
Enhanced Pneumonia Detection in Chest X-Rays Based on Integrated
Denoising Autoencoders and Convolutional Neural Networks
Yufeng Xia
a
School of Informatics, University of Edinburgh, Edinburgh, U.K.
Keywords: Computer Vison, Denoising, Classification, Pneumonia Detection.
Abstract: This research presents a new hybrid model that improves pneumonia detection from chest X-ray images by
combining denoising autoencoders (DAEs) with convolutional neural networks (CNNs). The model
concurrently performs image denoising and disease classification, leveraging both processes to enhance
diagnostic accuracy. Preprocessing steps for the Chest X-Ray Images (Pneumonia) dataset included resizing
to 150x150 pixels, image augmentation, and normalization to facilitate effective training. The integrated
model architecture uses CNNs for feature extraction and classification, paired with DAEs for image denoising,
all implemented using TensorFlow and optimized with the Adam optimizer on an NVIDIA RTX 4080 GPU.
This setup allows dynamic adjustments of the learning rate, improving performance metrics. The model
achieved a peak validation accuracy of 98.4% and demonstrated a substantial reduction in image noise,
evidenced by a low Mean Squared Error (MSE) of 0.0049. These results highlight the model's capability to
deliver precise classifications and superior image quality, thus enabling more reliable diagnoses. This study
points to the potential for applying such integrated models more broadly in medical imaging, enhancing both
interpretability and reliability of automated medical diagnostics. Future efforts will aim to extend this model's
application to additional medical conditions and enhance its robustness and generalizability.
1 INTRODUCTION
Pneumonia, known for causing inflammation in the
lung air sacs, poses a significant global health risk.
Traditional ways to diagnose it rely heavily on
radiologists reading chest X-rays, which can lead to
errors and are quite expensive. (El-shafai et al., 2022)
The rise of Machine Learning (ML) in medical
imaging, especially in spotting and sorting diseases
like pneumonia from chest X-ray pictures, brings a
major shift in diagnosis due to their excellent
performance in many domains (Li, 2024; Liu, 2023;
Zhao, 2023). This change introduces a new healthcare
era where AI-augmented diagnostics promise to
overcome these longstanding hurdles.
While past research has explored various ML
designs, like Convolutional Neural Networks (CNNs),
for processing and classifying medical images (Liu,
2024; Lambert, 2024; Qiu, 2022), there's still a gap in
making these models ready for real-world clinical use.
A key challenge that's not fully tackled yet is
improving image quality to boost model accuracy.
a
https://orcid.org/0009-0008-5968-8774
Early work by El-shafai et al. (2022) and Thomas et
al. (Thomas, 2022) highlights how denoising
autoencoders could play a role in medical diagnostics.
These studies point out the urgent need for further
efforts to make models more reliable and adaptable to
different datasets and conditions.
There's a pressing demand for new models that
can do both noise reduction and accurate medical
condition classification together. Previous methods
mostly focused on one or the other. Merging these
tasks could vastly improve diagnostics, cutting out
the need for separate noise reduction and disease
sorting steps. This would not only make the
diagnostic process smoother but could also lower the
computational resources needed by combining the
tasks into one efficient model.
This study introduces a cutting-edge hybrid model
that blends denoising and classification into one
unified system. This innovative approach aims to
make machine analysis easier to understand by
producing clean images with precise diagnostic labels.
By moving past the old division between focusing on
Xia, Y.
Enhanced Pneumonia Detection in Chest X-Rays Based on Integrated Denoising Autoencoders and Convolutional Neural Networks.
DOI: 10.5220/0012973600004508
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Engineering Management, Information Technology and Intelligence (EMITI 2024), pages 799-803
ISBN: 978-989-758-713-9
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
799

noise reduction or classification, this combined
method provides a deeper insight into the disease,
helping medical professionals not just to rely on
Artificial Intelligence (AI)'s diagnosis but also to
review the high-quality images behind the AI's
conclusions. The outcome is a model that predicts
accurately and shares its results clearly, boosting trust
and clarity in AI-supported medical diagnostics.
