
Possibilities of Simulation of the Socio-Political Conflicts Based on the
Mathematical Technique of the Langmuir Monolayers Theory
A. Y. Petukhov
1,2 a
, A. N. Morozov
3
, Yu. M. Selivantyev
3
, I. V. Vorotyntsev
3
, O. A. Raitman
3
and N. S. Morozov
4
1
Lomonosov Moscow State University, Leninskie Gory, 1, 119991, Moscow, Russia Federation
2
ANO «Neymark» Lobachevsky State University, Nizhniy Novgorod, Alexeevskaya 6/16, 603000, Russia Federation
3
Mendeleev University of Chemical Technology, Miusskaya sq., 9, Moscow, 125047, Russia Federation
4
Lobachevsky State University, Nizhniy Novgorod, Gagarina Ave., 23, 603022, Russia Federation
Keywords: Monolayer, Mathematical Simulation, Socio-Political Processes, Socio-Political Conflicts.
Abstract: The possibility of creating the model of social and political processes (in particular, that of conflicts) with use
of the chemical theory of monolayers is studied. The main theoretical approaches to simulation of the social
processes are analyzed. A formalized dynamic parameters of protest pro-cesses in the crowd are defined. The
mathematical model based on the chemical theory of mono-molecular layers and the coupling field parameter
(h) are proposed. In addition, the basic equations are derived, interpreted and applied to social processes. The
main effects in the studied pro-cesses are described.
1 INTRODUCTION
The question on forecasting the social and political
conflicts, including that by means of mathematical
simulation, is of the most actuality, especially in the
conditions of increasing geopolitical confrontation.
In terms of classical approaches developed earlier,
a social conflict is defined in general as a terminal
phase in increased confrontation of interests between
single individuals, groups of people or in society at
whole. This phase is featured by incongruity of
separate interests, goals and positions of the
interacted subjects. Conflicts can be hidden or
explicit, but they are always based on the
unwillingness to compromise or even on the complete
absence of dialogue between the opposing sides
(Dahrendorf, 1965; Gurr and Harff, 1994).
As to mathematical models derived from physical
and chemical analogies, they are still relatively rare in
sociological researches. Nevertheless, there are a
number of works concerning a question on simulation
of social and political processes, which a certain
success has been achieved in - (Mason, 2013; Traud
et al., 2011).
a
https://orcid.org/0000-0002-7412-5397
The models presented up to date can be divided
into three types:
The 1st type are the conceptual models based on
identification and analysis of general historical
regularities shaped as cognitive schemes and
described the logical links between various factors
which affect historical processes. Such models are
featured by high degree of generalization, but they are
still not mathematical ones, being rather logical and
conceptual in their nature.
The 2nd type are the particular mathematical
models of simulation type which are focused on the
certain historical event or phenomenon. In such
models, the main attention is paid to analysis and
detailed description of factors and processes that
effect the phenomena under research. The models of
that type work, as a rule, only if the event under
consideration is highly localized and limited by
duration. These models are "tied" to a specific
historical event and are not applicable for a long
period of time.
The 3rd type are the mixed models combining the
former two ones. These models simulate a certain
class of social processes, without claiming to describe
392
Petukhov, A., Morozov, A., Selivantyev, Y., Vorotyntsev, I., Raitman, O. and Morozov, N.
Possibilities of Simulation of the Socio-Political Conflicts Based on the Mathematical Technique of the Langmuir Monolayers Theory.
DOI: 10.5220/0012828500003758
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 14th International Conference on Simulation and Modeling Methodologies, Technologies and Applications (SIMULTECH 2024), pages 392-398
ISBN: 978-989-758-708-5; ISSN: 2184-2841
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

in detail the features for each specific historical case
(Malkov, 2004).
