
Electrical Properties of Filled PVA-C for Bioelectrical Impedance
Spectroscopy Phantoms
Anna Bublex, Amalric Montalibet, Bertrand Massot and Claudine Gehin
INSA Lyon, Ecole Centrale de Lyon, CNRS, Universite Claude Bernard Lyon 1,
CPE Lyon, INL, UMR5270, 69621 Villeurbanne, France
Keywords: PVA Phantom, Hydrogel, Bioelectrical Impedance Spectroscopy, Conductivity, Permittivity,
Living Tissue, Electrical Properties.
Abstract: This paper investigates the electrical properties of polyvinyl alcohol cryogel (PVA-C) filled with various
materials to develop biological phantoms for bioelectrical impedance spectroscopy (BIS) applications.
PVA-C is a hydrogel that undergoes cross-linking through freeze-thaw cycling, known for its long-term
stability and mechanical properties, which closely mimic those of living tissue, making it a superior alternative
to traditional gelling agents such as agar. To assess the impact of different fillers, samples with varying filler
proportions were prepared, and their electrical properties were analysed using BIS across a low-frequency
range (1 kHz to 1 MHz). The study was divided into two parts: the first one focused on the effects of PVA
concentration, the number of freeze-thaw cycles, molecular weight, and time-dependent behaviour on
PVA-C’s electrical properties. The second part compared the electrical properties of PVA-C combined with
various fillers, including particles, polymers, and ceramics. Finally, the results were compared with existing
published data on the electrical properties of living muscle and fat.
1 INTRODUCTION
Bioelectrical impedance spectroscopy (BIS) is a
valuable technique for assessing the composition,
structure, and functional properties of living tissues.
This is achieved by passing an alternating current
through a body part and measuring the resulting
voltage at low frequencies. To ensure the accuracy
and reliability of BIS sensors used in body
composition analysis, thorough validation is
essential. This involves conducting precise
measurements to compare the sensor outputs against
known standards or reference methods. However, the
inherent variability of human physiology poses
challenges to obtaining consistent in vivo data, as
short-term fluctuations within individuals
(intra-individual) and differences between
individuals (inter-individual) can influence results.
To address these challenges, researchers utilise
phantoms (artificial physical models) designed to
replicate the properties of biological tissues.
Phantoms provide a stable and repeatable
environment for testing and enable precise control
over tissue parameters during validation studies.
Phantoms have been extensively developed using
biological materials such as vegetable-based
substances or gelling agents filled with conductive
particles (Anand et al., 2019; Hess et al., 2022;
Mobashsher & Abbosh, 2014). While these materials
effectively replicate the electrical properties of living
tissues, they often suffer from short-term stability and
inadequate mechanical strength. To overcome these
limitations, researchers have explored alternative
materials such as silicones, elastomers, and filled
polymers (Dunne et al., 2018; Goyal et al., 2022).
Among these polymers, polyvinyl alcohol (PVA) has
attracted significant research interest. PVA is soluble
in hot water and can be cross-linked through
freeze-thaw cycles to form polyvinyl alcohol cryogel
(PVA-C), a hydrogel with mechanical properties and
texture similar to those of human tissue (Chen et al.,
2012; Goyal et al., 2022). While PVA-C exhibits
excellent mechanical properties, its electrical
properties are not inherently comparable to those of
living tissue. To address this, fillers are incorporated
into the hydrogel to achieve appropriate conductivity.
This paper investigates the electrical properties of
PVA-C samples loaded with various fillers.
Conductivity and permittivity measurements were
26
Bublex, A., Montalibet, A., Massot, B. and Gehin, C.
Electrical Properties of Filled PVA-C for Bioelectrical Impedance Spectroscopy Phantoms.
DOI: 10.5220/0013103800003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 26-34
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
performed to evaluate their potential application in
the development of electrical phantoms.
2 MATERIALS AND METHODS
2.1 Materials
The materials used in this study are summarised in
Table 1.
Table 1: Materials used in this study.
