
Progressing Toward Smart Brain Hemorrhage Detection: Machine
Learning-Based Advanced Medical Imaging Technologies
Jingya Li
a
School of Computer Science, Fudan University, Shanghai, China
Keywords: Brain Haemorrhage Detection, Machine Learning, Deep Learning, Medical Imaging.
Abstract: In the rapidly evolving field of neuroscience, early and accurate detection of brain hemorrhage remains a
significant challenge with profound implications for patient outcomes. The integration of Machine Learning
(ML) techniques into diagnostic processes represents a promising frontier, offering the potential to
revolutionize how brain hemorrhages are identified and treated, thereby reducing the associated morbidity
and mortality rates. This review explores the application of ML in detecting brain hemorrhage. Recognizing
the significance of early and accurate detection, the review outlines the general ML workflow encompassing
data collection, preprocessing, model development, training, and evaluation. It delves into specific ML
methods, including traditional algorithms like Support Vector Machines (SVM) and Random Forests,
alongside deep learning approaches such as Recurrent Neural Networks (RNN) and Convolutional Neural
Networks (CNN), assessing their strengths and limitations. The discussion highlights key challenges faced by
ML in this context, such as the "black box" nature of models affecting interpretability, issues with
generalization across diverse datasets, and concerns surrounding data privacy. Proposed solutions and future
prospects are offered to address these challenges, emphasizing the potential of cascading models and the
importance of integrating more complex modeling techniques for improved clinical efficacy. This review
extensively discusses various machine learning algorithms and their application to brain hemorrhage detection,
aiming to drive improvements in ML and foster the integration of computer-aided diagnosis (CAD) in medical
imaging.
1 INTRODUCTION
Intracerebral hemorrhage (ICH), also known as brain
bleed, is a kind of stroke that occurs when there is
bleeding either between the brain tissue and the skull
or within the brain tissue itself. In the realm of
neuroscience, Intracerebral hemorrhage stands out as
a life-threatening condition, marked by a high fatality
rate and the potential for severe sequelae (Chen,
2024). Based on the urgency of symptoms and the
severity of consequences associated with ICH, it
becomes imperative to ensure the utmost accuracy in
examining, categorizing, and quantifying various
aspects of brain hemorrhages, including the critical
task of accurately gauging the volume and extent of
bleeding.
The diagnosis of brain hemorrhage commonly
relies on a variety of medical imaging techniques,
primarily utilizing Computed Tomography (CT) and
a
https://orcid.org/0009-0009-4832-9191
Magnetic Resonance Imaging (MRI). While both CT
and MRI exhibit high sensitivity in detecting brain
hemorrhages, the preference often leans towards CT,
especially in time-sensitive situations. This
inclination arises due to the quicker turnaround time
of CT scans, making them more suitable for patients
in critical conditions. Despite the widespread use of
MRI for detailed assessments, its extended scanning
duration may limit its applicability during the acute
phase (McGurgan, 2021).
While the comparison between CT and MRI
highlights their respective strengths and limitations,
even with accurate CT results, the intricate nature and
variability in brain hemorrhage imaging pose
significant challenges to manual diagnosis. However,
this is precisely where deep learning demonstrates its
prowess. Given the complexity and variations in these
images, deep learning algorithms excel in discerning
patterns and extracting relevant features, making
422
Li, J.
Progressing Toward Smart Brain Hemorrhage Detection: Machine Learning-Based Advanced Medical Imaging Technologies.
DOI: 10.5220/0012939500004508
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Engineering Management, Information Technology and Intelligence (EMITI 2024), pages 422-428
ISBN: 978-989-758-713-9
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
them valuable tools in enhancing the accuracy and
efficiency of brain hemorrhage diagnosis.
In recognizing the importance of deep learning, it
is pivotal to position it within the broader context of
computer-aided detection (CAD) in the medical field.
Over the past few decades, the integration of CAD in
the analysis of medical datasets has become a
prominent area of research in medical imaging
(Gautam, 2021). This evolution has unfolded over a
span of time, gradually establishing CAD as a major
research focus. In the realm of clinical imaging
systems employing CAD, a spectrum of machine
learning algorithms is widely utilized, including
probability models like Naive Bayes and Gaussian
Mixture Model,as well as Support Vector Machine
(SVM), Artificial Neural Network (ANN), among
others.
