
Developing a Head-Attached Interface Device for Closed-Loop
Transcranial Ultrasound Stimulation in the Mouse Brain
Ryo Furukawa
1a
, Shuichi Murakami
2b
and Takashi Tateno
3c
1
Bioengineering and Bioinformatics, Graduate School of Information Science and Technology, Hokkaido University,
Kita 14, Nishi 9, Kita-ku, Sapporo, Hokkaido 060-0814, Japan
2
Osaka Research Institute of Industrial Science and Technology, 2-7-1, Ayumino, Izumi, Osaka, 594-1157, Japan
3
Bioengineering and Bioinformatics, Faculty of Information Science and Technology, Hokkaido University, Kita 14,
Nishi 9, Kita-ku, Sapporo, Hokkaido 060-0814, Japan
Keywords: Closed-Loop System, Event-Related Potential, Micromachined Transducer, Transcranial Ultrasound Stimulation,
Wearable Device.
Abstract: Transcranial ultrasound stimulation (TUS), which can be used to noninvasively stimulate local and deep brain
areas, holds significant promise for clinical applications. However, TUS apparatus is typically constructed
with several components, including a relatively large single-element ultrasound (US) transducer, a waveguide,
and a driving source. These components pose challenges when conducting experiments with freely moving
small animals, especially in the context of wearable devices. Additionally, conventional open-loop stimulation
systems do not allow for the simultaneous monitoring of neural activity, which can sometimes result in the
overactivation of neural responses. In this study, we developed a head-mounted piezoelectric micromachined
ultrasound transducer (PMUT) array with integrated monitoring electrodes to serve as a TUS interface for
mice. To determine effective array patterns for optimal US beam profiles, we first conducted beamforming
simulations. We then microfabricated the PMUT arrays according to the results of these simulations.
Subsequently, we performed electroencephalographic (EEG) recordings to evaluate the potential of TUS in
mice while simultaneously monitoring neural activities. Finally, we discuss future applications of a closed-
loop TUS system in the treatment of brain diseases.
1 INTRODUCTION
Neuromodulation has been utilized as a clinical tool
for treating brain disorders (Davidson et al., 2024;
Mattioli et al., 2024). However, conventional
neuromodulation techniques, including
electromagnetic stimulation, face challenges related
to spatial resolution, invasiveness, and effective
transmission to deep brain regions (Rezayat &
Toostani, 2016).
Transcranial ultrasound stimulation holds
promise for clinical applications owing to its low or
non-invasive nature, high spatial resolution, and
ability to transmit mechanical vibrations into the
brain to induce neuromodulation (Tufail et al., 2010).
Recently, miniaturized devices based on
a
https://orcid.org/0000-0001-8920-1025
b
https://orcid.org/0000-0002-8862-8446
c
https://orcid.org/0000-0001-9429-9880
microelectromechanical system (MEMS) technology
have been reported for the purpose of ultrasound (US)
neuromodulation (Jo et al., 2019). However, because
the conventional transcranial ultrasound stimulation
(TUS) devices used in animals are specialized for
brain stimulation itself, simultaneously monitoring
neural activity driven by these devices requires
separate recording systems from the stimulation
devices (H. Kim et al., 2019; Zhou et al., 2019).
Therefore, the integration of stimulation and
recording devices is beneficial for realizing an on-
demand stimulation paradigm, which would enable
brain stimulation as needed for short periods without
causing overstimulation. More specifically, deep
brain stimulation (DBS) is utilized as one of the
symptomatic treatments for Parkinson’s disease and
Furukawa, R., Murakami, S. and Tateno, T.
Developing a Head-Attached Interface Device for Closed-Loop Transcranial Ultrasound Stimulation in the Mouse Brain.
DOI: 10.5220/0013082900003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 17-25
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
17

has demonstrated clinical efficacy in alleviating
symptoms (Okun, 2012). Currently, DBS systems are
commonly implemented using an open-loop
configuration; however, this approach has associated
with side effects, including the induction of neural
hyperactivity. To address these challenges, a closed-
loop DBS system that monitors neural activity and
adaptively delivers stimulation as needed is being
explored as a promising solution (Little et al., 2016).
Conventional TUS devices are open-loop
stimulators, which can sometimes induce excessive
neural activity, whereas the on-demand stimulation
paradigm requires a closed-loop stimulation system
(Takeuchi & Berényi, 2020). Although several
studies have reported methods for closed-loop TUS
systems (Jo et al., 2022; Xie et al., 2022), in these
studies, the stimulator and monitoring electrodes
were not packaged together as one device. For future
applications for chronic conditions in small animal
models such as rodents, small-sized systems using an
integrated device are desired.
Here, we describe our development of a head-
attached interface device for TUS applied to mice in
a closed-loop manner. This paper primarily describes:
(i) the design of a closed-loop TUS device, (ii) a
fabrication method to realize this design, and (iii) a
demonstration of the device’s usefulness by
analyzing electroencephalographic (EEG) data
recorded from a mouse head through the incorporated
monitoring electrodes. First, we describe the design
of a piezoelectric micromachined ultrasound
transducer (PMUT) combined with monitoring
electrodes. Second, we explain our detailed
microfabrication method. Third, we present EEG
recordings to examine whether the head-attached
device could be used for monitoring neural activities.
Finally, we discuss future applications of a wearable
closed-loop TUS system for the treatment of human
brain diseases.
