the services related to the given and executed
consent that patients can access within the proposed
framework, provided they have the necessary creden-
tials. The primary goal of provenance services is to
ensure patients receive accurate and comprehensive
information and have confidence regarding their given
and executed informed consent.
6.1 Given Consent Services
In this scope, patients can access the list of all the
given consents for sharing healthcare data to date.
These consents are in their original state and may
or may not be executed for making data-sharing de-
cisions. Patients can see the list where each con-
sent contains information about who the sender is,
who the receiver is, what the protected healthcare
information is, and the purpose of sharing health-
care data when the sharing informed consent is given.
Given consent services can be delivered: (i) sender-
oriented, (ii) receiver-oriented, (iii) PHI-oriented,
and (iv) purpose-oriented. For example, patients can
have sender-oriented consent services that include all
the consents given to a particular sender or a group
of senders. Figure 8 depicts sender-oriented given
consents for Donald, who has permission to share
PHI with various receivers. Figure 9 shows the PHI-
oriented given consents for health record PHI-1008.
Donald
PHI-1003
PHI-1005
PHI-1007
PHI-1008
[
Lucy, Research
]
[Sam, Diagnosis]
[
Audrey, Treatment
]
[Steve, Treatment]
[Sam, Treatment]
[Steve, Diagnosis]
[
Hazel, Marketing
]
[
William, Diagnosis
]
Figure 8: Sender-oriented Given Consents.
PHI-1008
Diagnosis
Marketing
Treatmen t
Research
[Donald, Sam]
[Donald, Steve]
[Donald, Steve]
[Donald, Sam]
[Willow, Lena]
[Willow, Arden]
[Edith, Jane]
[Edith, James]
Figure 9: PHI-Oriented Given Consents.
6.2 Executed Consent Services
After generation, all consents may or may not be ex-
ecuted to share healthcare data. A consent is ex-
ecuted when a sender wants to share PHI with the
receiver when there is a need that serves the pur-
pose included in the consent. If consent is executed,
other information is stored in addition to the consent,
like an honest broker ID, a pertinent policy status
that the broker has certified, a timestamp, etc. Exe-
cuted consent services can be provided: (i) sender-
oriented, (ii) receiver-oriented, (iii) PHI-oriented,
and (iv) purpose-oriented. For example, a patient may
need to know the executed consent for a particular
receiver. Figure 10 shows receiver-oriented executed
consents for Steve with senders and timestamps. Fig-
ure 11 depicts purpose-oriented executed consents for
treatment with sender, receiver, and timestamp.
SECRYPT 2024 - 21st International Conference on Security and Cryptography
220
Steve
PHI-1003
PHI-1008
PHI-1007
PHI-1005
[Edith, Research , 02-25-23, 2:42 PM]
[Donald, Treatment , 02-03-24, 10:42 AM]
[Donald, Diagnosis , 02-02-24, 2:42 PM]
[Willow, Treatment , 02-03-24, 10:42 AM]
[Donald, Diagnosis , 02-02-24, 2:42 PM]
[Donald, Treatment , 02-03-24, 10:42 AM]
[Edith, Research , 02-25-23, 2:42 PM]
[Willow, Treatment , 02-03-24, 10:42 AM]
Figure 10: Receiver-oriented Executed Consents.
Tre atm ent
PHI-1007
PHI-1008
PHI-1003
PHI-1005
[Donald, Steve , 02-05-24, 11:28 AM]
[Donald, Sam , 01-25-24, 03:22 PM]
[Donald, Hazel , 01-28-24, 04:42 PM]
[Donald, Audrey , 02-03-24, 10:55 AM]
Figure 11: Purpose-oriented Executed Consents.
6.3 Service Delivery to Patients
Patients will interact with the system through inter-
faces like GUIs or apps supported by wallets like
Coinbase and MetaMask for transaction signing and
data access management. These wallets safeguard
users’ private keys and credentials. The system ac-
commodates various user types, including those re-
quiring tailored interfaces, such as seniors, physically
disabled individuals, minors, and others. Healthcare
providers may address the specific needs of these di-
verse users and can develop apps and software to pro-
vide services. Patients’ devices and apps are assumed
to be secure against unauthorized access, and commu-
nication with the blockchain is also protected.
