
Enhancing Brain Tumor Detection in Magnetic Resonance Imaging
Through Explainable Artificial Intelligence Techniques and Fusion
Models
Adwaita Sathrukkan
1
a
, Naveen Raaghavendran
1
b
, Sanjay Balamurugan
1
c
, Srivaishnavi J. V.
1
d
,
S. Raghul
2
e
and G. Jeyakumar
1
f
1
Department of Computer Science Engineering, Amrita School of Computing, Coimbatore,
Amrita Vishwa Vidyapeetham, India
2
Research and Engineering Scientist, Zoho Corporation Private Limited, India
Keywords: Image Recognition, Deep Learning, Convolutional Neural Networks, Tumor Detection, Explainable Artificial
Intelligence, Feature Extraction.
Abstract: Enhancing the detection of brain tumors in Magnetic Resonance Imaging (MRI) represents a critical frontier in
medical imaging and neuro-oncology. This paper introduces an innovative approach that leverages Explain-
able Artificial Intelligence (XAI) techniques and fusion models to significantly improve the accuracy and
interpretability of brain tumor detection. This paper proposes a novel framework integrating deep learning
models and fusion strategies for enhanced feature extraction from multiple MRI sequences, as detailed in sub-
sequent sections. By employing XAI methodologies, the approach presented in this paper not only enhances
detection performance but also provides meaningful explanations for its predictions, thereby increasing the
trustworthiness of automated diagnosis.
1 INTRODUCTION
Tumors of the brain and other parts of the nervous
system, such as glioblastomas (GBM), are among the
top causes of cancer mortality in adult populations.
Brain tumors, whether malignant or non-malignant,
constitute the second-highest cause of death linked to
cancer in adolescents and children. Standard
treatments for brain cancer encompass surgery,
radiation therapy, and chemotherapy. However,
surgically pinpointing and removing the diseased
areas is often exceedingly challenging due to the
complexity involved in distinguishing tumors from
the normal brain tissue visually. Magnetic resonance
imaging (MRI) is a crucial tool in clinical settings,
aiding doctors brain tumor identification. MRI
a
https://orcid.org/0009-0007-0509-8659
b
https://orcid.org/0009-0006-6253-558X
c
https://orcid.org/0009-0007-9025-9532
d
https://orcid.org/0009-0009-3993-0794
e
https://orcid.org/0000-0002-1306-6960
f
https://orcid.org/0009-0009-3993-0794
provides detailed images of soft tissues, which
enhances the ability to determine the location and
boundaries of tumors.
The advent of machine learning (ML) models in
medical imaging marks a significant leap forward in
diagnostics, particularly in the domain of brain
tumors. These advanced computational tools have
demonstrated the ability to analyze complex imaging
data with high precision, offering insights into tumor
characteristics that were previously unattainable
through traditional diagnostic methods. Despite these
advancements the integration of ML models into
clinical practice faces considerable challenges,
primarily due to “Black Box” nature of Artificial
Intelligence (AI) algorithms. This opacity in decision-
making processes poses a barrier to clinical adoption,
266
Sathrukkan, A., Jeyakumar, G., Balamurugan, S., J. V., S., Raghul, S. and Raaghavendran, N.
Enhancing Brain Tumor Detection in Magnetic Resonance Imaging Through Explainable Artificial Intelligence Techniques and Fusion Models.
DOI: 10.5220/0013344000004646
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Cognitive & Cloud Computing (IC3Com 2024), pages 266-275
ISBN: 978-989-758-739-9
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

as healthcare professionals require transparent and
interpretable systems to trust and effectively use
these technologies in patient care.
Explainable Artificial Intelligence (XAI) emerges
as a crucial area of research aimed at addressing these
challenges. By making AI’s decision making
transparent and understandable, XAI holds the
promise of bridging the gap between the technical
capabilities of ML models and the practical need of
clinical diagnosis. However, a comprehensive
literature survey reveals a significant lack of focused
research on the application of XAI in the domain of
medical imaging for brain tumor detection. While
several studies have underscored the potential of ML
in improving diagnostic accuracy, the exploration of
XAI models in elucidating the rationale behind AI-
generated diagnoses remains limited. This gap in
research underscores the significance of this study,
which seeks to investigate the integration of XAI
within the context of brain tumor detection from
medical images. The work presented in this paper
aims to not only highlight the potential of combining
ML models with XAI techniques to enhance
diagnostic accuracy but also to address the pressing
need for interpretability in medical AI applications.
By doing so, this study contributes to the broader
adoption of AI in healthcare, ensuring that the
benefits of these technologies can be fully leveraged
to improve patient outcomes while maintaining the
trust and confidence of medical practitioners in AI-
driven diagnostic tools.
The remaining part of the paper is organized as
follows. Section 2 explains various state-of-the- art
ML and XAI models. The Section 3 briefs about
various ML models used in medical image
processing. Section 4 briefs about the usage of XAI
models in medical image processing. Next, section 5
presents the details of the proposed methodology.