2 METHODS
2.1 Design
To achieve TUS in a mouse brain, we aimed to
develop a PMUT with several diaphragms, the design
and structure of which were based on a previous study
(Furukawa et al., 2024). The PMUT is designed to
meet the following three conditions: (i) a resonant
frequency of 500 kHz for each diaphragm, (ii)
ultrasound pressure generated by mechanical
oscillations greater than 100 kPa, and (iii) a focal
length of over 5 mm (Yuan et al., 2021). Additionally,
microelectrodes (200 × 200 μm) were designed on the
back side of the PMUT to monitor EEG signals from
a mouse. The size of the microelectrodes was
determined on the basis of a previous report that
demonstrated low electrode impedance with the same
size and materials (Furukawa et al., 2024).
The structure of the PMUT is similar to that of a
previously reported device (Furukawa et al., 2024),
and consists of the following five components: (1) a
lead zirconate titanate (PZT) plate, (2) a silicon (Si)
layer, (3) an SiO₂ membrane, (4) top and bottom Pt/Ti
electrodes, and (5) an Si supporting layer. To operate
the diaphragm as a transducer and generate acoustic
pressure, a thin film of piezoelectric material was
used to convert electrical (voltage) signals into
ultrasound pressure changes. To obtain a thin
diaphragm that could function as a vibrating plate,
circular openings were designed from the rear side of
the supportive Si substrate.
Before fabricating the device, we numerically
simulated the vibrations of diaphragms in the PMUT
using general-purpose physics simulation software
(COMSOL Multiphysics, Ver. 6.2, COMSOL AB,
Sweden) on a supercomputer system (PRIMERGY
CX400/CX2550, FUJITSU, Japan) at the Hokkaido
University Computer Centre. Using the finite element
method (FEM) in this simulation software, we
calculated the primary resonant frequency, targeting
500 kHz, and determined the required sizes for the
PMUT.
Next, to explore the effective design of a US array
for TUS within a restricted area (10 × 10 mm), we
conducted a beamforming simulation with two
variable parameters with the aim of achieving target
acoustic pressures and focal length: (i) the distance
between the centers of diaphragms (d) ranging from
1 to 2 mm in 0.5-mm steps; and (ii) the excitation time
delay (Δt) ranging from 0 to 1.8 μs in 0.2-μs steps
(Fig. 1A) when used as a phased array system. The
excitation time delay was used to steer the US beam.
For this numerical simulation, the combinations of
the distance d and the total number of diaphragms
(cells) in individual PMUTs for our explored design
are summarized in Table 1.
Table 1: Design of the PMUT phased array used in the
beamforming simulation.
d (mm) Total cell numbe
r
1.0 25
1.5 17
2.0 17
In our simulation, for the acoustic pressure p
t
, the
wave equation is described as:
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
18
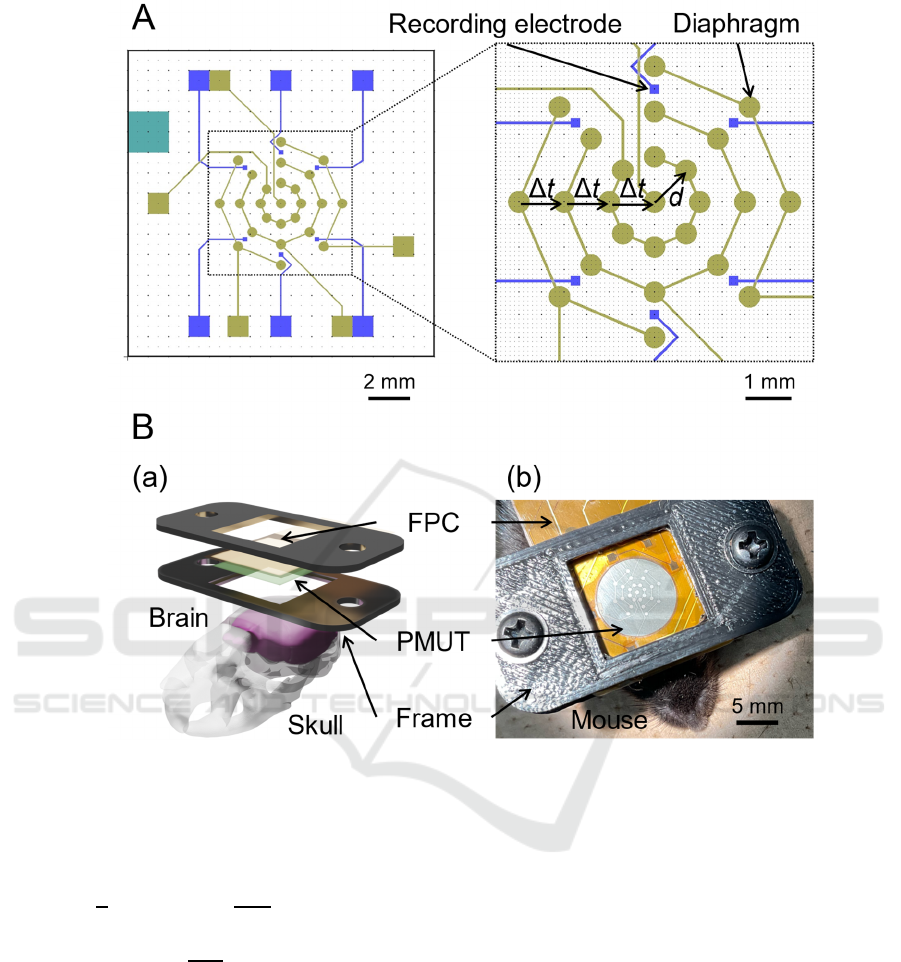
Figure 1: The design of a wearable PMUT phased array for closed-loop TUS. (A) Overall patterns of the PMUT design (left
view) and an enlarged view of the centre part (right panel). Gold lines, squares, and circles represent the upper electrodes and
their pads used to drive the transducer. Green squares are contact holes of the bottom electrode. Blue small and large squares
are microelectrodes and their pads used to acquire EEG signals from mice. (B) (a) Schematic of the head-attached type PMUT.