7 EXPERIMENTAL EVALUATION
The Ethereum Virtual Machine (EVM) based three
blockchain test networks (Arbitrum, Polygon, and
Optimism) are chosen for the experiments. We de-
veloped and deployed smart contracts for storing and
retrieving PPA integrity and informed consent in test
networks. Ethereum’s Remote Procedure Call (RPC)
API services are employed for deploying smart con-
tracts and performing transactions on these networks
(Kim and Hwang, 2023). Utilizing public RPC elim-
inates the need to maintain a blockchain node for
contract interaction, assuming minimal resource us-
age (CPU, HDD, bandwidth) on the local machine.
We used Metamask wallet to sign and authorize trans-
actions using ETH and MATIC faucet tokens as gas.
Healthcare providers may invest in infrastructure such
as blockchain nodes, web interfaces, and mobile ap-
plications for seamless service interaction between
patients and healthcare systems. Storing informed
consent on public blockchains like Ethereum incurs
direct monetary costs. Patients, insurance companies,
and others can split these costs, like those for doctor
visits, medications, and laboratory tests. The follow-
ing discusses gas consumption and time requirements.
7.1 Gas Consumption
Gas is needed for any activity on the Ethereum net-
work involving writing data or changing the state of
the blockchain. Smart contract deployment and func-