Section 6 presents the design details of the
experiments. The results and discussions are in
Section 7. The Section 8 concludes the paper.
2 LITERATURE SURVEY
The initial phase of medical imaging involves the
detection of tumors in the MRI scans and subsequent
extractions of essential features for classifications as
presented in (Abhilasha et al., 2022). Numerous
methodologies have been developed to address
challenges associated with variations in field
strength, dataset biases, mislabeled in- stances, and
other illustrative changes in the context of medical
imaging. The evolution from conventional hand-
written medical diagnosis, by the people in the field,
to deep learning-based models and AI have proven
advantageous, particularly in handling large data and
providing a robust feature representation,
segmentation, and classification. In the do- main of
XAI, innovative approaches of using XAI in deep
learning-based medical image analysis are de-
scribed in (Velden et al., 2022). As given in (Priya,
A. and V. Vasudevan, 2024), brain tumor
classification and detection are possible using a
suitable CNN structure (Eg. a hybrid AlexNet-GRU)
based on the given MRI data. This process involves
sharpening and denoising the MRI images using
local filters.
A feature extraction method from brain MRIs is
proposed in (Tas, 2023), and is used for brain tumor
detection. In this work the DenseNet201 is trained
using the exemplar method, and then the features are
extracted. The authors of (Amran et al., 2022),
proposes a brain tumor classification and detection
system using a GoogLeNet architecture. In the
proposed architecture, 5 layers of GoogLeNet are
eliminated and 14 new layers are added to extract the
features automatically. In (Apostolopoulos et al.,
2023), a novel approach of integrating CNN with
attention models and feature-fusion blocks is
presented. This integrated approach is demonstrated
on the brain tumor classification task, using MRI
data. This approach is named as Attention Feature
Fusion VGG19 (AFF- VGG19), and it was found
outperforming other state- of-the-art similar
approaches. Regarding the basis of segmentation, a
brain tumor segmentation system using modified
ResUNET architecture which combines the strengths
of the U-Net architecture is presented in (Pathak et
al., 2023). This system is known for its
effectiveness in bio-medical image segmentation,
with the residual learning framework to facilitate
training of deeper networks as evidenced in ((Pathak
et al., 2023).
The work presented in (Younis et al., 2022),
explored the usage of VGG (Visual Geometry
Group) and CNN for brain tumor related image
analysis. A new system is proposed and the same is
demonstrated for training and classifying brain
tumors based on different MRI images. Also, it is
found from the literature that different CNNs such as
VGG16/19, AlexNet, GoogLeNet and Resnet are
demonstrating well on MRI based image
classification tasks. In the field of XAI, automatic
segmentation of multimodal brain tumor images
based on classification of super- voxels which uses a
type of MRI sequence called Fluid Attenuated
Inversion Recovery (FLAIR) is popular. FLAIR is an
Enhancing Brain Tumor Detection in Magnetic Resonance Imaging Through Explainable Artificial Intelligence Techniques and Fusion
Models
267

imaging technique used to sup- press the effects of
fluid within the image, particularly cerebrospinal
fluid (CSF), to bring out the periventricular
hyperintensities (lesions near the ventricles of the
brain), as give in the study (Hu et al., 2021).
NeuroXAI uses seven advanced methods to clarify
deep neural networks in MRI brain tumor analysis,
providing visualization maps for transparency.
These include: Vanilla Gradient (VG) for
highlighting crucial image areas, Guided
Backpropagation (GBP) for alternative gradient
calculations, Integrated Gradients (IG) to tackle
gradient saturation, Guided Integrated Gradients
(GIG) for refined attribution paths, SmoothGrad for
sharper sensitivity maps, Gradient CAM (GCAM) for
model-agnostic visual explanations, and Guided
GCAM (GGCAM) for high-resolution detail capture,
as evidenced in (Zeineldin et al., 2022). A multi-
disease diagnosis model using the X-ray images of the
chest, with XAI, is presented in (Rani et al., 2022).
In-Hospital mortality prognosis, the usage of
XAI techniques is demonstrated using seven different
machine learning models in (Maheswari et al., 2023).
A model for classifying suprasellar lesions formed in
the brain is proposed in (Priyanka et al., 2023). This
study has used discharge summary of 422 patients.
The usage of machine learning models in diagnosis
other medical issues also notable. As an example, a
diabetic retinopathy detection using Gradient-
weighted class activation map (Grad-CAM),
presented in (Duvvuri et al., 2022), is added here.