In particular, machine learning algorithms based
on convolutional neural networks (CNNs) have
garnered significant attention. Leveraging their
exceptional feature learning and abstraction abilities,
remarkable achievements have been observed,
particularly in the segmentation of cerebral
hemorrhage in CT images (Qiu, 2019, Rao, 2021).
The utilization of CNNs in this context exemplifies
the potential of advanced machine learning
techniques in enhancing the accuracy and efficacy of
medical imaging analyses. Beyond these
advancements, ongoing research and exploration in
this field promise further innovations and
improvements in the diagnosis and understanding of
brain hemorrhages.
Overall, cerebral hemorrhages have received
comparatively less attention within the intersection of
AI and medicine, despite their medical significance.
However, recent years have witnessed substantial
progress, with an increasing number of studies and
algorithmic models significantly advancing the
accuracy, speed, and efficiency of ICH detection.
Thus, there is a crucial demand for a comprehensive
review within this specialized yet advancing field,
where AI converges with medicine.
The main objectives of this review encompass
providing a comprehensive overview of recent
advances in the application of deep learning
algorithms for the detection and classification of brain
hemorrhages. By scrutinizing diverse studies, the
emphasis lies in shedding light on the methodological
strides, performance benchmarks, and clinical
applicability of these technologies. Following this
introduction, the rest of this paper is organized as
follows. Afterward, it will proceed to detailed
analysis of various deep learning models with regard
to its design, training, and validation of brain
hemorrhage applications. The subsequent sections
will explore the inherent limitations and potential
challenges of these models, paving the way for a
comprehensive discussion on avenues for future
optimization and innovation.
2 METHODS
2.1 Framework of Machine
Learning-Based in Hemorrhage
Detection
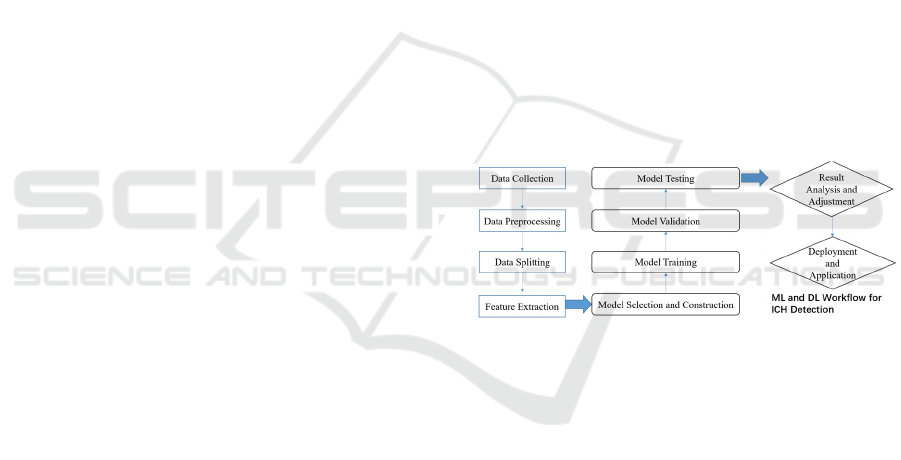
Figure 1. illustrates the workflow for machine
learning and deep learning in intracranial hemorrhage
detection. The process begins with data collection,
followed by data preprocessing, data splitting, and
feature extraction. Subsequently, the selection and
construction of the model take place. Once the model
is established, it undergoes training, validation, and
testing phases. The model is then optimized through
result analysis and adjustment, preparing it for
deployment and application. Further details can be
found in the subsections below.
Figure 1: The workflow of Machine Learning (ML) and
Deep Learning (DL)-based in hemorrhage detection
(Photo/Picture credit: Original).
Dataset Collection. Robust and varied datasets
underpin the successful development of AI
algorithms for cerebral hemorrhage detection. While
exploring publicly available datasets used in the field
of cerebral hemorrhage detection, a prime example of
such resources is the dataset provided by the RSNA
Intracranial Hemorrhage Detection Competition on
Kaggle, which features brain CT images annotated
with hemorrhage conditions, serving as an invaluable
asset for research in this domain (Kaggle, 2020).
Collecting detailed information on available dataset
resources, including specific time frames, case types,
and slice thickness, is crucial. This not only enhances
data quality but also fosters a model's nuanced
understanding and detection capabilities.