A surgically fixed bottom frame and removable top frame hold the PMUT. (b) Image of the fully packaged PMUT device.
∇∙
1
𝜌
∇𝑝
𝐪
𝑘
𝑝
𝜌
𝑄
,
(1)
𝑘
2𝜋𝑓
𝑐
(2)
where 𝜌 is the density of the medium, c is the speed
of sound, f is the driving US frequency, parameter q
d
is the dipole domain source (which represents a
domain volumetric force), and Q
m
is the monopole
domain source for a uniform strength in all directions.
Several PMUTs have recently been reported
for neuromodulation in rodents. A 1D-array PMUT
with a 275-μm radius for each diaphragm was
reported, with a single transducer generating 67.3 kPa
with an applied voltage of 66 V (Lee et al., 2019; Oh
et al., 2019). A 2D-array PMUT with a 580-μm radius
for each diaphragm was also reported, for which a
single transducer generated 65.6 ± 1.8 kPa with an
applied voltage of 70 V (Furukawa et al., 2024). In
these previous reports, the acoustic pressures were all
measured at a distance of approximately 1 mm away
from the devices. Therefore, in the structural model
of our simulation, we determined 60 kPa at 1 mm
from each diaphragm to be the desired acoustic
pressure value generated (i.e., 1.25 MPa).
2.2 Microfabrication and Packaging
Our microfabrication process was based on a previous
report on the standard MEMS technology (Kuwano et
al., 2020). The initial substrate was a silicon-on-
insulator (SOI) wafer consisting of the following
three layers: a device layer (Si, 15 μm), an insulating
Developing a Head-Attached Interface Device for Closed-Loop Transcranial Ultrasound Stimulation in the Mouse Brain
19

membrane (SiO
2
, 1 μm), and a handle layer (Si, 500
μm) with a 1-μm thermal-oxidized SiO
2
layer.
Briefly, our microfabrication process was as follows:
1. To form the microelectrode, a layer
consisting of a 100-nm-thick Pt coating and
a 10-nm-thick Ti coating was deposited on
the back side of the substrate using a
sputtering system (RSC-3ERD, Riken-sha
Co., Japan).
2. Subsequently, the recording electrodes and
their wires were patterned on the back side
by photolithography using an inductively
coupled plasma reactive ion etching (ICP-
RIE) system (RIE-101HU, SUMCO Co.,
Japan).
3. Next, the protective film (TMMR 2000SV,
TOKYO OHKA KOGYO CO., LTD.) for
the wires was formed on the bottom side.
4. A contact hole to expose the bottom
electrode of the PMUT was formed on the
front side of the substrate using a Deep-RIE
instrument (MUC-21 ASE-SRE, SPP
Technologies Co., Japan).
5. A PZT plate with a thickness of 100 μm (PI
Japan) was attached to the front side of the
SOI substrate with an epoxy adhesive (E205,
Konishi Co., Ltd.).
6. The thickness of the PZT plate was reduced
to 40 μm by using a griding machine
(Mechatec300 SPC, Kitagawa GRESTECH
Co., Ltd.).
7. In order to form the top electrodes of the
PZT and their lead wires, a layer consisting
of a 100-nm-thick Pt coating and a 10-nm-
thick Ti coating was deposited using a
sputtering system, and was patterned using a
lift-off technique.
8. To create the diaphragm shape, the Si handle
layer was removed from the back side using
a Deep-RIE instrument (MUC-21 ASE-
SRE, SPP Technologies Co., Japan).
After the process, the fabricated PMUT device
was packaged with the flexible printed circuit board
(Fig. 1B).
2.3 Measuring Event-Related
Potentials
2.3.1 Surgical Procedure
In this study, all animal experiments were carried out
in accordance with the NIH Guide for the Care and
Use of Laboratory Animals and with approval from
the International Animal Care and Use Committee of
Hokkaido University. For the animal experiments,
two female and one male C57BL/6J mice (Japan
SLC, Japan; 7 to 8 weeks old) were used.
Intraperitoneal injections of a mixture of
medetomidine (0.3 mg/kg), midazolam (4.0 mg/kg),
and butorphanol (5.0 mg/kg) were used to initiate
anesthesia, and anesthesia levels were confirmed by
the level of response when pinching the leg
(Yoshikawa et al., 2023).
The fur on top of the skull was gently removed,
then the scalp was carefully excised to expose the
skull. The custom-made 3D-printed bottom frame (20
× 40 mm; the center was positioned at the lambda)
was attached to hold the packaged PMUT by
sandwiching it with the top frame (Fig. 1B). The gap
between the PMUT and skull was filled with US gel.