As a rule, the classical theoretical model of
dynamic processes in nonlinear systems (an example
of which can be social movements too) operates with
multidimensional differential equations,
mathematical technique of cellular automata,
approaches of theory of catastrophes, and that of the
theory of self-organized criticality. Stochastic
differential equations by Langevin and Ito-
Stratonovich are used too, as well as analysis of
chaotic systems with reconstruction of stable states
(attractors) by time series (Malkov, 2004; Weber et
al., 2015).
A number of studies were devoted to ethnical
diversity and its impact on economic, social, cultural
development and related processes (Weber et al.,
2015). These are cross-cutting studies on mutual
influence of ethnic and social problems and the
economy. They take into account ethnic and cultural
factors and their combined impact on possible and
running conflicts.
Forecasting and description of social and political
processes uses of variety of methods (Mikhailov and
Gorbatikov, 2012).
Since social conflicts have a significant impact on
society and processes in it, the development of
methods and approaches to describe such phenomena,
as well as to predict them, is of great im-portance.
The use of the mathematical techniques of
Langmuir Monolayers Theory (LMT) for simulations
of social conflicts and their acute phase, which the
mass protests are, was attempted in this study.
2 THEORETICAL BASES OF
SIMULATION
It is easy to see that the parametrization of
mathematical tools used in physics and chemistry
needs to be adjusted in relation to the social process
and should be adapted to the phenomenon that is
currently being considered. Processes in physics and
chemistry and processes in society and politics are not
the same thing. However, we will hypothetically
proceed from the fact that common patterns and
phenomena (threshold effects, self-organization,
fractals, etc.) are spread in nature almost everywhere,
and, consequently, almost the same processes (i.e. the
similar ones) can be observed in society, since people
are also part of nature and must obey the laws of it
too. However, the question on whether the model
does function properly or does not (at least in
particular cases) can be answered only after a
numerical experiment is over and its results (which
are a short- or medium-term forecast) are compared
with what actually happened.
From a mathematical point of view, social and
political processes are not what can be detailed and
completely characterized by a set of parameters, since
the latter undergo constant changes and deformations.
Here, the analogy with Brownian motion seems to be
close enough: it seems that a single particle moves by
well-defined trajectory, but only before it is examined
more closely (Hołyst et al., 2000; Petukhov et al.,
2020; Petukhov and Kaminchenko, 2021; Petukhovm
2021): the real shapes of the tracks turn out to be
tortuous and nonlinear. These smaller changes in
motion (trajectory fluctuations) are influenced by
closer and smaller particles (molecules), which,
randomly moving nearby a large particle, correct the
trajectory of its movement. In social processes, such
fluctuations are caused by manifestations of the free
will of its participants, as well as by other accidental
environmental influences. The next step in
developing such a model should be to consider
external influences that are not accidental, but
external interference.
The proposed model is based on the assumption
that individuals interact in society through a
conditional "communication field" – h. This concept
was introduced earlier (Hołyst et al., 2000), but had a
different type of parametrization and other initial
equations.
The necessity of introducing that concept is
caused by a number of factors:
i) Any live cognitive system (which is both a
society and a person) acts through exchange of in-
formation carried out through nerve impulses in the
brain or in the opioid system of the cerebellum, or in
the form of Internet information flows, or in any other
suitable form.
ii) As follows from the above, the model of
individuals’ behaviour in society is directly related to
the model of information exchange.
iii) That is why it is desirable to use the function
of information exchange as the basis for the model.
For this purpose, the h-function has been
introduced into model. Actually, this is a function of
information exchange occurring in individuals’
medium. But from a mathematical point of view, this
function is to be considered as an informational field
created by any person in society, which modulates the
information interaction itself.
Possibilities of Simulation of the Socio-Political Conflicts Based on the Mathematical Technique of the Langmuir Monolayers Theory
393

Also, from the point of view of the physical and
mathematical description, it should be borne in mind
that here we are talking about a society that cannot be
attributed to a separate object in spatial topology, as
it happens in classical physics. Indeed, from the point
of view of information exchange between individuals,
social space should combine both classical spatial
coordinates and additional specific parameters and
features. This is due to the fact that in the modern
information environment there is no need to be
physically closer to the object of influence in order to
transmit information to it.