Preprocessing. During the data preprocessing
phase shown in Figure 2, several techniques are
commonly employed to enhance image quality and
Progressing Toward Smart Brain Hemorrhage Detection: Machine Learning-Based Advanced Medical Imaging Technologies
423
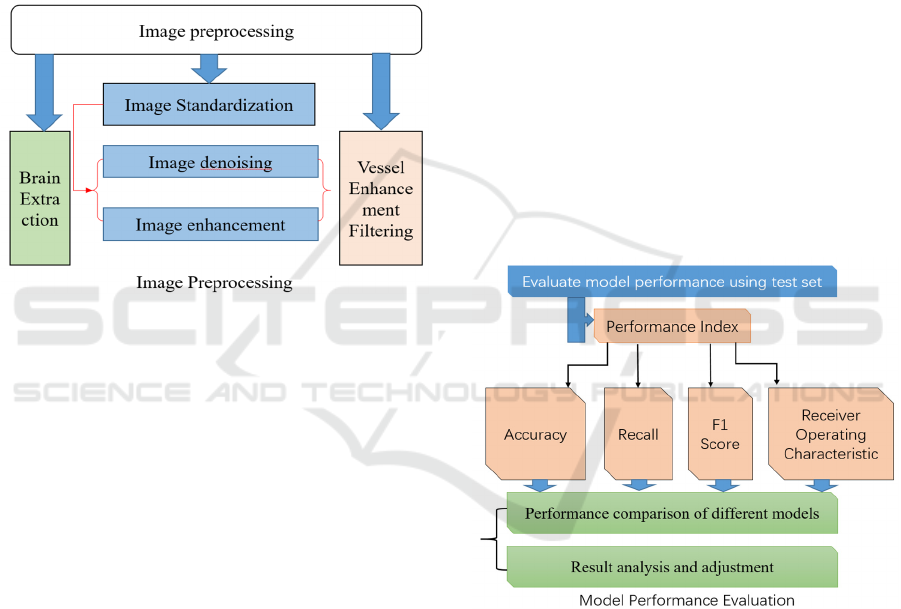
optimize training outcomes, including image
denoising, which aims to reduce random variations
within images, and image enhancement methods like
contrast adjustment and edge enhancement to
improve visual clarity and highlight critical features.
Additionally, normalization and standardization
processes ensure the uniformity of image data in
terms of scale and value range. Furthermore, data
augmentation techniques such as rotation, scaling,
and flipping are utilized to introduce diversity into the
dataset. This is particularly crucial for deep learning
models, enabling them to learn a broader
representation of features.
Figure 2: The workflow of image preprocessing
(Photo/Picture credit: Original).
Data Splitting. Following preprocessing, data is
typically divided into training, validation, and testing
sets. This strategic segmentation is crucial for
assessing the model's performance and robustness,
ensuring it performs well not just on familiar data but
also on unseen datasets. For instance, a study in South
Korea on deep learning for detecting Acute
Intracranial Hemorrhage (AIH) stands out not only
for its collection of a large number of slices with
detailed cerebral hemorrhage information from
various medical institutions but also for its meticulous
categorization of data into three distinct datasets: a
development dataset, an external validation dataset,
and a reader study dataset. This approach not only
ensured the comprehensiveness of the datasets but
also laid a solid foundation for the enhancement of
the algorithm's accuracy and generalizability. A
noteworthy aspect of the research was the
adjudication of imaging standards via a tripartite
radiologist consensus, which bolstered the
annotation's accuracy and trustworthiness. This phase
is pivotal for the formulation of efficacious and
precise AI models since the caliber of annotations
directly correlates with the model's learning
efficiency (Yun, 2023).
Feature Extraction. Finally, in the feature
extraction phase, traditional machine learning
methods and deep learning approaches utilize manual
and automatic feature extraction, respectively. This
allows for the more effective capture and utilization
of key information within image data, enhancing the
model's ability to discern relevant patterns and
characteristics.
Model Training and Analysis. The subsequent
steps largely align with those typical of most machine
learning algorithm applications, which involve
selecting an algorithm to build the model. For
machine learning, this might include algorithms like
SVM and Random Forests, while for deeper learning,
this extends to ANN and CNN. Each of these
algorithms will be elaborated on in further sections.
Following this, the previously segregated training set
is utilized to train and test the model, adjusting
parameters such as the learning rate and the size of
hidden layers. Before deploying the model into a
clinical setting, it's crucial to compare and analyze the
model's performance shown in Figure 3, ensuring it
meets the necessary standards for accuracy and
reliability.