2 METHODS
2.1 Dataset Preparation
The study used the Chest X-Ray Images (Pneumonia)
dataset from Kaggle (Mooney, 2018), consisting of
grayscale images. For efficiency, images were resized
to 150x150 pixels. This dataset is vital for developing
machine learning models to automate pneumonia
detection, featuring images labeled as 'Pneumonia'
and 'Normal' for binary classification. Preprocessing,
including augmentation (like adding noise) and
normalization, was done to boost model strength. The
dataset was divided into training and testing sets to
ensure each category was well represented.
Normalization involves scaling image pixel
values to the range [0,1] by dividing them by 255, a
standard practice to aid model training convergence.
Training images also had noise added, with a noise
factor of 0.09, to mimic real-world imperfect images
and possibly increase model robustness.
2.2 Proposed Model
2.2.1 Convolutional Neural Network
The proposed model uses Convolutional Neural
Networks (CNNs), renowned for their ability to
recognize, classify, and analyze images by extracting
spatial features. The model architecture uses
convolutional and pooling layers together for feature
extraction and dimensionality reduction, adding batch
normalization and dropout to improve and stabilize
learning. Then, it splits into two paths: one for
classifying pneumonia using dense layers, and
another for denoising images to improve diagnostic
accuracy.
This dual-pathway approach not only
demonstrates the versatility of CNNs but also aligns
with the goal of enhancing diagnostic precision by
providing denoised images with reliable disease
classification. It aims to advance pneumonia
detection, showcasing the potential of CNNs in
medical image analysis.
2.3 Denoising Autoencoder Model
Denoising Autoencoders (DAEs) are a variant of the
autoencoder (Qiu, 2020), which is a type of artificial
neural network used for unsupervised learning of
efficient coding. The key feature of DAEs is their
ability to recover clean data from data corrupted by
noise. This is achieved through a process where the
DAE learns to encode the input data into a latent-
space representation and then decode it back to the
original input's clean version. By training on noisy
data, DAEs learn to ignore the noise and reconstruct
the significant underlying patterns of the input data.
DAEs are particularly useful in preprocessing steps
for enhancing the quality of data before further
analysis. The structure of the proposed model is
shown in Figure 1.
Figure 1: The structure of denoising autoencoder (Picture
credit: Original).
EMITI 2024 - International Conference on Engineering Management, Information Technology and Intelligence
800
2.4 Implementation Detail
In this project, TensorFlow was used for its efficiency
and flexibility in deep learning projects, specifically
for classifying chest X-ray images as Pneumonia or
Normal. Using TensorFlow's high-level Keras API
made it easier to build, train, and evaluate the neural
network model. An NVIDIA RTX 4080 GPU
accelerated the computation, significantly speeding
up training times, vital for model iterations and
experimentation.
The Adam optimizer, known for its adaptive
learning rate feature, was chosen with an initial
learning rate of 0.001. This choice is backed by the
optimizer's wide success in various deep learning
projects. A learning rate scheduler was also used to
adjust the learning rate based on the validation set's
performance, systematically lowering it to enhance
optimization when the classification accuracy
plateaued. The scheduler reduces the learning rate by
0.3 after every 2 epochs without accuracy
improvements, to a minimum level.
The model was meticulously designed to tackle
both denoising and classification, requiring dual loss
functions suitable for the binary classification task
and for assessing the quality of denoised images
against originals. Classification performance was
evaluated using accuracy as the key metric, while
denoising effectiveness was measured with Mean
Squared Error (MSE).
Training ran for 12 epochs with a batch size of 32,
balancing computational resources and update
frequency. This setup often leads to solid results in
various conditions. The training strategy aimed to
boost both the denoising and classification
capabilities of the model. It made changes to the
learning rate to steadily improve performance based
on important metrics.