2.3.2 Signal Recording and Sound Stimuli
The sensing microelectrodes were examined to detect
neural activities in extracellular field potentials
through EEG recordings. Pure-tone burst sounds
(frequencies: 2, 4, 8, 16, and 32 kHz; sound intensity:
80 dB sound pressure level [SPL]; duration: 100 ms)
were used to record the sound event-related potentials
(ERPs) in response to the acoustic stimulation. We
used linearly increasing onset and decreasing offset
stimulus envelopes set at 10% of the total duration of
each stimulus. Sound stimuli were presented via a
speaker (MF1; Tucker-Davis Technologies). The
EEG signals were recorded at a sampling rate of 1
kHz. Fifty trials were conducted under the same
conditions.
2.4 Data Analysis
All statistical analyses were performed using order
statistics without assuming a specific distribution,
employing non-parametric statistical methods. EEG
data were compared using the Wilcoxon signed-rank
test with Python (Ver. 3.12.1). The statistical analyses
were conducted for data acquired at two channels
(Chs 4 and 6) located at the inferior colliculus (IC).
We defined the baseline amplitude as the averaged
pre-stimulus peak amplitude before the stimulation
onset. In contrast, an ERP amplitude for 0.5 s after the
onset of a stimulus was defined as the post-stimulus
peak amplitude.
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
20
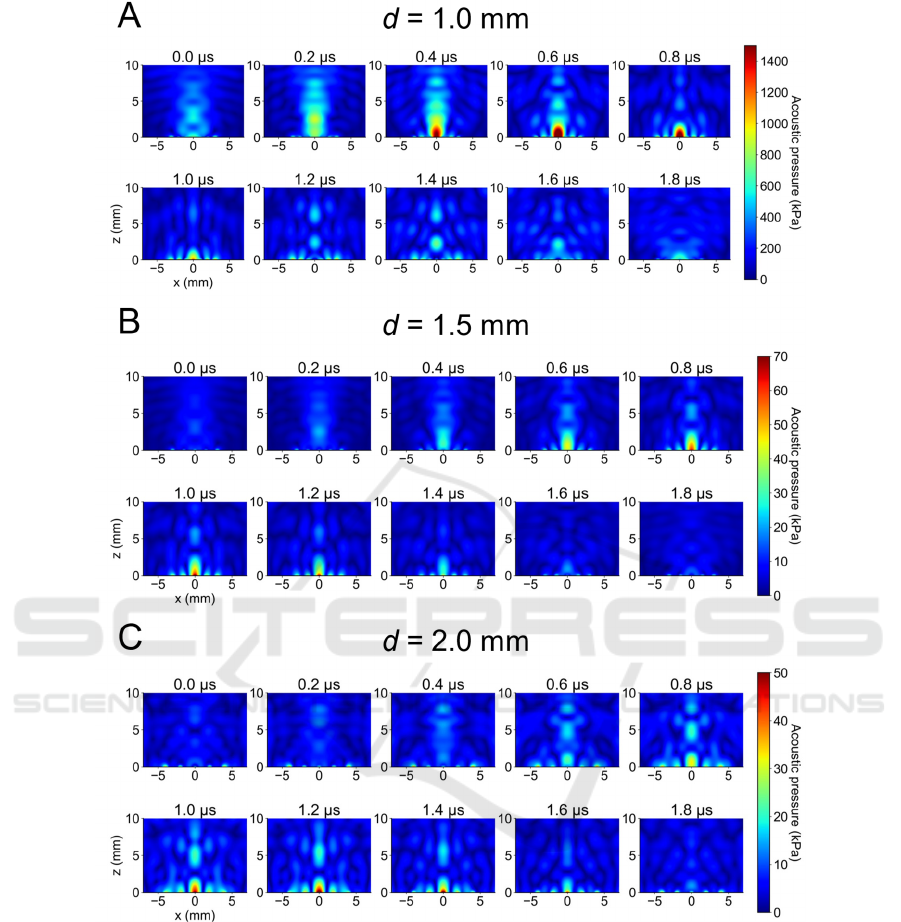
Figure 2: Simulated US beam profiles of the PMUT phased array. The distance (d) is represented in the right panel of Fig.
1A.
3 RESULTS
3.1 Ultrasound Beamforming
On the basis of the results of the numerical
calculations for our PMUT structural model with a
resonant frequency of 500 kHz, we selected the
following size parameters: a diaphragm radius of 235
μm, a PZT layer thickness of 40 μm, and a Si layer
thickness of 15 μm. Subsequently, using these
determined diaphragm sizes, we conducted a
beamforming simulation of the designed PMUT (Fig.
1A), in which each diaphragm array had different
nearest distances (d) between diaphragms (d = 1.0,
1.5, and 2.0 mm) and/or different total numbers of
diaphragms (17 or 25 cells, see Table 1). For example,
for the array pattern of d = 1.0 mm and 27 diaphragms,
Fig. 2A illustrates the two-dimensional (2D; x-z field)
acoustic pressure distributions at y = 0 mm in the 3D
acoustic field, resulting in a far-field peak at over 5
mm with a peak pressure exceeding hundreds of kPa.