tion calling costs to write data on the blockchain net-
work are considered in this work. A contract is de-
ployed for each patient separately to manage consent-
related queries efficiently. The cost of smart contract
deployment is proportional to the size of the code
(Albert et al., 2020). This is a one-time cost for a
single-contract deployment. How much it costs to
call a function depends on how many times it is called
and how much data needs to be stored or changed on
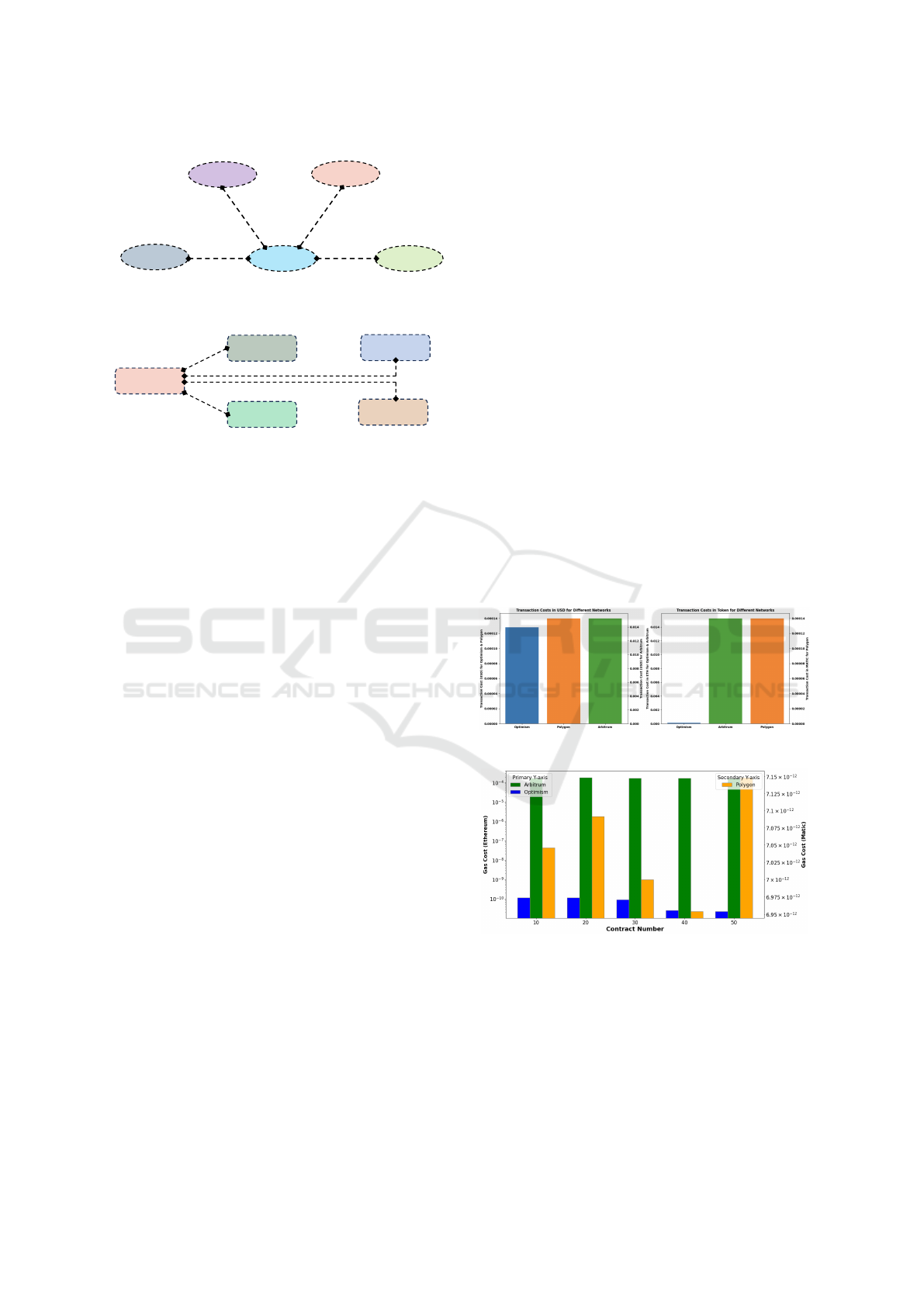
the blockchain network. Figure 12, 13, 14, 15, and
16 show the contract deployment and consent storage
costs in gas (token) and USD for three test networks.
Figure 12: PPA Integrity Storage Cost.
Figure 13: Contract Deployment Gas Cost.
7.2 Time Requirements
Blockchain-based applications require block data
writing and reading time requirements. Writing time
includes smart contract deployment and data addition.
Table 3 shows the writing time for various consent
numbers for the test networks. The reading time in-
dicates the required time to get data from the block
Balancing Patient Privacy and Health Data Security: The Role of Compliance in Protected Health Information (PHI) Sharing
221
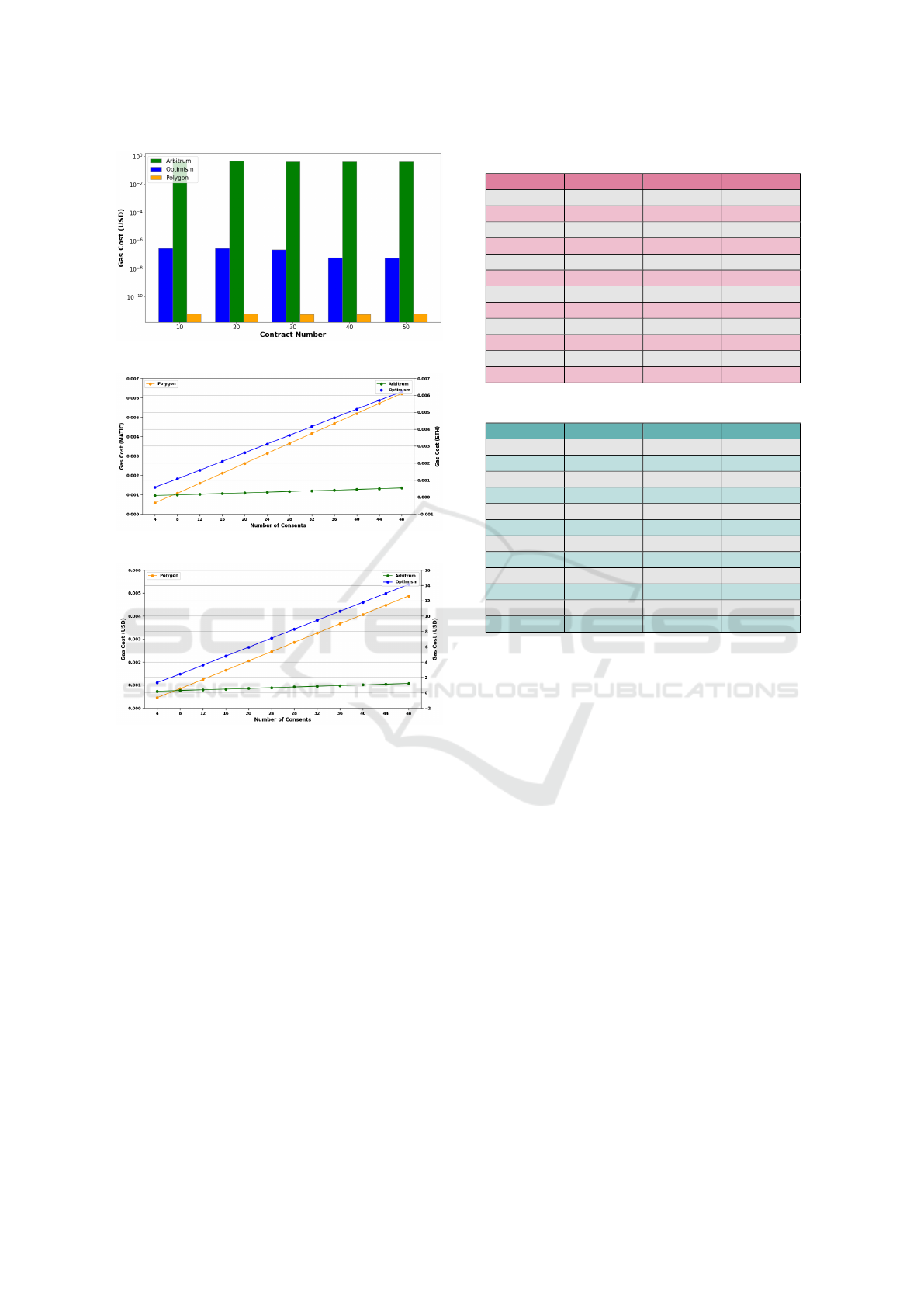
Figure 14: Contract Deployment USD Cost.
Figure 15: Consent Storage Gas Cost.
Figure 16: Consent Storage USD Cost.