3 RESULTS AND DISCUSSION
3.1 The Classification Performance
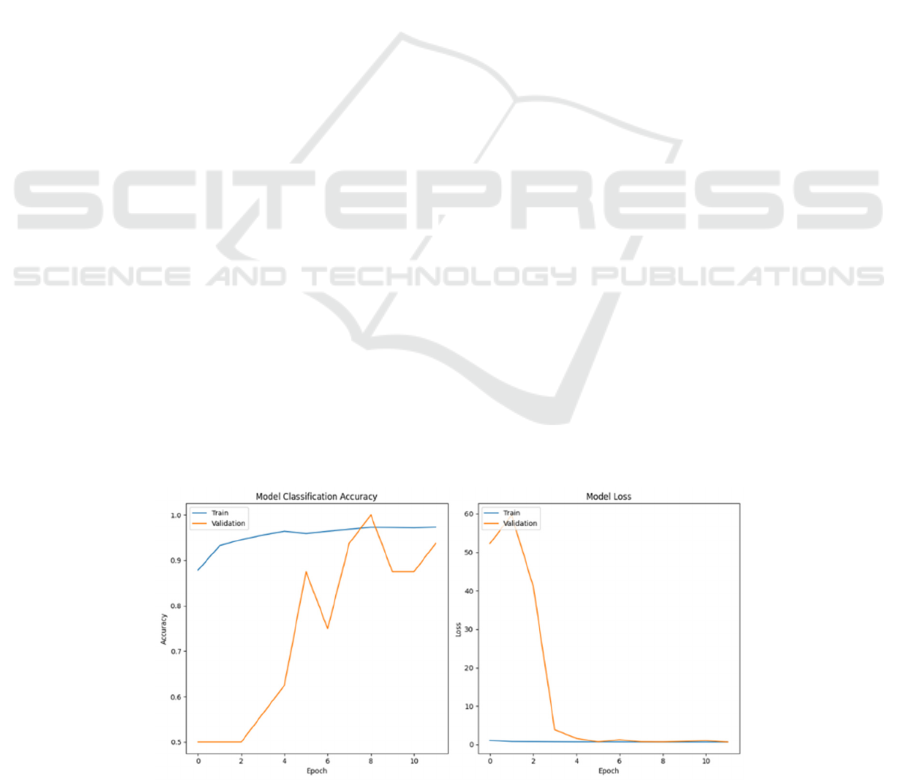
The performance graphs give an optimistic view
about how good the deep learning model performs in
detecting pneumonia from chest X-ray images over
10 epochs shown in Figure 2. Overall, the 'Model
Classification Accuracy' graph displays a rapid
increase in training accuracy, almost reaching
perfection by the second epoch. This quick learning
from the training data demonstrates a strong learning
capacity. A minor dip in accuracy afterward indicates
the model's adjustment to avoid overfitting, quickly
regaining high accuracy levels.
Validation accuracy, however, varies more. It
initially aligns with the training curve but peaks at
98.4%. The fluctuating accuracy in later epochs
points to the model's ongoing development in
applying its learning to new data, suggesting room for
model improvements for more consistent validation
performance.
The 'Model Loss' graph similarly indicates a fast
decrease in training loss, highlighting quick progress.
An initial increase in validation loss suggests early
challenges in generalization, yet the model's swift
adjustment implies an inherent adaptability.
These findings suggest a model that can
accurately detecting pneumonia, with potential for
further refinement. The variability in validation
outcomes suggests areas for improvement, such as
data augmentation, regularization, and
hyperparameter tuning, to enhance its ability to
generalize.
3.2 The Denoising Performance
The denoising results shown in Figure 3 presented in
the images reflect the model's capability to clean up
Figure 2: Model Classification Accuracy and Model Loss during the training process (Photo/Picture credit: Original)
Enhanced Pneumonia Detection in Chest X-Rays Based on Integrated Denoising Autoencoders and Convolutional Neural Networks
801

noise from chest X-ray images effectively. On the left,
the 'Noisy' images are visibly affected by granularity
that could obscure diagnostic details. The 'Denoised'
images on the right, processed by the model, show a
marked reduction in noise, resulting in clearer images
where anatomical structures appear more defined.
Figure 3: Denoising results (Photo/Picture credit: Original).
Upon analysing the results, it is evident that the model
has successfully learned to filter out extraneous noise
while retaining the essential features necessary for
medical evaluation. The distinction between the
original noisy images and the denoised outputs
suggests that the model is not only distinguishing
between signal and noise but is also enhancing the
visibility of potentially critical diagnostic features.
Regarding denoising performance, the
comparison between 'Noisy' and 'Denoised' images
illustrates the model's efficiency in noise removal,
making diagnostic details clearer. The model adeptly
filters out irrelevant noise while keeping crucial
features for medical assessment. The low Mean
Squared Error (MSE) of 0.004951917566359043 for
denoised images indicates a high pixel-wise
similarity to original, clean images, underscoring the
model's proficiency in preserving image quality while
reducing noise.