Figure 3: The workflow of model performance evaluation
(Photo/Picture credit: Original).
2.2 Machine Learning
Algorithms-Based Hemorrhage
Detection
2.2.1 SVM
Support Vector Machines are a supervised learning
algorithm well-suited for classifying high-
dimensional data, making them particularly valuable
in medical image analysis. For instance, a study from
Qingdao, China, utilized SVM among four machine
EMITI 2024 - International Conference on Engineering Management, Information Technology and Intelligence
424
learning models to construct a prognostic prediction
model for spontaneous cerebral hemorrhage
outcomes. The findings revealed that SVM
outperformed in overall predictive efficiency,
demonstrating significantly higher accuracy,
specificity, and sensitivity compared to other models
(Li, 2024). SVM's ability to tackle complex nonlinear
problems by selecting appropriate kernel functions
enables it to distinguish effectively between healthy
and damaged tissues in cerebral hemorrhage
detection.
2.2.2 Random Forest
Random Forest, is an ensemble learning technique
that utilizes multiple decision trees for classification
or regression analysis. This method selects random
data subsets and features for each tree during training,
with the final decision derived from a majority vote
or average of all trees' predictions. A study from
Beijing, China, showcased Random Forest's
effectiveness in predicting outcomes of cerebral
hemorrhage surgery, too. Using the Random Forest
model allowed for integrating extensive variables,
like patient condition changes and blood sugar levels
in this study, and therefore, the model demonstrated
high accuracy and consistent probability distribution
between the test and training sets against real-world
outcomes, highlighting its excellent calibration
capability (Gao, 2023). The robustness of Random
Forest in handling overfitting, along with its ability to
process substantial amounts of data, makes it an ideal
choice for classifying types of cerebral hemorrhage.
2.3 Deep Learning Algorithms-Based
Hemorrhage Detection
2.3.1 CNN
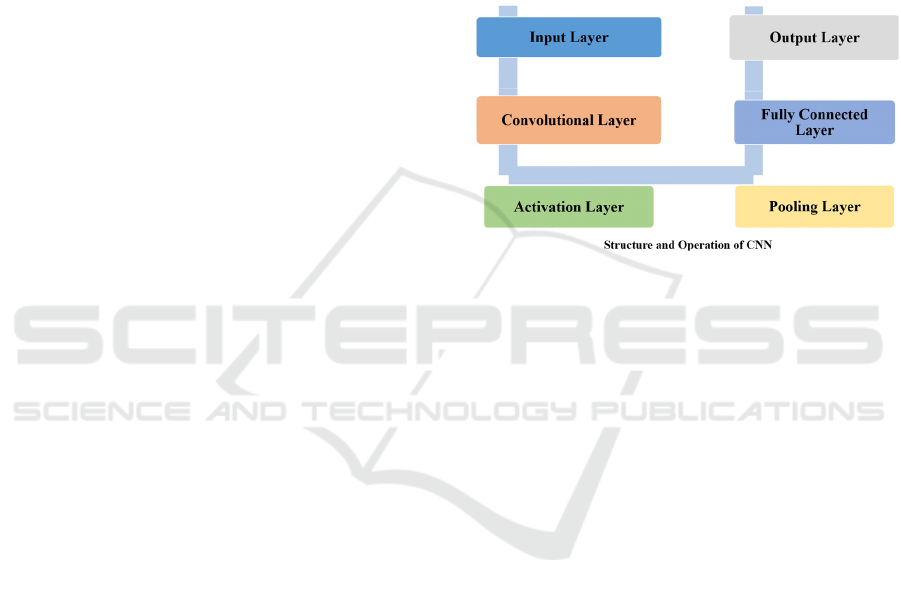
The Convolutional Neural Network depicted in
Figure 4 is tailored for the nuanced task of intracranial
hemorrhage detection from medical imaging.
Beginning with the input layer, the CNN processes
image data, extracting salient features through its
convolutional layers. Activation functions then
introduce non-linearity, allowing for complex
patterns to be captured, while pooling layers reduce
dimensionality, focusing on the most relevant
features. In the fully connected layers, the network
classifies the images, leveraging the distilled features
to accurately distinguish between hemorrhagic and
non-hemorrhagic cases. CNNs, in the context of
medical imaging analysis, have been pivotal, with
algorithms achieving accuracy rates above 99% in
some studies (Mahjoubi, 2023). The inherent
capability of CNNs to autonomously learn and refine
feature recognition empowers the model to uncover
potentially critical biomarkers for intracranial
hemorrhages that might have been previously
underestimated or missed by traditional analytical
methods. By harnessing the intricate feature detection
and classification capabilities of CNNs as outlined in
Figure 4, it is possible to achieve more nuanced and
precise identification of intracranial hemorrhages,
which is critical for timely and effective patient
treatment.