of the blockchain ledger. All the read calls of smart
contracts are gas-free. Table 4 shows the test net-
work’s reading time for various consent numbers. The
same smart contracts and consents are used for all test
networks. Maintaining a node locally can reduce the
reading time from the network where block data can
be accessed in real-time. The system continuously
synchronizes with the blockchain network to update
the ledger data. The providers can maintain local
nodes for faster authorizations.
8 CONCLUSIONS
Sharing patient health data is beneficial for improv-
ing medical care, diagnosis, and other essential ser-
vices. However, keeping this information private and
secure is important. Different policies from various
authorities help ensure the privacy and security of this
health data. Complying with these policies ensures
Table 3: Consent Writing Time to Blockchain Network.
Consents # Polygon Arbitrum Optimism
4 6.719 Sec 6.854 Sec 8.459 Sec
8 5.961 Sec 6.068 Sec 7.785 Sec
12 5.972 Sec 6.338 Sec 7.738 Sec
16 6.309 Sec 6.063 Sec 7.762 Sec
20 6.085 Sec 6.081 Sec 8.163 Sec
24 6.015 Sec 2.476 Sec 7.482 Sec
28 10.117 Sec 6.521 Sec 7.718 Sec
32 10.041 Sec 2.451 Sec 8.268 Sec
36 10.045 Sec 6.662 Sec 7.736 Sec
40 14.039 Sec 2.458 Sec 7.797 Sec
44 10.048 Sec 6.201 Sec 7.881 Sec
48 10.138 Sec 6.174 Sec 8.971 Sec
Table 4: Consent Reading Time from Blockchain Network.