This literature survey summarizes advancements in
brain tumor detection and classification through
medical imaging, tracing the shift from traditional
diagnostic methods to deep learning and AI
technologies. It discusses challenges such as
imaging variability and dataset biases, and the
evolution towards automated feature extraction and
classification using deep learning architectures like
Dense-Net, GoogLeNet, and VGG-16. Highlighting
the role of XAI in making neural network decisions
transparent, the survey underscores the necessity for
methods like Vanilla Gradient, Guided
Backpropagation, and Gradient CAM to ensure
model reliability and acceptance by medical
professionals. Building on this, this paper proposes a
novel approach that merges the latest in deep learning
with XAI to enhance diagnostic accuracy and
interpretability, aiming to revolutionize AI-driven
medical imaging for brain tumor analysis.
3 MACHINE LEARNING MODEL
In the adapted implementation of the AlexNet
architecture for experimental purposes, the model
features five convolutional layers with kernel sizes
11x11 for the first layer and 3x3 for subsequent layers,
followed by three maxpooling layers and enhanced
with batch normalization to improve training
efficiency. The architecture includes four dense
layers with a substantial number of neurons (4096 for
the first two dense layers, 1000 for the third) and
employs dropout with a rate of 0.4 after each dense
layer for regularization. The GoogLeNet architecture
is utilized for brain tumor classification from MRI
images, featuring multiple inceptions blocks that
parallelly process input through convolutional layers
of varying kernel sizes and a max-pooling layer,
enhancing feature extraction efficiently. This
implementation starts with a 7x7 convolution,
progresses through strategic inception blocks and
max pooling for depth and dimensionality reduction,
and concludes with global average pooling and a
SoftMax classification layer.
This GoogLeNet model, which is optimized with
Adam and has Early Stop-ping and Model Checkpoint
callbacks, is designed for high accuracy in multi-
class classification tasks, indicating the potential of
deep learning in medical diagnostics. For efficient
MRI brain tumor segmentation, the VGG19+UNet
architecture combines the reliable feature extraction
of VGG19 with the accurate localization of UNet.
With VGG19 pre-trained on ImageNet for deep
feature extraction, this model performs exceptionally
well at identifying complex patterns in MRI pictures.
The UNet decoder uses these features for
reconstructing the segmentation maps and to identify
tumors. There is a preprocessing, augmentation, and
normalization procedure applied to the MRI pictures
and the segmentation masks before the training. The
performance metrics and the visual evaluations prove
that this hybrid approach shows high precision in
tumor delineation.
ResNet’s deep feature extraction procedure is
merged with UNet’s accurate localization. This
merging uses ResNet’s residual connections,
prevents the vanishing gradient problem, and
enhances learning efficiency. This approach used a
custom data generator for data handling. The MRI
images and masks are processed by resizing and
normalization. The dynamic learning rate
adjustments and early stopping through callbacks are
the important training techniques used. The ability
of this model to successfully segment brain tumors
highlights the usefulness of integrating residual
IC3Com 2024 - International Conference on Cognitive & Cloud Computing
268

learning with UNet’s architecture and highlights the
model’s potential for improving clinical diagnostics
and medical imaging research. The modified VGG16
architecture, which uses 3x3 filters with ReLU
activation, keeps its basic structure of 13
convolutional layers arranged into five blocks, each
of which ends with max pooling to reduce
dimensions and classify brain tumors from MRI
images. A customized classifier, consisting of a
flatten layer, dropout for regularization, and dense
layers culminating in a SoftMax activation for
multiclass prediction, replaces the original fully
connected layers. By making the final convolutional
block train- able to capture tumor-specific features,
the model is refined.
4 XAI MODELS
To make the decision-making of neural networks
transparent, the NeuroXAI framework combines an
explanation generation module with a deep neural
network for processing MRI brain scans. The
process starts with MRI scans that are run through a
convolutional neural network (CNN), which produces
feature maps and outcomes such as tumor
segmentations or classifications. Medical experts
review these results, and upon request, the system
employs advanced XAI techniques to generate visual
explanation maps. These techniques have Vanilla
Gradient (VG) and Gradient CAM (Grad-CAM). The
VG is to create a saliency map and to identify
influential image parts. The Grad-CAM is to
highlight important regions for predictions. Also, the
techniques of Integrated Gradients (IG) and Guided
Backpropagation (GBP) are included in the
framework. They are used to identify which areas of
the images have a major impact on the decisions. The
Guided Integrated Gradients (GIG) are used to
further improve the feature relevancy attribution.
The GIG uses the computation of gradients between
the baseline and the input image. Smooth Grad and
Guided Grad-CAM techniques average gradients of
noise-perturbed input images and combine macro
and micro-level visualizations, respectively, offering
clearer, more interpretable visualizations. These
sophisticated XAI approaches help bridge the gap
between AI outputs and clinical decision-making,
fostering trust and collaboration in AI-assisted
diagnostics.
5 PROPOSED METHODOLOGIES
To address the challenge of MRI brain tumor
classification and segmentation, this paper integrates
advanced adaptations of deep learning architectures.