Figure 4: The structure and operation of CNN (Photo/
Picture credit: Original).
2.3.2 RNN
Recurrent Neural Networks (RNNs) are deep learning
models equipped with internal memory, making them
sensitive to sequential dependencies of events. Their
architecture allows them to apply the same operation
across each element in a sequence, where
computations for the current state are influenced by
both the present input and results from previous steps
(Fang, 2021). Although RNNs are not as
predominantly used in image analysis as CNNs, their
proficiency in handling sequential data offers
substantial benefits in specific scenarios related to
ICH detection. Particularly in analyzing time-series
medical imaging data, such as monitoring the
progression of bleeding or assessing treatment
effects, RNNs can account for temporal variations,
capturing changes in hemorrhagic areas over time.
3 DISCUSSION
3.1 Advantages and Disadvantages of
Traditional ML and DL
In the field of neuroscience, machine learning
technologies have demonstrated significant research
Progressing Toward Smart Brain Hemorrhage Detection: Machine Learning-Based Advanced Medical Imaging Technologies
425

Table 1: The Strengths and limitations of ML and DL.
ML (SVM, Random Forest) DL (RNN, CNN)
Limitations 1.Dependence on feature engineering.
2.Limited capability in handling high-dimensional data.
1.High demand for computational resources.
2.Poor interpretability.
Strengths 1.Interpretability.
2.Computational efficiency.
1.Automatic feature extraction.
2.Capability to handle complex patterns.
and application potential. Traditional ML methods
show their powerful capabilities in scenarios
involving smaller datasets with clear feature
structures, as visually summarized in Table 1. Their
prominent advantages lie in their high interpretability
and lower computational costs, which are particularly
important for foundational brain science research in
its exploratory stages. For instance, in preliminary
neuroimaging studies, researchers can use traditional
ML methods to intuitively and thoroughly analyze the
complex relationships between brain region activities
and behavioral responses. Meanwhile, deep learning
technologies, with their excellent ability to
automatically learn features, have shown unparalleled
performance in handling large and complex brain
imaging datasets.
However, as outlined in Table 1, both approaches
have their distinct limitations. Traditional machine
learning models often fall short in dealing with
problems involving nonlinear relationships, high-
dimensional features, and complex data structures,
where deep learning models tend to excel. On the
other hand, deep learning models, despite their
significant performance advantages, require
substantial amounts of training data and suffer from
interpretability issues due to their internal
complexity. These challenges are particularly
pronounced in the field of neuroscience, where
research demands not just high-precision predictive
outcomes but also a deep understanding of the
biological mechanisms behind these results. This
necessitates models that are not only accurate but also
possess a degree of interpretability.
In summary, the choice between machine learning
approaches hinges on the study's goals and the data's
nature and size. Traditional machine learning is suited
for early, small-scale studies with clear features, like
initial brain hemorrhage detection research, offering
ease of interpretation and lower computational needs.
Conversely, deep learning excels in analyzing
extensive datasets and complex patterns, crucial for
advanced brain hemorrhage analysis. Understanding
these methods' strengths and limitations is key to their
effective application in neuroscience, especially for
brain hemorrhage detection.
3.2 Challenges
3.2.1 Lack of Interpretability
The 'black box' nature of deep learning models poses
a significant challenge in neuroscience applications.
This opacity hinders the ability to understand and
explain the rationale behind a model's decisions,
posing problems for trust and validation in scientific
research. When models incorrectly identify or miss
brain hemorrhages, the lack of interpretability
complicates the process of debugging and refining
these algorithms to enhance their performance.
Furthermore, for applications as critical as medical
diagnostics, the inability to elucidate the decision-
making process can impede regulatory approval and
broader acceptance within the medical community.