Thus, society is a multidimensional socio-
physical space that models the ability of one
individual to cover another with a personal
communicative field, i.e. to exert an informational
influence on him, change his parameters and the
ability to move in this space. And, thus, the mutual
position of those who act in this space also models the
level of relationship between them and involvement
in the exchange of information.
From the point of view of modelling, the conflict
control by means of external influence or any other
way of mediating it (Dahrendorf, 1965) may be
represented as an additional function that depends at
least on the spatial coordinate and that affects overall
stability of the social system and structure of the last
one. There are a number of analogies acting in
physical systems, for example, the dissipative
function, which can appear and work by a number of
ways depending on physical conditions (Hołyst et al.,
2000).
2.1 Monolayer Theory
The Langmuir Monolayer Theory (LMT) is one of the
most successful theories describing the behaviour of
separate molecules on the plane (or at the interface),
as well as their influence on each other upon
concentration increasing. It describes well the
physical and chemical properties of 2D systems,
taking into account the cooperative interaction of
molecules in them. However, before using the LMT
mathematical tools to describe the processes under
study, it is necessary to briefly explain its physical
meaning and draw an analogy with the phenomena
under study.
Monolayers on the aqueous subphase, which are
the films at the air/water interface constructed of
amphiphilic compounds with a thickness of just one
molecule, have a special structure and exhibit unusual
physical and chemical properties (Arslanov et al.,
2022). Depending on number of molecules the
monolayer is formed from, it can be sparse or tightly
packed. The thermodynamic properties of the film are
usually studied using a Langmuir device. It consists
of the following elements: a "trough", which is a
container filled with aqueous subphase; movable
barriers designed to change the surface water area;
and a Langmuir film balance with a Wilhelmy plate
for surface tension measurement (Moehwald and
Brezesinski, 2016). In order to determine the
thermodynamic parameters of a monolayers, Irving
Langmuir has introduced the concept of surface
pressure which is the mathematical difference
between the values of surface tension of pure water
and that with the surfactant present. In addition,
surface pressure is a two-dimensional analogue of
common thermodynamic pressure; that is the force
acting on the unit length of the measuring plate.
Knowing exactly the amount of substance placed on
the surface and, consequently, the number of
"floating" molecules, it is possible to build the so-
called monolayer compression isotherm by smoother
changing the water surface area (using movable
barriers). The isotherm represents the dependence of
the monolayer surface pressure (π, mN/m) on the
water surface area available to each molecule (A, Å
2
).
Analyzing this function (insert in Fig. 1), it is possible
to make conclusions about the processes occurring in
the monolayer, such as interaction of molecules, their
reorientation and conformational rearrangement, as
well as about phase transitions in the film.
If the area provided to one molecule is large
enough, the molecules do not interact with each other,
and the system as a whole can be represented as a
two-dimensional ideal gas (see Figure 1a), which
conforms the equation:
𝜋𝐴 = 𝑘𝑇 (1)
This equation is a two-dimensional analogue of
the Mendeleev and Clapeyron equations for two
degrees of freedom for translational motion of
molecules.