Consents # Polygon Arbitrum Optimism
4 0.426 Sec 0.234 Sec 0.399 Sec
8 0.366 Sec 0.201 Sec 0.423 Sec
12 0.337 Sec 0.239 Sec 0.425 Sec
16 0.346 Sec 0.259 Sec 0.423 Sec
20 0.327 Sec 0.288 Sec 0.442 Sec
24 0.344 Sec 0.241 Sec 0.579 Sec
28 0.358 Sec 0.221 Sec 0.536 Sec
32 0.361 Sec 0.288 Sec 0.495 Sec
36 0.401 Sec 0.225 Sec 0.512 Sec
40 0.36 Sec 0.206 Sec 0.482 Sec
44 0.361 Sec 0.233 Sec 0.462 Sec
48 0.522 Sec 0.224 Sec 0.434 Sec
that safety measures are working. Getting patients’
informed consent is also critical to protecting their
privacy and giving them control over sharing their in-
formation. Patients need to understand fully how their
data is shared. Patients should also feel confident that
strong safeguards are in place to protect their data.
Using smart contracts to manage patient consent is a
promising way to securely and privately share health
data. These systems let patients control their health
records and agree to how doctors and others use them.
Blockchain technology improves these systems by
providing security, efficiency, decentralization, trans-
parency, and immutability. This enhances the trust-
worthiness and responsibility of sharing healthcare
data among everyone involved.
Looking forward, our objective is to provide func-
tional mechanisms for essential consent management
operations for data sharing and enhancing patient care
and services. Management operations generate, mod-
ify, withdraw, expire, and archive consent. Improper
consent can cause sensitive data disclosure or prevent
getting services. Consent generation must be done
carefully. It is necessary to modify a given consent
due to improper components like the receivers or pur-
poses. In this situation, a modified new consent must
be deployed, while the old consent must be moved to
the achieving repository.
SECRYPT 2024 - 21st International Conference on Security and Cryptography
222

ACKNOWLEDGEMENTS
This work was partially supported by the U.S. Na-
tional Science Foundation under Grant No. 1822118
and 2226232, the member partners of the NSF IU-
CRC Center for Cyber Security Analytics and Au-
tomation – Statnett, AMI, NewPush, Cyber Risk Re-
search, NIST, and ARL – the State of Colorado (grant
#SB 18-086), and the authors’ institutions. Any opin-
ions, findings, conclusions, or recommendations ex-
pressed in this material are those of the authors and
do not necessarily reflect the views of the National
Science Foundation or other organizations and agen-
cies.
REFERENCES
Al Amin, M., Altarawneh, A., and Ray, I. (2023). Informed
consent as patient driven policy for clinical diagno-
sis and treatment: A smart contract based approach.
In Proceedings of the 20th International Conference
on Security and Cryptography-SECRYPT, pages 159–
170.
Alarcon, M. L., Nguyen, M., Debroy, S., Bhamidipati,
N. R., Calyam, P., and Mosa, A. (2021). Trust model
for efficient honest broker based healthcare data ac-
cess and processing. In 2021 IEEE International Con-
ference on Pervasive Computing and Communications
Workshops and other Affiliated Events (PerCom Work-
shops), pages 201–206. IEEE.
Albert, E., Correas, J., Gordillo, P., Rom
´
an-D
´
ıez, G., and
Rubio, A. (2020). Gasol: Gas analysis and opti-
mization for ethereum smart contracts. In Interna-
tional Conference on Tools and Algorithms for the
Construction and Analysis of Systems, pages 118–125.
Springer.
Alhajri, M., Salehi Shahraki, A., and Rudolph, C. (2022).
Privacy of fitness applications and consent manage-
ment in blockchain. Proceedings of the 2022 Aus-
tralasian Computer Science Week, pages 65–73.
Aljabri, M., Aldossary, M., Al-Homeed, N., Alhetelah, B.,
Althubiany, M., Alotaibi, O., and Alsaqer, S. (2022).