Material Descri
p
tion Su
pp
lier
PVA
145 kDa
Fully hydrolysed Sigma-Aldrich
PVA
60 kDa
Fully hydrolysed Sigma-Aldrich
Li
g
nin Alkali Si
g
ma-Aldrich
1,2-propanediol
ACS reagent,
≥99.5%
Sigma-Aldrich
Barium titanate
Powder, <3 µm,
99%,
M=233.19 g/mol
Sigma-Aldrich
Agar
Industrial agar,
CONDALAB
Dutscher
NaCl
M=58.44 g/mol,
APPLICHEM
Dutscher
Graphite
Purity 99.9%,
particle size
5-10
µ
m
NanoGrafi
Plaste
r
Parexlanko Local market
Glycerine Coope
r
Local market
Corn flou
r
Maïzena Local market
“Sommières” earth
‘’La fée du logis
vert’’
Local market
Diatomaceous earth Novatera Local market
Glass beads
5-20 µm and
150-200 µm
Local market
2.2 PVA-Cryogel: A Brief Overview
Polyvinyl alcohol (PVA) is a versatile synthetic
polymer widely used in medical applications such as
cartilage replacement and the fabrication of
cardiovascular devices (Kobayashi & Hyu, 2010;
Wan et al., 2014). Its ability to form stable hydrogels
through freeze-thaw cross-linking has made it a
material of significant interest for tissue engineering
and biomedical research. This process results in the
formation of polyvinyl alcohol cryogel (PVA-C),
which closely mimics the texture and mechanical
properties of human tissues, as confirmed in multiple
studies (Chen et al., 2012; Duboeuf et al., 2009;
Fromageau et al., 2007; Gautam et al., 2021; Goyal
et al., 2022; Khaled et al., 2007).
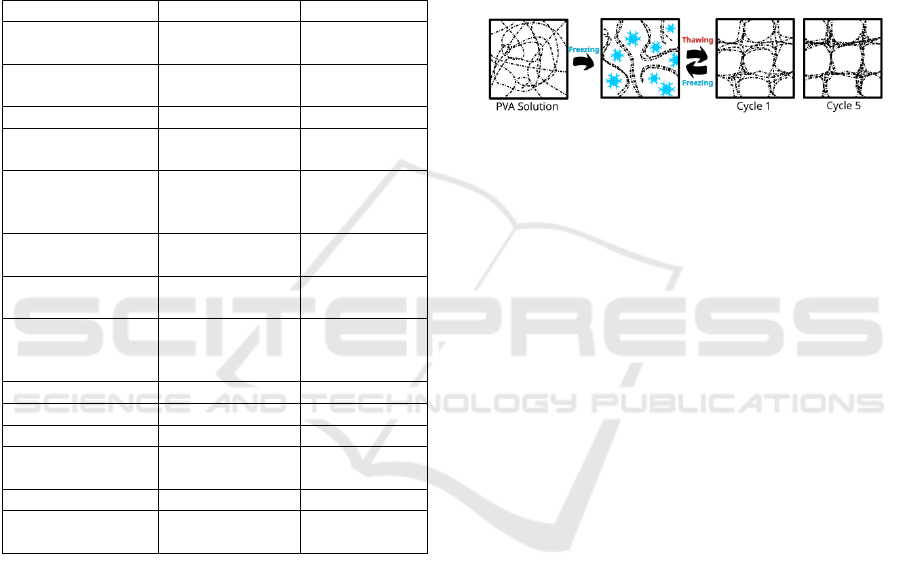
The process of crosslinking PVA to form PVA-C
is illustrated in Figure 1. During the freezing process,
the formation of ice crystals forces the PVA chains
into a more compact configuration, resulting in the
creation of a PVA-rich phase. The PVA regions
become increasingly concentrated, and the formation
of hydrogel bonds is facilitated by an increase in the
number of freeze-thaw cycles. Given the slow
movement of macromolecules, multiple cycles are
required to achieve a robust hydrogel. The strength of
the resulting hydrogel also depends on the molecular
weight and concentration of PVA in solution (Adelnia
et al., 2022; Wan et al., 2014).
Figure 1: Effect of cycling on the microstructure of PVA-C.
The mechanical and electrical properties of
PVA-C are influenced by several factors, including
the degree of hydrolysis, molecular weight,
concentration, and the freeze-thaw process. These
properties change during the course of the cycles
(Adelnia et al., 2022; Getangama et al., 2020; Wan et
al., 2014). Notably, below a concentration of 5% w/w
of PVA, the hydrogel will not form. Furthermore, the
mechanical strength of the hydrogel significantly
decreases when the concentration is below 10% w/w,
whereas concentrations above 30% w/w make the
mixture difficult to manipulate (Adelnia et al., 2022).