When the surface area becomes too small and,
consequently, the pressure increases, equation (1)
stops working properly, and behaviour of monolayer
can be described with a certain accuracy by two-
dimensional analogue of Van-der-Waals equation:
𝜋+
𝐴−𝑏
= 𝑘𝑇 (2)
Here the coefficient a is introduced for correcting
the pressure reduced due to interaction of amphiphilic
molecules with each other. This interaction consists
in dispersed attraction of hydrocarbon chains,
accompanied by Coulomb repulsion of charged
carboxyl groups. The coefficient b corresponds to
SIMULTECH 2024 - 14th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
394
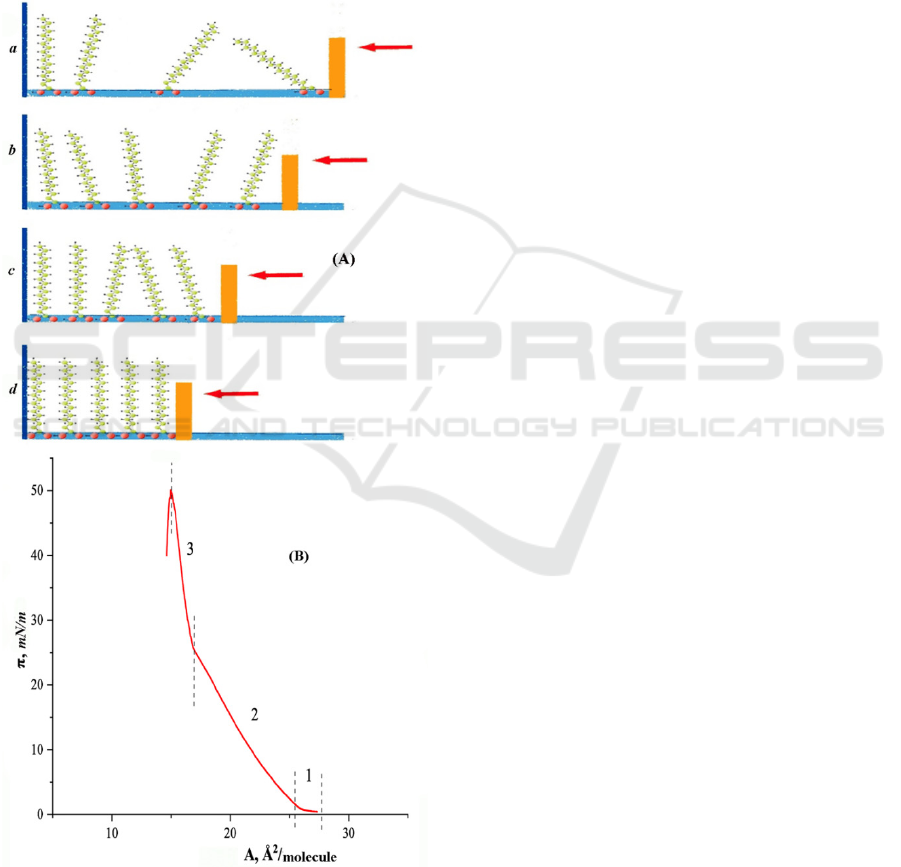
minimum value of area per molecule in a tightly
packed monolayer.
An increase in pressure transforms the monolayer
into a two-dimensional liquid state, in which the
molecules come close enough to interact with each
other, but there is still no definite order in the film
structure (Fig. 1, b).
By reducing the surface area available per
molecule, the monolayer is first converted into a
liquid crystal phase, and then into a two-dimensional
solid state (Fig. 1, c and d, respectively). Hydrocarbon
Figure 1: (A): States of phase in monolayer of stearic acid
at various surface pressure: 2D gas (a), 2D liquid (b), liquid
crystal (c), 2D solid state (d). (B): An isotherm of stearic
acid compression at a temperature of 20°C.
chains become less flexible and line up almost
perpendicular to the surface. Such transformations are
phase transitions of the first kind. Further limitation
of the subphase surface and, consequently, reduction
of the monolayer area is accompanied by a rapid
increase in pressure. As a result, the system,
previously structured in two dimensions, breaks
down, being unable to withstand such a load (Figure
1). So called collapse of the monolayer occur. The
structure loses its integrity: one part of the molecules
moves into the water, while the second begins to
crawl on top of each other, forming both regular
multi-layered ensembles and disordered aggregates
(Oliveira et al., 2022).
Until now, the physical aspects of phase
transitions in monolayers, placed on the surface of
water, have not been well studied, and there is still no
numerical theory explaining the multilevel
supramolecular interactions of amphiphilic molecules
with each other, as well as with subphase molecules.