Moreover, with the array pattern of d = 1.5 mm (or
Developing a Head-Attached Interface Device for Closed-Loop Transcranial Ultrasound Stimulation in the Mouse Brain
21
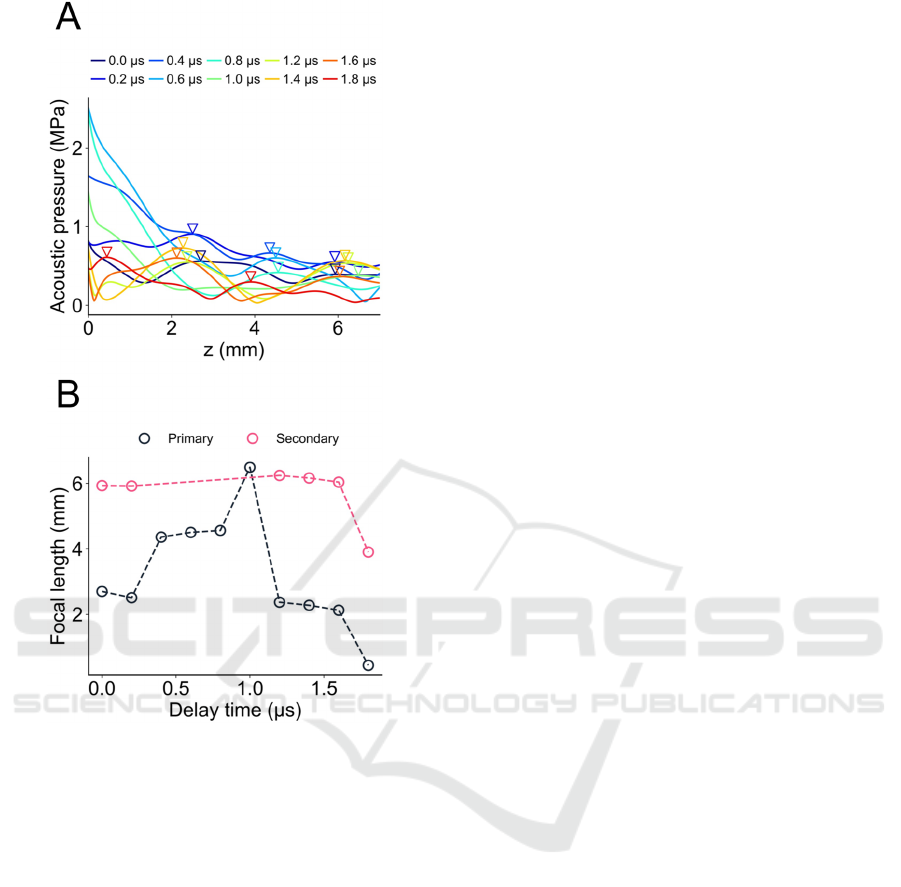
Figure 3: US beam profile for the axial distance in Fig. 2.
(A) Acoustic pressure distribution with different delay
times in the array pattern of d = 1.0 mm (Fig. 2A). Each
inverted triangle marker represents a local peak under the
delay time. (B) Focal length and delay times for primary
and secondary peaks indicated in panel (A). For some delay
time conditions, no secondary peaks were detected.
2.0 mm) and 17 diaphragms, although a far-field peak
exists at approximately 5 mm in the 2D field with the
axial direction (perpendicular to the PMUT array
surface), the simulated acoustic pressures were
smaller (in the tens of kPa) compared with the array
pattern of d = 1.0 mm (Figs. 2B, C). The acoustic field
in the axial direction (i.e., z direction) for the array
pattern of d = 1.0 mm is shown in Fig. 3A. The
inverted triangle markers represent the primary or
secondary peaks (local maxima). The focal lengths
depending on the delay times (Δt) of the phased array
are summarized in Fig. 3B. We found that using a
delay time Δt of 1.0 μs, a primary peak in the acoustic
field showed a local maximum at the farthest-most
field. This result suggests that using the delay time
(e.g., Δt = 0.6 μs for the surface or Δt = 1.4 μs for the
center) and a 70-V input voltage to the PMUT, the US
beam generated by the array of d = 1.0 mm can reach
a brain target within a range from the surface to the
center of a mouse brain.
3.2 Packaging of the Wearable PMUT
We successfully microfabricated the PMUT phased
array and integrated it with a custom-made flexible
printed circuit (FPC). The total weight of the
packaged device, including the head frames, PMUT,
FPC, and any other components, was 1.40 g.
3.3 Event-Related Potentials
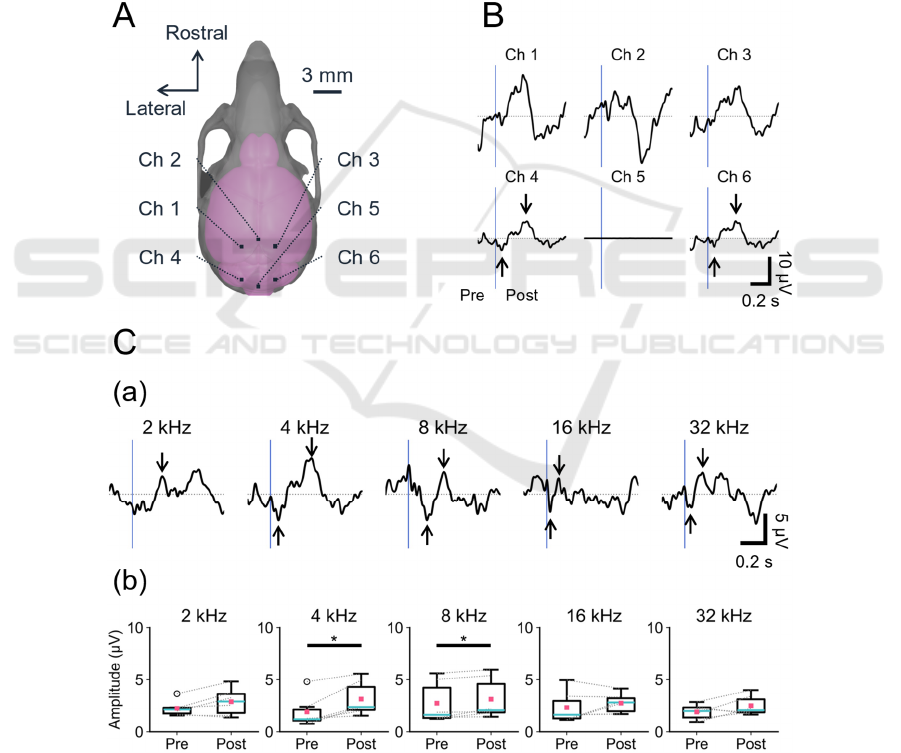
To examine the device’s ability to detect neural
activity from EEG signals, we conducted ERP
recordings in response to sound stimulation. The
schematic for the EEG recordings with six monitoring
electrodes (Chs 1 to 6) is illustrated in Fig. 4A.