Initially, a comprehensive dataset of MRI images is
compiled and subjected to meticulous preprocessing,
including normalization, resizing, and augmentation,
to prepare for model training. This paper adapts and
optimizes several renowned architectures for specific
tasks: AlexNet is tailored for binary classification
with adjusted convolutional layers and dropout rates;
GoogLeNet is configured with inception blocks for
efficient multi-class classification; VGG19 is
combined with UNet for precise tumor segmentation
through deep feature extraction and localization;
ResUNet leverages ResNet’s residual connections
with UNet’s segmentation accuracy; and VGG16 is
modified with custom classifiers for enhanced tumor
feature recognition. Each model undergoes fine-
tuning, employing strategies like dynamic learning
rate adjustments and early stopping, to ensure
optimal performance.
This comprehensive approach, focusing on the
customization of CNNs, aims to enhance the
accuracy, efficiency, and reliability of MRI brain
tumor diagnosis and segmentation, demonstrating
the potential of deep learning in medical diagnostics
and imaging analysis. Additionally, this paper
integrates Explainable Artificial Intelligence (XAI)
methods to enhance model transparency and
interpretability. Techniques such as Vanilla
Gradient, Grad-CAM, Guided Backpropagation,
Integrated Gradients, Guided Integrated Gradients,
Smooth Grad, and Guided Grad-CAM generate
visual explanation maps, aiding medical experts in
understanding AI decisions. The NeuroXAI
framework processes MRI scans through a CNN,
creating feature maps and results with clear visual
explanations. Model performance is evaluated using
metrics like Intersection over Union (IoU) and
accuracy, comparing predictions with manual
segmentations. Clinical validation through trials and
feedback from medical professionals ensures real-
world applicability and reliability. This approach
highlights the potential of XAI techniques and fusion
models to im- prove diagnostic accuracy, clinical
decision-making, and patient outcomes in neuro-
oncology.
Enhancing Brain Tumor Detection in Magnetic Resonance Imaging Through Explainable Artificial Intelligence Techniques and Fusion
Models
269

6 DESIGNS OF EXPERIMENTS
Different datasets have been used during model
selection and in the stage of implementation of the
final XAI model. Brain MRI images with manual
FLAIR abnormality along with segmentation masks
are obtained from (Buda, Mateusz, 2022). This
dataset was obtained from The Cancer Imaging
Archive (TCIA). This dataset has the details of 110
patients. This dataset is used as a base dataset to find
the best model among the 6 chosen models. The
datasets used for training, validation and testing are
available in (Shah, 2019). This includes pre-
operative multimodal MRI scan of glioblastoma
(HGG) and lower grade glioma (LGG). This BRATs
2019 dataset is used for Classification task and the
image format of the dataset is 2-dimensional. BRATs
2021 dataset is used for segmentation task with 3-
dimensional images obtained from (Schettler, 2021).
This study considers the VGG16, VGG19+unet,
ResUnet, Alexnet, Googlenet and CNN ML Models.
The machine learning algorithms are dependent on
parameters, initial values and the training/testing
process depends on updating the values until the
requirements are met. Parameters are particularly
important for fine tuning of the model, making
proper predictions and defining the skill of the model
on the given problem. Table 1 presents the
information of all the parameters that are important
to detect the anomaly in the brain MRI image and get
the highly accurate machine learning models with the
help of performance metrics. The experiments are
done with SmoothGrad, Guided Grad-CAM, Guided
Backpropagation, Vanilla Grad, Grad-CAM and
Guided Integrated Gradients, which are the XAI
Models.
Parameters in XAI models are important in
shaping the interpretability and transparency of the
model, to understand the decision-making process
efficiently. The parameter sample size plays a key
role here because it generates accurate heatmap in the
output of all the 8 different XAI models and it defines
the number of trainings also. Table 2 presents a
detailed breakdown of the important parameters used
in the models.
7 RESULTS AND DISCUSSIONS
Machine learning performance measures are
essential determinants of model’s efficacy and
capacity to complete the assigned task. Precision,
accuracy, and F1 score are the commonly used
metrics in machine learning and data analysis for
evaluating the performance of classification models.
These metrics are used together to provide a
comprehensive evaluation of the model’s
performance, considering distinct aspects of
classification accuracy and error. Table 3 results help
us to get the best model, which is a fusion of two base
models namely, VGG19 and Unet. These results are
used as a base model for the other 8 XAI models for
generating heat maps to detect anomaly in the brain’s
MRI images.
Table 4 results in the performance metrics used
in the image segmentation process. The metric
Intersection over Union (IoU) and Monte Carlo
prediction are specific for XAI methods. The ratio of
the inter- section area between the predicted and the
ground truth masks to the union of both the masks, is
used as the measure for IoU. Monte Carlo
predictions are often used for uncertainty prediction
particularly for a Bayesian deep learning.