The long-term stability of PVA-C is particularly
noteworthy, making it a preferred option over
traditional gelling agents such as agar or gelatine
(Hess et al., 2022). Additionally, PVA is considered
an environmentally friendly material due to its
biocompatibility, biodegradability, and non-toxicity
during both the manufacturing and cross-linking
processes (Belay, 2023).
2.3 Preparation of the Samples
This paper presents an analysis of two distinct sample
types. Both sample types were placed in a standard
measuring cell (refer to section 2.4.2) to compare the
conductivity and permittivity values of the materials
with those of muscle reported in the literature.
The first set of samples enables the investigation
of the electrical properties of PVA-C based on
various parameters, including the PVA concentration
in solution, the number of freeze-thaw cycles, the
molecular weight of PVA and the evolution of
Electrical Properties of Filled PVA-C for Bioelectrical Impedance Spectroscopy Phantoms
27
PVA-C properties over time. This investigation was
conducted on three distinct samples.
The first PVA-C sample was cross-linked from a
10% w/w PVA solution and underwent a single
freeze-thaw cycle. The remaining two samples were
of considerable size, from which smaller sub-samples
were extracted for bioimpedance measurements
across varying cycles. One sub-sample was
specifically examined to assess its stability over time.
During the intervals between measurements, the
sample was stored in a plastic film at room
temperature. In the case of the 60 kDa sample, the
first freeze-thaw cycle resulted in the formation of a
hydrogel that lacked the required robustness for
handling and measurement. Table 2 summarises the
characteristics of the studied PVA-C samples. In this
study, weight proportions (% w/w) are expressed in
relation to the weight of the material relative to the
weight of the solvent (deionised water).
Table 2: Summary of characteristics for the studied PVA-C
samples.
Molecular
weight
Cycle
Concentration
(% w/w)
Study
of
stabilit
y
1
145 kDa
1
10
2
15
x
2 15
3 15
4 15
3 60 kDa
2 15
3 15
4 15
The second set of samples was prepared to
investigate the electrical properties of PVA-C filled
with different types of particles, polymers, and other
materials. The objective was to examine the effect of
varying the proportions of fillers in a solution of
PVA, with a molecular weight of 145 kDa and a
concentration of 10% w/w, on its electrical
properties. All samples were subjected to a single
freeze-thaw cycle.
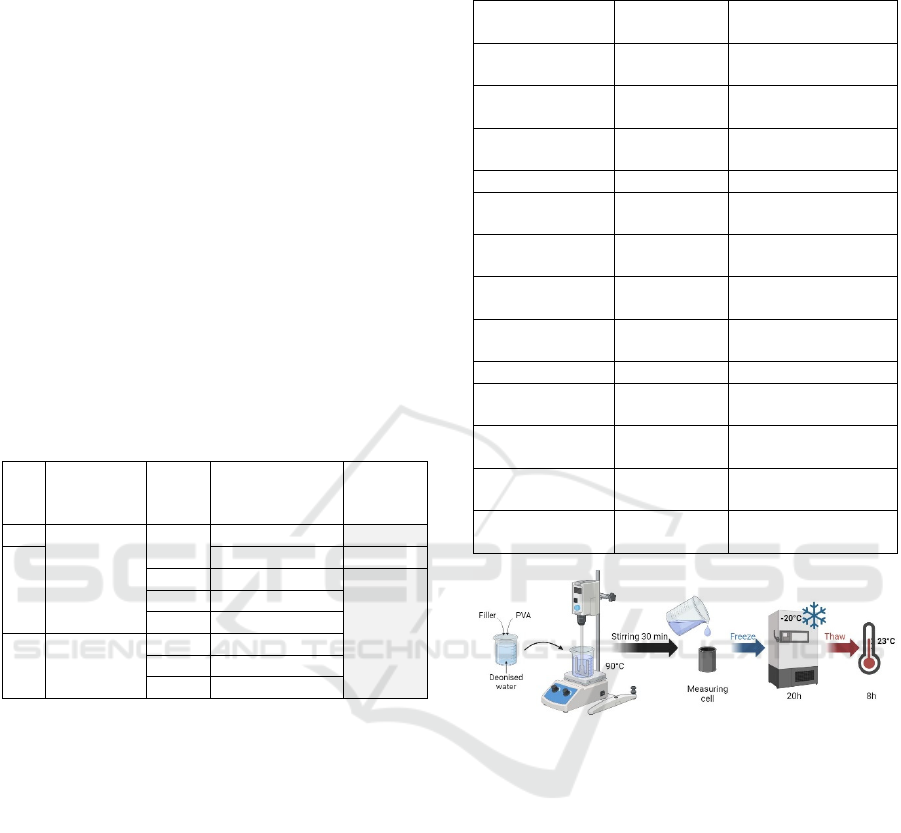
Samples were prepared according to the material
proportions detailed in Table 3.