Additionally, a typical example of averaged
waveforms evoked by sound stimuli (4-kHz pure-
tone burst, 80 dB SPL) is shown in Fig. 4B. The
waveforms obtained from two channels (e.g., Chs 4
and 6 in Fig. 4B) located around the IC showed
negative and positive peaks after sound stimulation
onset (−4.9 μV and 6.6 μV at Ch 4 in Fig. 4B).
Furthermore, averaged waveforms obtained from Ch
4 in response to sound stimulation with different
frequencies are illustrated in Fig. 4Ca. Negative or
positive peaks after the onset of the stimulation were
observed in response to the pure-tone bursts
examined (Fig. 4Ca). In particular, ERP peaks (post-
amplitudes) larger than the corresponding baseline
amplitudes (pre-amplitudes) were detected in
response to 4- and 8-kHz pure-tone burst stimuli (*p
< 0.05, n = 6 from three animals). In contrast, no
significant differences were found between pre and
post peak amplitudes in responses to the stimuli with
2, 16, and 32 kHz.
4 DISCUSSION
The total weights of previously reported wearable
transducers for rodents were 0.765 and 20 g for rats
(E. Kim et al., 2021; H. Kim et al., 2019), and 2 g for
mice (Zhou et al., 2019). Since our developed device
has a total weight of 1.4 g, we suggest that it is
suitable for application in experiments with freely
moving mice. However, the signal generator and
driving voltage source are not included in our weight
measurement.
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
22
In this study, we describe the development of
a MEMS-based PMUT phased array as a wearable
TUS interface device for closed-loop stimulation. The
resonant frequency of a single element transducer was
first calculated, followed by determination of the
sizes and the structure. Subsequently, US
beamforming was simulated with 25 or 17 cells with
a variable delay time for the driving signals of the
phased array. Guided by the numerical findings, we
proceeded to design and microfabricate wearable
PMUTs featuring 25 circular diaphragms, and
incorporating six square monitoring electrodes. To
our knowledge, this is the first report of the packaging
of monitoring electrodes onto a wearable PMUT.
With regard to the results of the beamforming
simulation, we expect to be able to modulate neural
activity with the output US beam. In addition, we
suggest that the focal length can be manipulated
across a wide range of regions in the mouse brain by
adjusting the excitation delay time (Figs. 3A, B). To
experimentally confirm the simulated beam profiles,
we will measure the acoustic pressure distribution
with a needle hydrophone (Furukawa et al., 2022).
Next, we demonstrated sound-driven EEG
recordings as a step towards the future application of
a wearable closed-loop TUS system. We successfully
detected the sound ERPs with the incorporated
electrodes, and since the IC is located 2–3 mm caudal
to the lambda and 2 mm lateral to the midline, the
observed ERPs could possibly be attributed to
Figure 4: Sound ERP waveforms at six sites on the mouse skull. (A) The six recording sites on the mouse skull are illustrated
with channel numbers (Chs 1 to 6). (B) Averaged ERP waveforms in response to sound stimulation (pure-tone burst, 4 kHz,
80 dB SPL). (C) ERP waveforms recorded at (a) Ch 4 and (b) comparison between pre- and post-maximum amplitudes of the
pure-tone burst with different frequencies (*p < 0.05, n = 6 from three animals). The timings of the sound onset are represented
by vertical blue bars.
Developing a Head-Attached Interface Device for Closed-Loop Transcranial Ultrasound Stimulation in the Mouse Brain
23

neural activity in the IC. Lower frequencies (e.g., 4
kHz) tend to evoke neural activity in shallow laminae
in the mouse IC (Sato et al., 2024), which is consistent
with the frequency characteristic of our observed
ERPs (Figs. 4Ca, 4b).
In future applications, we plan to test this device
in closed-loop TUS as a treatment method for brain
diseases. In line with this purpose, the oscillatory
power of specific frequencies is utilized as a reliable
EEG biomarker. Further, EEG biomarkers play a
pivotal role in diagnosing and understanding
neurological disorders, including epilepsy
(Buchhalter et al., 2022; Saeedinia et al., 2024),
Alzheimer’s disease (Chetty et al., 2024; Meghdadi et
al., 2021), and psychiatric disorders (Abi-Dargham &
Horga, 2016). In future applications of our device,
EEG biomarkers could provide PMUT device uses
with invaluable data for early detection and
intervention by capturing aberrations in brain activity
characteristics.