The mean prediction shape represents the
average, or the expected value of the predictions
generated by the segmentation process for a particular
slice index. The mean prediction matrix represents
the predicted values for each vowel in the input
volume, in this case particularly it has taken
intensities for each 4 of the classes.
In general, a machine learning model defines a
representation of a training process. It does the task of
discovering the patterns from the input training data,
it structures a ML model which can understand these
patterns and makes predictions on new inputs.
There are three types of learning algorithms that
can be followed in a machine learning model -
Supervised learning, Unsupervised learning, and
Reinforcement learning. The chosen 6 classification
models follow super-vised learning where the
relationship between the input and output is
designed, and it uses labeled datasets to train the
algorithm to predict the outcomes and recognize
patterns.
Table 1: Parameter configuration of chosen ML models.
S
no.
Model Name
Epoch
Batch
size
Train:Test:Valid
Total
p
arams
1 VGG16 7 32 2828:393:708 128590
2
VGG19+unet
2
36 1167:103:103 31172033
3 ResUnet 100 16 1167:103:103 1210513
4
Alexnet 150 17 2611:650:329 26052829
5 Goo
g
lenet 20 30 2611:653:326 5977692
6
CN
N
35 32 2504:835:590 18818113
IC3Com 2024 - International Conference on Cognitive & Cloud Computing
270
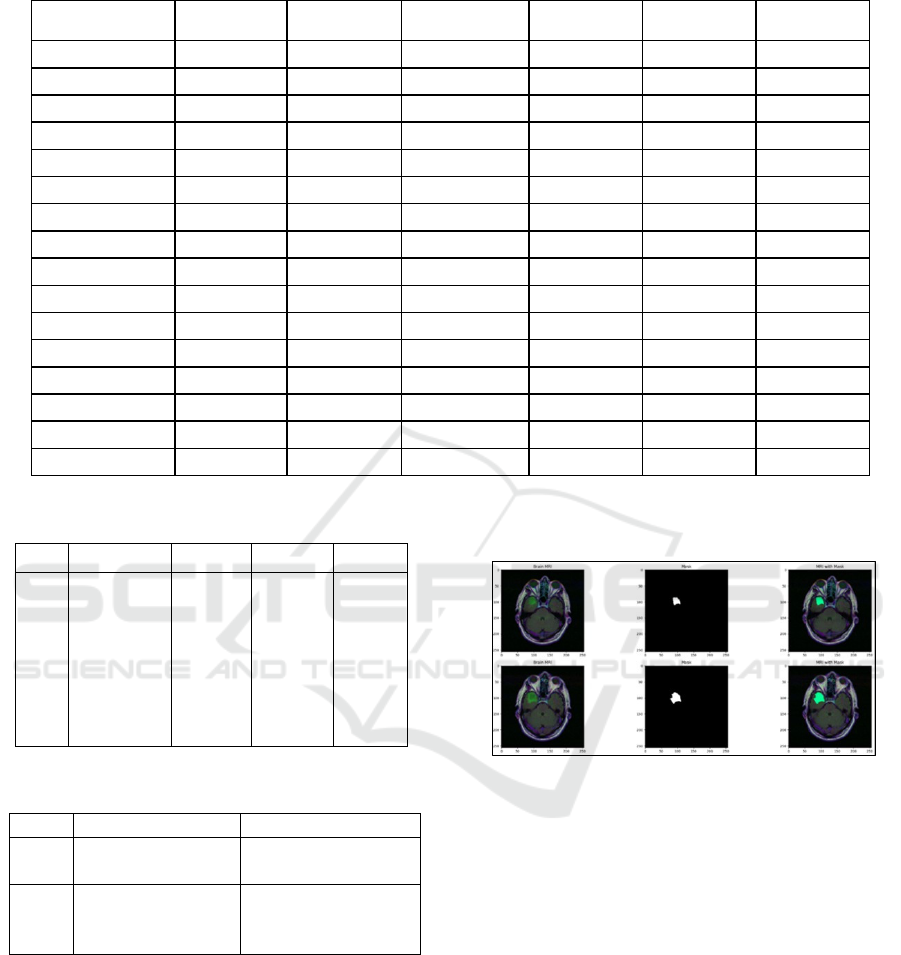
Table 2: Parameter Requirements for Various XAI Models.