Testing and exploiting tools to improve owasp top ten
security vulnerabilities detection. In 2022 14th In-
ternational Conference on Computational Intelligence
and Communication Networks (CICN), pages 797–
803. IEEE.
Amofa, S., Sifah, E. B., Kwame, O.-B., Abla, S., Xia, Q.,
Gee, J. C., and Gao, J. (2018). A blockchain-based
architecture framework for secure sharing of personal
health data. In 2018 IEEE 20th international confer-
ence on e-Health networking, applications and ser-
vices (Healthcom), pages 1–6. IEEE.
Balistri, E., Casellato, F., Giannelli, C., and Stefanelli,
C. (2021). Blockhealth: Blockchain-based secure
and peer-to-peer health information sharing with data
protection and right to be forgotten. ICT Express,
7(3):308–315.
Buterin, V. et al. (2014). A next-generation smart contract
and decentralized application platform. white paper,
3(37):2–1.
Chung, K., Chung, D., and Joo, Y. (2006). Overview of ad-
ministrative simplification provisions of hipaa. Jour-
nal of medical systems, 30:51–55.
Fan, K., Wang, S., Ren, Y., Li, H., and Yang, Y. (2018).
Medblock: Efficient and secure medical data shar-
ing via blockchain. Journal of medical systems,
42(8):136.
Hutchings, E., Loomes, M., Butow, P., and Boyle, F. M.
(2021). A systematic literature review of attitudes to-
wards secondary use and sharing of health administra-
tive and clinical trial data: a focus on consent. System-
atic Reviews, 10:1–44.
Kim, S. and Hwang, S. (2023). Etherdiffer: Differential
testing on rpc services of ethereum nodes. In Pro-
ceedings of the 31st ACM Joint European Software
Engineering Conference and Symposium on the Foun-
dations of Software Engineering, pages 1333–1344.
Le, T.-V. and Hsu, C.-L. (2021). A systematic literature re-
view of blockchain technology: Security properties,
applications and challenges. Journal of Internet Tech-
nology, 22(4):789–802.
Lopez Martinez, A., Gil P
´
erez, M., and Ruiz-Mart
´
ınez, A.
(2023). A comprehensive review of the state-of-the-
art on security and privacy issues in healthcare. ACM
Computing Surveys, 55(12):1–38.
Mulamba, D. and Ray, I. (2017). Resilient reference moni-
tor for distributed access control via moving target de-
fense. In Data and Applications Security and Privacy
XXXI: 31st Annual IFIP WG 11.3 Conference, DBSec
2017, Philadelphia, PA, USA, July 19-21, 2017, Pro-
ceedings 31, pages 20–40. Springer.
Rights (OCR), O. f. C. (2008). HIPAA Enforcement. Last
Modified: 2021-06-28T08:59:34-0400.
Samuel, C. N., Glock, S., Verdier, F., and Guitton-
Ouhamou, P. (2021). Choice of ethereum clients for
private blockchain: Assessment from proof of author-
ity perspective. In 2021 IEEE International Con-
ference on Blockchain and Cryptocurrency (ICBC),
pages 1–5. IEEE.
Shah, M., Li, C., Sheng, M., Zhang, Y., and Xing, C.
(2019). Crowdmed: A blockchain-based approach to
consent management for health data sharing. In Smart
Health: International Conference, ICSH 2019, Shen-
zhen, China, July 1–2, 2019, Proceedings 7, pages
345–356. Springer.
Timmermans, S. (2020). The engaged patient: The
relevance of patient–physician communication for
twenty-first-century health. Journal of Health and So-
cial Behavior, 61(3):259–273.
Zhuang, Y., Sheets, L. R., Chen, Y.-W., Shae, Z.-Y., Tsai,
J. J., and Shyu, C.-R. (2020). A patient-centric health
information exchange framework using blockchain
technology. IEEE journal of biomedical and health
informatics, 24(8):2169–2176.
Balancing Patient Privacy and Health Data Security: The Role of Compliance in Protected Health Information (PHI) Sharing
223