ACKNOWLEDGEMENTS
R.F. was supported by Grant-in-Aid for JSPS Fellows
[grant number JP23KJ0047]. T.T. was supported by
supported by the Murata Science Foundation, the
Suzuken Memorial Foundation, the Nakatani
Foundation for Advancement of Measuring
Technologies in Biomedical Engineering, a Grant-in-
Aid for Exploratory Research [grant number
21K19755], and a Grant-in-Aid for Scientific
Research (B) [grant number 23H03416] (Japan). The
authors appreciate Mr. Kawakatsu for his kind advice
and support.
REFERENCES
Abi-Dargham, A., & Horga, G. (2016). The search for
imaging biomarkers in psychiatric disorders. Nature
Medicine, 22(11), 1248–1255. https://doi.org/10.
1038/nm.4190
Buchhalter, J., Neuray, C., Cheng, J. Y., D’Cruz, O., Datta,
A. N., Dlugos, D., French, J., Haubenberger, D.,
Hulihan, J., Klein, P., Komorowski, R. W., Kramer, L.,
Lothe, A., Nabbout, R., Perucca, E., & der Ark, P. V.
(2022). EEG parameters as endpoints in epilepsy
clinical trials—An expert panel opinion paper. Epilepsy
Research, 187, 107028. https://doi.org/10.
1016/j.eplepsyres.2022.107028
Chetty, C. A., Bhardwaj, H., Kumar, G. P., Devanand, T.,
Sekhar, C. S. A., Aktürk, T., Kiyi, I., Yener, G.,
Güntekin, B., Joseph, J., & Adaikkan, C. (2024). EEG
biomarkers in Alzheimer’s and prodromal Alzheimer’s:
A comprehensive analysis of spectral and connectivity
features. Alzheimer’s Research & Therapy, 16(1), 236.
https://doi.org/10.1186/s13195-024-01582-w
Davidson, B., Bhattacharya, A., Sarica, C., Darmani, G.,
Raies, N., Chen, R., & Lozano, A. M. (2024).
Neuromodulation techniques – From non-invasive
brain stimulation to deep brain stimulation.
Neurotherapeutics, 21(3), e00330. https://doi.
org/10.1016/j.neurot.2024.e00330
Furukawa, R., Kaneta, H., & Tateno, T. (2022). A
Multielectrode Array-Based Recording System for
Analyzing Ultrasound-Driven Neural Responses in
Brain Slices in vitro. Frontiers in Neuroscience, 16.
https://www.frontiersin.org/articles/10.3389/fnins.202
2.824142
Furukawa, R., Yoshikawa, T., Murakami, S., & Tateno, T.
(2024). A piezoelectric micromachined ultrasound
transducer combined with recording electrodes for
acute brain preparations in vitro. Journal of
Neuroscience Methods, 403, 110048.
https://doi.org/10.1016/j.jneumeth.2023.110048
Jo, Y., Lee, S.-M., Jung, T., Park, G., Lee, C., Im, G. H.,
Lee, S., Park, J. S., Oh, C., Kook, G., Kim, H., Kim, S.,
Lee, B. C., Suh, G. S. B., Kim, S.-G., Kim, J., & Lee,
H. J. (2022). General-Purpose Ultrasound
Neuromodulation System for Chronic, Closed-Loop
Preclinical Studies in Freely Behaving Rodents.
Advanced Science, 9(34), 2202345. https://doi.org/10.
1002/advs.202202345
Jo, Y., Oh, C., & Lee, H. J. (2019). Microelectromechanical
Systems-Based Neurotools for Non-Invasive
Ultrasound Brain Stimulation. Chronobiology in
Medicine, 1(2), 55–59. https://doi.org/10.
33069/cim.2019.0009
Kim, E., Anguluan, E., Kum, J., Sanchez-Casanova, J.,
Park, T. Y., Kim, J. G., & Kim, H. (2021). Wearable
transcranial ultrasound system for remote stimulation
of freely moving animal. IEEE Transactions on
Biomedical Engineering, 68(7), 2195–2202.
https://doi.org/10.1109/TBME.2020.3038018
Kim, H., Kim, S., Sim, N. S., Pasquinelli, C., Thielscher,
A., Lee, J. H., & Lee, H. J. (2019). Miniature ultrasound
ring array transducers for transcranial ultrasound
neuromodulation of freely-moving small animals.
Brain Stimulation, 12(2), 251–255. https://doi.org/10.
1016/j.brs.2018.11.007
Kuwano, T., Kaneta, H., Nishikawa, J., Satoh, K.,
Murakami, S., & Tateno, T. (2020). Developing a
Frequency-selective Piezoelectric Acoustic Sensor
Sensitive to the Audible Frequency Range of Rodents.
IEEJ Transactions on Electrical and Electronic
Engineering, 15(12), 1816–1823. https://doi.org/10.
1002/tee.23260
Lee, J., Ko, K., Shin, H., Oh, S.-J., Lee, C. J., Chou, N.,
Choi, N., Tack Oh, M., Chul Lee, B., Chan Jun, S., &
Cho, I.-J. (2019). A MEMS ultrasound stimulation
system for modulation of neural circuits with high
spatial resolution in vitro. Microsystems &
Nanoengineering, 5(1), Article 1. https://doi.org/10.