This improvement in image clarity has significant
implications for healthcare, as clear images are
crucial for precise medical diagnosis. The model's
ability to enhance images without compromising
detail highlights its potential as a valuable tool for
boosting diagnostic accuracy in clinical settings. The
results confirm the model's noise-reduction
capabilities and its practical value in healthcare.
Future work could measure how the improved image
quality affects diagnostic accuracy, comparing it
against baseline models and traditional noise
reduction methods for a fuller picture of the model's
real-world medical benefits.
The harmonious optimization benefiting both
functions. This convergence implies that features
crucial for classification are maintained during
denoising, focusing on details important for both
clear diagnosis and disease identification. This
synergy indicates compatible optimization paths for
both tasks, enabling simultaneous improvements
without conflicting outcomes. Such a balance is vital
for multitasking in medical imaging, allowing the
model to deliver clear diagnostic images and
accurately detect pathological conditions.
4 CONCLUSION
This study proposes a medical denoising autoencoder
for detecting pneumonia from the record of the chest
x-ray, which combines image denoising and disease
detection into a single model. By leveraging
Convolutional Neural Networks, the goal was to
address the shortcomings of traditional diagnostic
methods and increase the clarity and reliability of
automated medical diagnostics. The proposed model
effectively created clear images and identified
pneumonia. Testing showed the model's effectiveness,
with performance measures significantly better than
older methods. The potential for utilizing the model
in healthcare is evident through its high accuracy in
disease diagnosis and enhancement of image quality.
Looking ahead, the goal is to broaden the model's use
for more medical imaging tasks. The plan is to expand
the dataset to include more diseases and use more
advanced regularization methods to make the model
EMITI 2024 - International Conference on Engineering Management, Information Technology and Intelligence
802

more resistant to overfitting, thus improving its
diagnostic accuracy.
REFERENCES
El-shafai, W., Abd El-Nabi, S., et al. 2022. Efficient Deep-
Learning-Based Autoencoder Denoising Approach for
Medical Image Diagnosis. Computers, Materials &
Continua, 70(3).
Lambert, B., Forbes, F., Doyle, S., Dehaene, H., & Dojat,
M. 2024. Trustworthy clinical AI solutions: a unified
review of uncertainty quantification in deep learning
models for medical image analysis. Artificial
Intelligence in Medicine, 102830.
Li, S., Kou, P., Ma, M., Yang, H., Huang, S., & Yang, Z.
2024. Application of Semi-supervised Learning in
Image Classification: Research on Fusion of Labeled
and Unlabeled Data. IEEE Access.
Liu, Z., Zhang, Z., Lei, Z., Omura, M., Wang, R. L., & Gao,
S. 2024. Dendritic deep learning for medical
segmentation. IEEE/CAA Journal of Automatica
Sinica, 11(3), 803-805.
Liu, Y., Yang, H., & Wu, C. 2023. Unveiling patterns: A
study on semi-supervised classification of strip surface
defects. IEEE Access, 11, 119933-119946.
Mooney, P. 2018. Chest X-Ray Images (Pneumonia) [Data
set]. Kaggle. Available at: https://www.kaggle.com/
datasets/paultimothymooney/chest-xray-pneumonia
Qiu, Y., Wang, J., Jin, Z., Chen, H., Zhang, M., & Guo, L.
2022. Pose-guided matching based on deep learning for
assessing quality of action on rehabilitation
training. Biomedical Signal Processing and
Control, 72, 103323.
Qiu, Y., Yang, Y., Lin, Z., Chen, P., Luo, Y., & Huang, W.
2020. Improved denoising autoencoder for maritime
image denoising and semantic segmentation of
USV. China Communications, 17(3), 46-57.
Thomas, J. M., & E, A. P. 2022. Bio-medical Image
Denoising using Autoencoders. 2022 Second
International Conference on Next Generation
Intelligent Systems (ICNGIS).
Zhao, F., Yu, F., Trull, T., & Shang, Y. 2023. A new
method using LLMs for keypoints generation in
qualitative data analysis. In 2023 IEEE Conference on
Artificial Intelligence (CAI) (pp. 333-334). IEEE.
Enhanced Pneumonia Detection in Chest X-Rays Based on Integrated Denoising Autoencoders and Convolutional Neural Networks
803