3.2.2 Generalization Issues
Generalization issues challenge machine learning
models' efficacy in neuroscience due to the
significant variability in datasets, brain states, and
disease conditions. Differences in demographics,
genetic backgrounds, environmental factors, and
disease stages can impede a model's performance
across diverse populations. Additionally, variations
in brain imaging techniques and protocols introduce
further complexity. A study from Japan illustrates a
promising approach to overcoming these hurdles:
researchers developed machine learning predictive
models for hematoma expansion in acute
intracerebral hemorrhage, utilizing multicenter data
and multivendor CT images (Tanioka, 2022). While
this study demonstrates efforts to enhance model
generalizability and applicability across diverse
neurological conditions, it also underscores the
broader issue: the difficulty of developing models that
perform well across varied datasets, brain states, and
disease conditions. Generalization remains a
significant challenge in applying machine learning to
neuroscience.
3.2.3 Data Acquisition and Privacy
The creation and application of machine learning
EMITI 2024 - International Conference on Engineering Management, Information Technology and Intelligence
426

models, especially in neuroscience, demand large
datasets and substantial computational power. Yet,
the high costs of gathering quality data, alongside
privacy and ethical issues, restrict the formation of
extensive datasets, impeding the models' training and
validation process. Moreover, even well-trained ML
models face risks from various adversarial attacks,
such as membership, attribute, and model inversion
attacks, highlighting the crucial need for robust
privacy protection. A notable study introduced a
Phase, Guarantee, and Utility (PGU) triad-based
model after a comprehensive review, emphasizing the
importance of safeguarding data and privacy
throughout the ML process (Xu, 2021). Addressing
these challenges is a vital step for future exploration
and advancement in the field.
3.3 Future Prospects and Possible
Solutions
3.3.1 Linking ML Decisions to Their
Underlying Logic in ICH Detection
Addressing the black box issue in ML for ICH
detection involves enhancing model transparency and
interpretability, notably through integrating
explainable AI (XAI) techniques (Highton, 2023).
Methods like Layer-wise Relevance Propagation
(LRP) and SHAP (SHapley Additive exPlanations)
help visualize and understand influential features in
model predictions. Moreover, developing models
with inherently interpretable structures, such as
decision trees or Generalized Additive Models
(GAMs), allows for a direct understanding of how
inputs affect outputs. The black box issue in ML
transcends technical challenges, encompassing
ethical considerations as well. A study examines
model interpretability through the lens of four ethical
principles—autonomy, beneficence, non-
maleficence, and justice—to assess the necessity and
role of interpretability (Amann, 2020). These
solutions are crucial to ensure that developed models
are not only accurate but also understandable and
trustworthy for healthcare practitioners, integrating
ethical oversight into technological advancements.
3.3.2 Addressing Generalization in ICH
Detection via Transfer Learning and
Domain Adaptation
Incorporating transfer learning and domain
adaptation into ICH deep learning detection enhances
model generalization by utilizing knowledge from
extensive datasets, such as MRI or CT images, and
fine-tuning with a smaller, specific dataset for
hemorrhage detection. Transfer learning addresses
the scarcity of labeled data, while domain adaptation
further tailors models to align with target data
distributions, effectively managing discrepancies
caused by different imaging devices or protocols
across institutions (Xu, 2020).
3.3.3 Leveraging Federated Learning for
Brain Hemorrhage Detection
Incorporating big models into brain hemorrhage
detection, demands a nuanced approach to data
privacy and security. Federated learning emerges as a
pivotal solution in this context. It enables
decentralized model training, allowing for the
collaborative utilization of data across various
locations without the need for direct data exchange.
By ensuring that data remains local and only model
updates are shared, federated learning effectively
addresses privacy and security concerns, facilitating
the use of powerful computational models in sensitive
medical fields.
4 CONCLUSIONS
This article systematically explores the application of
ML in the detection of brain hemorrhage, covering
the cutting-edge developments of ML in brain
hemorrhage detection and emphasizing the diversity
and depth of ML applications in enhancing diagnostic
accuracy and facilitating timely intervention. The
main contribution is a critical analysis of various
machine learning methods, from traditional machine
learning models to advanced deep learning networks.
This review evaluated their effectiveness, limitations,
and the potential for integration into clinical
workflows, providing insights for future research
directions.
This review is limited to discussing individual
models without fully addressing the potential of
cascading models, which layer processes for
enhanced precision. For instance, a cascading
approach might use CNNs for initial hemorrhage
detection and then apply FCNs for nuanced subtyping
and lesion mapping, offering a path to significantly
refine outcomes. Future updates should delve into
complex models like cascading systems, comparing
their impact on clinical practice, and incorporating
case studies to illustrate real-world applications and
advancements in machine learning for neuroscience.