From a chemical point of view, in order to
combine all the influencing factors into a single
system, this is necessary to take into account the
following phenomena: steric repulsion and dispersion
attraction of hydrocarbon chains; Coulomb and
dipole-dipole interaction between polar fragments of
molecules; formation of two-dimensional hydrates
and hydrogen bonds between surfactants and water;
changes in the structure of the aqueous layer surface
affected by monolayer; and so on. Moreover, the
introduction of ions (regardless on the charge sign)
into the subphase should drastically change the
potential of the double electric layer at the air/water
interface, affecting the thermodynamic properties of
two-dimensional phase transitions and phase states.
In this regard, approximate calculation methods are
currently mainly used, such as the mean field method,
the scaling method and other approaches, including
the Monte-Carlo method, grid models and other
microscopic approaches (Lösche M. et al. 1985).
It is important to note here that a number of
interesting phenomena are observed in such systems,
including threshold effects associated with the
processes of collapsing, self-organization of
molecules (clustering), etc. These effects are of
interest as they are the analogues of processes
occurring in a crowd, for example, during mass riots,
protests, etc., i.e. when previously unorganized crowd
begins to organize itself to give separate groups and
leaders appearance, or when a relatively peaceful
protest goes far and destructive actions occur
(threshold effect, collapse). Of course, these are the
cases when protest has no serious preliminary
preparation, being just spontaneous one.
Possibilities of Simulation of the Socio-Political Conflicts Based on the Mathematical Technique of the Langmuir Monolayers Theory
395

3 MATHEMATICAL
REPRESENTATION OF THE
SYSTEM
Within the framework of this study, a type of
mathematical simulation based on concept of
monolayers’ behaviour in liquid and liquid-crystal
state is used. Here, the hydrophobic parts of
amphiphilic molecules can be considered as elements
of the individual's communication field. The phase
transition from the 2D-gas state to that of 2D-liquid
corresponds to process of information exchange
between separate individuals or groups of them. The
absence of interactions itself is out of interest for this
study since the complete individuals' isolation is an
analogue of monolayer’s two-dimensional gas state.
Thus, the transition from communication to the
unification of individuals into groups (the liquid
crystal state of a monolayer) is the first phase
transition that is the subject of further research. Also
here, the general alignment of molecules in similar
spatial positions (Fig.1. b-d) can be interpreted as
mutual coordination of communication fields, that is,
a certain alignment of the general information in the
crowd (coordination of requirements, alignment of
position, self-organization).
Of most interest is the simulation of the second
phase transition., i.e. from liquid crystal state to that
of crystal lattice. Exploration of this process may help
to predict the maximum social stress, after which the
system goes to self-organization. Of the greatest
interest are also cases when self-organization (crystal
formation) is localized in defined areas or even
points, but has not yet spread over the surface in
whole. At the same time, it is worth noting once again
that to solve this problem a lot of resources are
required and, as to numerical methods, at present
there are only partial solutions based on them, and a
complete analytical solution is still not available.
At the first stage, we will limit ourselves by
developing a model with a single-phase transition
(from a liquid state to a liquid-crystal state) and
consider it in context of conditions for consolidation
of a part of society (or a protest crowd) into well-
structured groups.
To perform the simulation procedure, it is
necessary to set the parameter h, which corresponds
to radius of the communication field, and b, which
represents the radius of a person's personal space. If p
is taken as a measure of social tension, then equation
(2) can be transformed as follows:
𝑝 =
()
(3)
where T parameter (acting as the temperature factor
(kT) in initial model) is a measure of social activity of
individuals, i.e. the ability of individual to undertake
a socially significant action.
When individuals (or any related elements) come
each other at a distance less than h, they unite and
form an "island" with an area equal to bn, where n is
a number of individuals in group. Using a chemical
analogy, the situation can be represented as a tightly
packed layer. In the future, it is planned to explore
various structures of such systems, which can be
oligomeric, dendritic and insular. From the point of
view of social sciences, this means the emergence of
various types of associating the individuals into
groups.