Parameter Name Smooth Grad
Guided Grad-
CAM
Guided
Back
p
ro
p
a
g
ation
Vanilla Grad Grad-CAM
Guided Integrate
d
Gradients
model Required Required Required Required Required Required
Io_ imgs Required Required Required Required Required Required
Class_id Required Required Required Required Required Required
LAYER_NAME Optional Optional Optional Optional Optional Optional
MODALITY Optional Optional Optional Optional Optional Optional
XAI_MODE Optional Optional Optional Optional Optional Optional
DIMENSION Optional Optional Optional Optional Optional Optional
STDEV_SPREAD Optional Not applicable Not applicable Not applicable Not applicable Not applicable
N_ SAMPLS Optional Not applicable Not applicable Not applicable Not applicable Not applicable
MAGNITUDE Optional Not applicable Not applicable Not applicable Not applicable Not applicable
CLASS_IDs Not applicable Optional Not applicable Not applicable Not applicable Not applicable
TUMOR_LABEL Not applicable Optional Not applicable Not applicable Not applicable Not applicable
eps Not applicable Optional Not applicable Not applicable Not applicable Not applicable
STEPS Not applicable Not applicable Not applicable Not applicable Not applicable Required
FRAC Not applicable Not applicable Not applicable Not applicable Not applicable Required
MAX_DIST Not applicable Not applicable Not applicable Not applicable Not applicable Required
Table 3: Evaluation metrics for ML models.
S no. Model Name Precision Accuracy F1 score
1 VGG16 85 82.19 83
2 VGG19+unet 98.5 98.27 97
3 ResUnet 98 96 97
4 Alexnet 57 60 72
5 Googlenet 88 87 87
6 CNN 95.42 95.96 95.42
Table 4: Evaluation Metrics for XAI Methods.
S.No Metrics
N
ame Values
1
Intersection over
Union (IoU)
-1.035
2
Monte Carlo
Prediction
Mean prediction
shape
(2,1,192,244,160,4)
Figure 1 shows a clear view of the fusion
model’s accuracy and its high performance.
Selecting VGG19 and UNet as a combinational
model is the base and the next step is to work with
the XAI models and check the accuracy of them
along with this machine learning model.
The two types of XAI methods are model-
specific and model agnostic. Among these two, the
former ones are tailored to unique characteristics and
architectures of a particular machine learning model.
These methods aim to provide explanations
specifically designed for the internal working of the
chosen model.
Figure 1: Accurate prediction of the tumor in the brain im-
age using the mask component by the fusion model. A)
Input MRI. B) Mask used. C) MRI with the mask.
Model-agnostic approaches, on the other hand,
are designed to be versatile and applicable to various
machine learning models. The chosen XAI methods
are FLAIR, Vanilla, Back propagation, IG, Guided
IG, Smooth Grad, Grad-CAM, Overlay Grad-CAM,
Guided Grad-CAM, Prediction and Prediction-
Overlay. These models fall under model-agnostic
methods as they rely on computing the gradients and
based on intricate details about the architecture. The
difference among these methods lies in their specific
techniques for highlighting and explaining distinct
aspects of machine learning model’s decision-making
process. Tables 5 and 6 explain the functionality of
each method and its contribution in classification and
segmentation.
Enhancing Brain Tumor Detection in Magnetic Resonance Imaging Through Explainable Artificial Intelligence Techniques and Fusion
Models
271

Table 5: Explanation of selected XAI methods – Part I.
S.No. Method
Name
Explanation
Methodology
Application
in Classification and
Se
g
mentation
1
FLAIR
Highlights
notable
features in
an image.
Reveals the important
feature that contributes
significantly to the
model decision.
2
Vanilla
Computed
gradients o
f
the outpu
t
concerning
the inpu
t
p
ixels.
Useful in both the tasks
and highlights areas
where slight changes i
n
pixel value occur.
3
Integrated
Gradients
(
IG
)
Computed
the integral
of gradients
along the
path.
Offers a holistic view
of pixel importance.
4
Guided IG
Restricts the
backpropaga
tion of
gradients.
Emphasizes
positive influence on
the model’s decision.
Table 6: Explanation of selected XAI methods – Part II.
S.no Method
Name
Explanation Methodology an
d
Application in Classification an
d
Segmentation
1
Smooth
Grad
Adding random noise to the inpu
t
image and averaging the resulting
gradients.
2
Grad-
CAM(class
activation
map)
Generates a heatmap highlighting
the regions where the model
focused during the decisio
n
making.
3
Overlay
Grad-CAM
This overlays the generate
d
heatmap onto the original image
for more intuitive visualizations.
Effective in classification tasks.
4
Guided
Grad-CAM
Combines the guided back-
propagation with Grad-CAM an
d
provides localization an
d
guidance on which features
contribute
p
ositively.
5
Prediction
Overlay
Overlays the predicted class
onto the input image and gives
a direct image of the model’s
decision.
The implementation details of the selected XAI
models are discussed under the following three
categories:
(1) classification (based on VGG19 model)
(2) segmentation
(3) CNN segmentation (convolutional neural
network)
7.1 Classification Based on VGG19
Model
The dataset classifies images as either indicating
glioma (labeled as 1) or its absence (labeled as 0).