1038/s41378-019-0070-5
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
24

Little, S., Tripoliti, E., Beudel, M., Pogosyan, A., Cagnan,
H., Herz, D., Bestmann, S., Aziz, T., Cheeran, B.,
Zrinzo, L., Hariz, M., Hyam, J., Limousin, P., Foltynie,
T., & Brown, P. (2016). Adaptive deep brain
stimulation for Parkinson’s disease demonstrates
reduced speech side effects compared to conventional
stimulation in the acute setting. Journal of Neurology,
Neurosurgery & Psychiatry, 87(12), 1388–1389.
https://doi.org/10.1136/jnnp-2016-313518
Mattioli, F., Maglianella, V., D’Antonio, S., Trimarco, E.,
& Caligiore, D. (2024). Non-invasive brain stimulation
for patients and healthy subjects: Current challenges
and future perspectives. Journal of the Neurological
Sciences, 456, 122825. https://doi.org/10.1016/j.
jns.2023.122825
Meghdadi, A. H., Karić, M. S., McConnell, M., Rupp, G.,
Richard, C., Hamilton, J., Salat, D., & Berka, C. (2021).
Resting state EEG biomarkers of cognitive decline
associated with Alzheimer’s disease and mild cognitive
impairment. PLOS ONE, 16(2), e0244180.
https://doi.org/10.1371/journal.pone.0244180
Oh, S.-J., Lee, J. M., Kim, H.-B., Lee, J., Han, S., Bae, J.
Y., Hong, G.-S., Koh, W., Kwon, J., Hwang, E.-S.,
Woo, D. H., Youn, I., Cho, I.-J., Bae, Y. C., Lee, S.,
Shim, J. W., Park, J.-H., & Lee, C. J. (2019). Ultrasonic
Neuromodulation via Astrocytic TRPA1. Current
Biology, 29(20), 3386-3401.e8. https://doi.org/10.
1016/j.cub.2019.08.021
Okun, M. S. (2012). Deep-Brain Stimulation for
Parkinson’s Disease. New England Journal of
Medicine, 367(16), 1529–1538. https://doi.org/10.
1056/NEJMct1208070
Rezayat, E., & Toostani, I. G. (2016). A review on brain
stimulation using low intensity focused ultrasound.
Basic and Clinical Neuroscience, 7(3), 187–194.
https://doi.org/10.15412/J.BCN.03070303
Saeedinia, S. A., Jahed-Motlagh, M. R., Tafakhori, A., &
Kasabov, N. K. (2024). Diagnostic biomarker
discovery from brain EEG data using LSTM, reservoir-
SNN, and NeuCube methods in a pilot study comparing
epilepsy and migraine. Scientific Reports, 14(1), 10667.
https://doi.org/10.1038/s41598-024-60996-6
Sato, H., Sugimoto, F., Furukawa, R., & Tateno, T. (2024).
Modulatory Effects on Laminar Neural Activity
Induced by Near-Infrared Light Stimulation with a
Continuous Waveform to the Mouse Inferior Colliculus
In Vivo. eNeuro, 11(5). https://doi.org/10.
1523/ENEURO.0521-23.2024
Takeuchi, Y., & Berényi, A. (2020). Oscillotherapeutics –
Time-targeted interventions in epilepsy and beyond.
Neuroscience Research, 152, 87–107. https://doi.
org/10.1016/j.neures.2020.01.002
Tufail, Y., Matyushov, A., Baldwin, N., Tauchmann, M. L.,
Georges, J., Yoshihiro, A., Tillery, S. I. H., & Tyler, W.
J. (2010). Transcranial Pulsed Ultrasound Stimulates
Intact Brain Circuits. Neuron, 66(5), 681–694.
https://doi.org/10.1016/j.neuron.2010.05.008
Xie, Z., Yan, J., Dong, S., Ji, H., & Yuan, Y. (2022). Phase-
locked closed-loop ultrasound stimulation modulates
theta and gamma rhythms in the mouse hippocampus.
Frontiers in Neuroscience, 16. https://www.
frontiersin.org/articles/10.3389/fnins.2022.994570
Yoshikawa, T., Sato, H., Kawakatsu, K., & Tateno, T.
(2023). Low-Cost Electroencephalographic Recording
System Combined with a Millimeter-Sized Coil to
Transcranially Stimulate the Mouse Brain In Vivo.
JoVE (Journal of Visualized Experiments), 195,
e65302. https://doi.org/10.3791/65302
Yuan, Y., Zhang, K., Zhang, Y., Yan, J., Wang, Z., Wang,
X., Liu, M., & Li, X. (2021). The Effect of Low-
Intensity Transcranial Ultrasound Stimulation on
Neural Oscillation and Hemodynamics in the Mouse
Visual Cortex Depends on Anesthesia Level and
Ultrasound Intensity. IEEE Transactions on
Biomedical Engineering, 68(5), 1619–1626. IEEE
Transactions on Biomedical Engineering.
https://doi.org/10.1109/TBME.2021.3050797
Zhou, H., Niu, L., Xia, X., Lin, Z., Liu, X., Su, M., Guo, R.,
Meng, L., & Zheng, H. (2019). Wearable Ultrasound
Improves Motor Function in an MPTP Mouse Model of
Parkinson’s Disease. IEEE Transactions on Biomedical
Engineering, 66(11), 3006–3013. IEEE Transactions
on Biomedical Engineering. https://doi.org/10.
1109/TBME.2019.2899631.
Developing a Head-Attached Interface Device for Closed-Loop Transcranial Ultrasound Stimulation in the Mouse Brain
25