With this approach, the pressure will gradually
increase with decreasing area, the trend will be
similar to 1 /x (when moving along the x axis), but
there are sharp changes caused by formation of the
groups, followed by releasing spaces for residual
actors.
It is important to add that in this model the process
of reducing the monolayer area (leading to pressure
increase potentially explainable by use of appropriate
thermodynamic function analogue) is interpreted as
two factors:
i) The indoor influence which is the growth of
social tension due to internal processes in crowd
(so called "massing" effects in classic researches
by Lebon and Moscovici).
ii) The outdoor influence, i.e. provocation,
incitement, etc. (or at least part of such external
influence, which can be interpreted as
temperature factor).
These factors may work together or separately and are
to be the subject for further investigation.
Bearing in mind the above, expression (3) should
be corrected. The parameter of surface area A works
as a function of the two-factor parameter u, which is
a measure of social tension, and, as a first
approximation, a circular distribution equation can be
used:
𝐴
(
𝑢
)
= 𝜋
(
𝑅
−𝑢
)
(4)
where R
o
is the initial value.
The number of individuals, N (or the number of
particles in the chemical model), is randomly
distributed over this surface. Each individual
occupies an area that is part of the whole surface and
under normal conditions, i.e. when the local tension
is lower than its threshold value, corresponds to
parameter h, which is the relative comfortable social
SIMULTECH 2024 - 14th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
396

distance. When the strain exceeds, this parameter
changes drastically. As mentioned above, at the first
stage we will consider the transition to a tightly
stacked layer. This means that area occupied by a
single person will change to b, which is the minimum
area occupied by compact structure:
𝑆
(
𝑢
)
=
𝜋ℎ
, 𝑢
< 𝑢
𝑏, 𝑢
≥ 𝑢
(5)
The local intensity of u
local
depends on
concentration of actors on the mutual surface area:
𝑢
(
𝑟
)
= 𝑃
∑
𝑓
(𝑅
, 𝑟
, 𝑟)
(6)
where P is the proportionality coefficient,
R
interact
(T) is the distance from selected point r(x,
y), which characterizes the maximum radius of
interaction (the average path length during Brown
motion of the particle) and depends on measure of
social mobility T, r
i
(x, y) are the coordinates of the
actor i, and the f
inc
is function which can be written
like this:
𝑓
(
𝑅
, 𝑟
, 𝑟
)
=
1,
|
𝑟−𝑟
|
≤𝑅
0,
|
𝑟−𝑟
|
> 𝑅
(7)
And finally, the model itself may be represented
as the following:
𝑝 (𝑢)=
(
)
(8)
When appropriate parameters for this simulation
are well set, the model can be used to assess and
analyze critical phenomena in order to maintain the
stability of social pressure under random initial
distribution of individuals. It still requires further
refinement since it does not consider the structure of
compact connections between actors. Such structures
can be simulated with use of well-designed methods
of fractal theory, and the processes occurring inside
them can be described in terms of theory of
percolation (Koposov and Iudin, 2008). When local
intensity parameter u
loc
changes, these structures can
undergo the transformations from sparse state to tight
one. Here, the compact monolayer formation is
concerned as the terminate phase, after which the
destruction of the particles themselves (representing
individuals in given model) begins. This can be
interpreted as terminal level of protest followed by
acute conflict, leading to collisions and human
casualties
4 CONCLUSIONS
The social hyper-clasterization of society, the sharp
separation in the informational and social
environment of individuals, as well as cultural and
interethnic disunity, creates conditions for social
conflicts and subsequent protests, riots, etc.
Prevention of social conflicts, determination of their
threshold conditions and search for the most effective
scenarios for their suppression is an important task of
modern social sciences.
In this article, the main approaches to modeling
social processes were briefly considered, and a
formalized definition of parameters describing the
dynamics of mass protest processes was given.