Additionally, the severity of glioma is determined by
the confidence levels associated with High-Grade
Glioma (HGG), which includes grade III and IV
gliomas associated with higher fatality rates, and
Low-Grade Glioma (LGG), comprising grade I and II
gliomas with generally longer patient survival. The
images (in Figure 2) show the classification levels of
low-grade glaucoma (LGG) and high-grade glaucoma
(HGG), with each method highlighting significant
features in the image representation.
In the image visualization, shown in Figure 2, the
LGG level is greater than that of HGG, which shows
that this region might not grow rapidly but is in a 3
rd
or 4
th
grade of glioma.
The image visualization shown in Figure 3 also
says that the LGG level is greater than that of HGG,
which shows that this region might not grow rapidly
but is in a 3
rd
or 4
th
grade of glioma. Sample images
for the LGG and HGG classifications are shown in
Figure 4 and Figure 5, respectively.
Figure 2: Predicted class 1, confidence of HGG: 0.3698,
confidence of LGG: 0.63.
Figure 3: Predicted class 1, confidence of HGG: 0.4010,
confidence of LGG: 0.59891.
Figure 4: Samples images which are classified as LGG
grade.
Figure 5: Sample images which are classified as HGG
grade.
IC3Com 2024 - International Conference on Cognitive & Cloud Computing
272

7.2 Segmentation based on UNet model
Here the deep brain glioma sub-region segmentation
has been interpreted using multimodal MRIs from the
BraTS 2021 validation dataset. The parameters are
defined according to numerous factors, such as
dimension, modality, XAI Mode, Class IDs, Tumor
Label, Layer name, and segmentation model
parameters, including dataset path and img_shape.
In the images shown in Figure 6, the XAI Mode
specifies the mode of explainability. Here it is set to
segmentation, as it deals with XAI methods applied to
the segmentation process. The modality being used is
FLAIR, a type of MRI sequence used in brain
imaging that suppresses the effects of cerebrospinal
fluid on the MRI image. The specific image ID for
a particular MRI case is picked.
In the images shown in Figure 7, the XAI Mode
is like the previous segmentation, but the modality is
not specified to FLAIR alone, it may be either T1,
TICE or T2. T1 shows the longitudinal relaxation,
while TICE does the same with a contrast agent and
finally T2 shows the transverse relaxation time.
Figure 6: Example of segmentation with FLAIR modality
and XAI methods applied.
Figure 7: XAI Mode, Modality: All Models (FLAIR,
T1, TICE, T2).
In the images of Figure 8, a set of IDs have
been considered with 3 different MRI cases from the
BraTS dataset. Here the last layer G-CAM has been
highlighted shown in red regions which corresponds
to a high score for the tumor region. Image has been
iterated over the ID’s 3 times for the set of tumor
labels.
Figure 8: XAI IDs = [”BraTS2021 01652”, ”BraTS2021
00542”,
”BraTS2021 01381”],
Modality: ”FLAIR”.
In the images of Figure 9, a set of IDs have been
considered with 3 different MRI cases from the BraTS
dataset classified as Good Slice IDs and the tumor la-
bels are as a set of values 0,1,2,3. Here it considers
all the modalities as dis-cussed earlier in 2nd case of
images. The last layer, G-CAM, has been highlighted
shown in red regions. This resulted high score for the
regions with tumor. Image has been iterated over the
IDs 3 times for the set of tumor labels.
Figure 9: XAI IDs = [“BraTS2021 01652”,
“BraTS2021 00542”, “BraTS2021 01381”], Modality:
All Models.
7.3 Segmentation using CNN based
UNet model
This segmentation result is done by providing
information flow visualization on the internal layers
of a segmentation CNN with the modality of FLAIR
and the red regions comprising the XAI’s last layer
G- CAM. CNN follows a systematic approach for
detecting brain gliomas by learning the abstract
features available in the network such as the brain
boundaries and identifies finely detailed tumor
boundaries.
In the image visualization of Figure 10, a sample
case has been considered from the BraTs dataset
where segmentation have been done using CNN.
Figure 10: Segmentation CNN (Sample case).
Enhancing Brain Tumor Detection in Magnetic Resonance Imaging Through Explainable Artificial Intelligence Techniques and Fusion
Models
273

With the IDs collected from the BraTS dataset, it
is partitioned into classification and segmentation.
From the classification method, the VGG19 model
was processed comprising HGG and LGG and from
the segmentation method the UNet model was
processed comprising T1, TICE, T2 and FLAIR
modalities.
8 CONCLUSIONS
The diagnosis of brain tumors from medical images is
critical, as the images are varying greatly. The avail-
ability of convolution neural networks (CNNs) makes
the brain tumor detection task easier. The CNN based
approaches for brain tumor classification have paved
the way for better tumor detection with increased ac-
curacy. Using MRI images for detecting and
classifying brain tumors is the recent focus.
Interestingly, the combination of more than one types
of CNN models has proved their performance for
better feature extraction from the MRI images. In this
study, a CNN is designed for brain tumor detection.