Progressing Toward Smart Brain Hemorrhage Detection: Machine Learning-Based Advanced Medical Imaging Technologies
427

REFERENCES
Amann, J., Blasimme, A., Vayena, E., Frey, D., Madai, V.
I., & Precise4Q Consortium. 2020. Explainability for
artificial intelligence in healthcare: a multidisciplinary
perspective. BMC medical informatics and decision
making, 20, 1-9.
Chen, Y., Tang, W., Huang, X., An, Y., Li, J., Yuan, S., ...
& Zhang, M. 2024. Mitophagy in intracerebral
hemorrhage: a new target for therapeutic intervention.
Neural Regeneration Research, 19(2), 316-323.
Fang, W., Chen, Y., & Xue, Q. 2021. Survey on research of
RNN-based spatio-temporal sequence prediction
algorithms. Journal on Big Data, 3(3), 97.
Gao, D., Feng, W., Qiao, Y., Jiang, X., & Zhang, Y. 2023.
Development and validation of a random forest model
to predict functional outcome in patients with
intracerebral hemorrhage. Neurological Sciences,
44(10), 3615-3627.
Gautam, A., & Raman, B. 2021. Towards effective
classification of brain hemorrhagic and ischemic stroke
using CNN. Biomedical Signal Processing and Control,
63, 102178.
Highton, J., Chong, Q. Z., Crawley, R., Schnabel, J. A., &
Bhatia, K. K. 2023. Evaluation of Randomized Input
Sampling for Explanation (RISE) for 3D XAI-Proof of
Concept for Black-Box Brain-Hemorrhage
Classification. In International Conference on Medical
Imaging and Computer-Aided Diagnosis (pp. 41-51).
Singapore: Springer Nature Singapore.
Kaggle. 2020. RSNA Intracranial Hemorrhage Detection.
https://www.kaggle.com/c/rsna-intracerebral-hemorrha
ge-detection/data.
Li, S., Zhang, J., Hou, X., Wang, Y., Li, T., Xu, Z., ... &
Liu, M. 2024. Prediction model for unfavorable
outcome in spontaneous intracerebral hemorrhage
based on machine learning. Journal of Korean
Neurosurgical Society, 67(1), 94.
Mahjoubi, M. A., Hamida, S., Siani, L. E., Cherradi, B., El
Abbassi, A., & Raihani, A. 2023. Deep Learning for
Cerebral Hemorrhage Detection and Classification in
Head CT Scans Using CNN. In 2023 3rd International
Conference on Innovative Research in Applied Science,
Engineering and Technology (IRASET) (pp. 1-8).
IEEE.
McGurgan, I. J., Ziai, W. C., Werring, D. J., Salman, R. A.
S., & Parry-Jones, A. R. 2021. Acute intracerebral
haemorrhage: diagnosis and management. Practical
Neurology, 21(2), 128-136.
Qiu, Y., Chang, C. S., Yan, J. L., Ko, L., & Chang, T. S.
2019. Semantic segmentation of intracranial
hemorrhages in head CT scans. In 2019 IEEE 10th
International Conference on Software Engineering and
Service Science (ICSESS) (pp. 112-115). IEEE.
Rao, B., Zohrabian, V., Cedeno, P., Saha, A., Pahade, J., &
Davis, M. A. 2021. Utility of artificial intelligence tool
as a prospective radiology peer reviewer—detection of
unreported intracranial hemorrhage. Academic
radiology, 28(1), 85-93.
Tanioka, S., Yago, T., Tanaka, K., Ishida, F., Kishimoto, T.,
Tsuda, K., ... & Suzuki, H. 2022. Machine learning
prediction of hematoma expansion in acute intra-
cerebral hemorrhage. Scientific Reports, 12(1), 12452.
Xu, R., Baracaldo, N., & Joshi, J. 2021. Privacy-preserving
machine learning: Methods, challenges and directions.
arXiv preprint arXiv:2108.04417.
Xu, W., He, J., & Shu, Y. 2020. Transfer learning and deep
domain adaptation. Advances and applications in deep
learning, 45.
Yun, T. J., Choi, J. W., Han, M., Jung, W. S., Choi, S. H.,
Yoo, R. E., & Hwang, I. P. 2023. Deep learning based
automatic detection algorithm for acute intracran
EMITI 2024 - International Conference on Engineering Management, Information Technology and Intelligence
428