A mathematical model based on the chemical
theory of Langmuir monolayers and the coupling
field parameter h is proposed. In addition, the basic
equations are given, the interpretation and adaptation
of the obtained formulas for social phenomena are
proposed, and the main effects in the processes under
study are described.
The next step planned for refinement of the model
will include its approbation based on a numerical
experiment in order to compare its results with data
on real protest processes.
ACKNOWLEDGEMENTS
The research was carried out with the support of the
Expert Institute for Social Research (research projects
in the form of a state assignment, in the field of socio-
political sciences, competition 2024).
REFERENCES
Dahrendorf R. (1965) Elemente eines Theorie des sozialen
Konflikts. Dahrendorf R. Gesellschaft und Freiheit.
Munchen.
Gurr Т., Harff B. (1994) Ethnic Conflict in World Politics.
Boulder, San Francisco, Oxford.
Mason J.W. D. (2013) Consciousness and the Structuring
Property of Typical Data. Complexity, Volume 18,
Issue 3, pages 28–37, January/February. DOI:
10.1002/cplx.21431
Traud, A. L., Kelsic, E. D., Mucha, P. J. & Porter, M. A.
(2011) Comparing Community Structure to
Characteristics in Online Collegiate Social Networks.
SIAM Review, 53, 526–543.
Malkov S. Yu. (2004) Mathematical Modeling of Historical
Dynamics. Approaches and processes. Ed. M. G.
Dmitriev, RSSU.
Possibilities of Simulation of the Socio-Political Conflicts Based on the Mathematical Technique of the Langmuir Monolayers Theory
397

Weber Sh., Davydov D.V., Dover P.A. Transfers and
Conflict Prevention: pros and cons. Economics and
Mathematical Methods. 2015, 51(2), 60-69.
Mikhailov, A. P. Gorbatikov E. A. (2012). The Basic Model
of the Muumvirate in the System "Power-Society"”,
Mathem. modeling, 24:1, 33-45
Hołyst J. A., Kacperski K., Schweitzer F. (2000). Phase
transitions in social impact models of opinion
formation. Physica A: Statistical Mechanics and its
Applications. 285 (1–2)199–210.
Petukhov A. Y., Malkhanov A.O., Sandalov V.M.,
Petukhov Y.V. (2020) Mathematical modeling ethno-
social conflicts with the introduction of the control
function. Simulation. 96(3) 337–346, DOI:
10.1177/0037549719884629
Petukhov A. Y., Kaminchenko D.I. (2021) Forecasting the
dynamics of socio-political protests in the Republic of
Belarus in 2020. Bulletin of the Tomsk State University,
64, 214-223 DOI: 10.17223/1998863Х/64/20
Petukhov A.Y. (2021) Development of the Russian state in
the 20th and 21st centuries: mathematical modeling
based on the socio-energy approach. Izvestiya Vysshikh
Uchebnykh Zavedeniy. Prikladnaya Nelineynaya
Dinamika, 2021, 29(3), 365–375. DOI:
10.18500/0869-6632-2021-29-3-365-375
Arslanov V. V. et al. (2022) Planar Supramolecular
Systems: Assembly and Functional Potential. Colloid
Journal.. Vol. 84, № 5. P. 581–610.
Moehwald H., Brezesinski G. (2016). From Langmuir
Monolayers to Multilayer Films. Langmuir. 32(41).
10445–10458.
Oliveira O.N., Caseli L., Ariga K. (2022) The Past and the
Future of Langmuir and Langmuir–Blodgett Films.
Chem Rev.. 122(6). 6459–6513.
Lösche M. et al. (1985). Formation of Langmuir-Blodgett
films via electrostatic control of the lipid/water
interface. Thin Solid Films. 133 (1–4), 51–64.
Koposov E.V., Iudin D.I. (2008). Percolation mechanism of
gravitational instability of dispersed systems. Volga
Scientific Journal. 1, 102–109.
SIMULTECH 2024 - 14th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
398