The de- signed net-work is trained using two
pretrained models - VGG19 and UNet, for faster and
more convenient training. VGG19 is known for its
deep architecture, while UNet is renowned for its
capability in semantic segmentation tasks, making
them a complementary and potent pair for brain tumor
classification. This paper’s aim is to detect brain tumor
using this fusion model along with XAI methods to
give proper visualization about how the tumor is
detected with their own significant methods. The use
of MRI as an imaging modality ensures detailed and
informative data for accurate classification. Thus,
this combinational approach addressed the concerns
related to the “black box” nature of deep learning
models in medical ap- plications.
In summary, the success of combining different
convolutional models along with the methods of XAI
suggests the potential for further exploration of
fusion strategies in neural networks for medical
imaging. This study contributes to the advancement
of brain tumor detection methodologies, providing a
foundation for future research.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to
their institution (Amrita School of Computing,
Amrita Vishwa Vidyapeetham, India), for the
support provided in completing this project and paper
submission.
REFERENCES
Abhilasha, K., S. Swati, and M. Kumar (2022). “Brain Tumor
Classification Using Modified AlexNet Network”. In:
Advances in Distributed Computing and Machine
Learning. Springer.
Amran, G.A. et al. (2022). “Brain Tumor Classification and
Detection Using Hybrid Deep Tumor Network”. In:
Electronics 11.3457.
Apostolopoulos, I.D., S. Aznaouridis, and M. Tzani (2023).
“An Attention-Based Deep Convolutional Neural
Network for Brain Tumor and Disorder Classification
and Grading in Magnetic Reso- nance Imaging”. In:
Information 14.174.
Buda, Mateusz (2022). LGG MRI Segmentation Dataset.
https : / / www . kaggle . com / datasets /
mateuszbuda/lgg-mri-segmentation.
Duvvuri, K. et al. (2022). “Grad-CAM for Visualizing
Diabetic Retinopathy”. In: 2022 3rd International
Conference for Emerging Technology (IN- CET), pp. 1–
4.
Hu, H. et al. (2021). “Brain Tumor Diagnose Applying
CNN through MRI”. In: 2021 2nd International
Conference on Artificial Intelligence and Computer
Engineering (ICAICE).
Maheswari, B.U. et al. (2023). “In-Hospital Mortality
Prognosis: Unmasking Patterns Using Data Science and
Explainable AI”. In: 2023 9th International Conference
on Signal Processing and Communication (ICSC), pp.
356–361.
Nair, Priyanka C. et al. (2023). “Building an Explain- able
Diagnostic Classification Model for Brain Tumor
Using Discharge Summaries”. In: Proce- dia Computer
Science 218, pp. 2058–2070.
Pathak, A., M. Kamani, and R. Priyanka (2023). “Brain
Tumor Segmentation Using Modified Re- sUNET
Architecture”. In: 2023 International Conference on
Sustainable Communication Networks and Application
(ICSCNA).
Priya, A. and V. Vasudevan (2024). “Brain Tumor
Classification and Detection via Hybrid AlexNet- GRU
Based on Deep Learning”. In: Biomedical Signal
Processing and Control 89.
Rani, N.S. et al. (2022). “Multi-Disease Diagnosis Model
for Chest X-ray Images with Explain- able AI – Grad-
CAM Feature Map Visualization”. In: 2022
International Conference on Futuristic Technologies
(INCOFT), pp. 1–5.
Schettler, David (2021). BraTS 2021 Task 1 Dataset.
https://www.kaggle.com/datasets/dschettler8845/ brats-
2021-task1.
Shah, Arya (2019). Brain Tumor Segmentation - BraTS
2019. https://www.kaggle.com/datasets/ aryashah2k /
brain - tumor - segmentation - brats - 2019/data.
IC3Com 2024 - International Conference on Cognitive & Cloud Computing
274

Tas¸cı, B. (2023). “Attention Deep Feature Extraction from
Brain MRIs in Explainable Mode: DGX- AINet”. In:
Diagnostics (Basel) 13.5, pp. 859.
Velden, Bas H.M. van der et al. (2022). “Explainable
Artificial Intelligence (XAI) in Deep Learning- Based
Medical Image Analysis”. In: Medical Im- age Analysis
79.
Younis, A. et al. (2022). “Brain Tumor Analysis Using
Deep Learning and VGG-16 Ensembling Learning
Approaches”. In: Applied Sciences 12.7282.
Zeineldin, R.A., M.E. Karar, Z. Elshaer, et al. (2022).
“Explainability of Deep Neural Networks for MRI
Analysis of Brain Tumors”. In: International Journal of
Computer Assisted Radiology and Surgery (CARS) 17,
pp. 1673–1683.
Enhancing Brain Tumor Detection in Magnetic Resonance Imaging Through Explainable Artificial Intelligence Techniques and Fusion
Models
275