For each sample, the materials were mixed with
deionised water in a beaker and mechanically agitated
for 30 minutes at 90°C. The resulting mixture was
then transferred into the standard measuring cell for a
single freeze-thaw cycle, consisting of freezing
at -20°C for 20 hours, followed by thawing at room
temperature (23°C) for 8 hours (see Figure 2).
Table 3: Summary of sample composition based on PVA
(145 kDa, 10% w/w, one freeze-thaw cycle).
Materials
Proportions
(% w/w)
Remarks
Lignin
1-5-10-
20% w/w
-
1,2-propanediol
5-10-
20% w/w
Also known as
p
ro
py
lene
g
l
y
col
Barium titanate 5-15% w/w
Must be handled
with
p
rotection
A
g
a
r
1-2-3% w/w Gellin
g
a
g
ent
NaCl
0.1-0.5-
1% w/w
Enhance ionic
conductivit
y
Graphite
0.1-0.5-1-2-
5% w/w
Plaster
0.1-0.5-2-5-
10% w/w
Glycerine 1-2-5% w/w
Also known as
g
l
y
cerol
Corn flou
r
1-2-5% w/w
“Sommières”
earth
2-5-10-
20% w/w
Clay known for its
absorbent properties
Diatomaceous
earth
2-5-10-
20% w/w
Fossilized remains
of diatoms (algae)
Glass beads
5-20
µ
m
2-5-10-
20% w/w
Glass beads
150-200
µ
m
2-5-10-
20% w/w
Figure 2: Sample preparation steps. Created in BioRender.
Bublex, A. (2025) https://BioRender.com/u47q709.
2.4 Impedance Spectroscopy
Measurement
2.4.1 Bioelectrical Impedance Spectroscopy
Bioelectrical impedance spectroscopy (BIS) is a
non-invasive technology used to measure the
volumes of various body compartments, including
total body water and extracellular water. BIS
measures impedance at multiple frequencies to
analyse cellular membrane integrity and fluid
distribution, thereby offering insights into cellular
health and hydration status. Impedance, a
generalisation of Ohm's law to alternating current, is
a complex quantity and is expressed as: (Bera, 2014;
Grimnes & Martinsen, 2015)
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
28

𝑍 𝑅 𝑗𝑋 (1)
where 𝑍: impedance
𝑅: resistance
𝑋: reactance
2.4.2 Measurement Tools
In this study, the Multifrequency Impedance
Analyzer (MFIA 5 MHz model) from Zurich
Instrument served as the reference standard for
bioimpedance measurements. The device is capable
of performing bioimpedance measurements across a
frequency range of 1 mHz to 5 MHz and within a
measurement range of 1 mΩ to 1 TΩ. In the present
investigation, measurements were conducted over a
frequency range of 1 kHz to 1 MHz, with 100 data
points acquired at logarithmic intervals. Standard
measuring cells were developed based on the model
established by Suga (Suga et al., 2013). The cells
consist of transparent, extruded polycarbonate
cylinders with a length of 50 mm and an inner
diameter of 24 mm. Measurements were performed
using these standard measuring cells, as illustrated in
Figure 3.
Figure 3: Schematic of a standard measuring cell. Length
d = 50 mm, inner surface area A = 4.52 cm².
The electrodes were 30 mm diameter, gold-plated
discs, manufactured by JLCPCB, China. The gold
coating was specified as ENIG 2U (Electroless Nickel
Immersion Gold) with a thickness of 2 µm. The
support structure was designed using Autodesk
Fusion 360 and 3D printed using polylactic acid
(PLA). A spring mechanism was incorporated into
the design to ensure a constant force applied to the
sample.
2.4.3 Two-Electrode Measurements: Model
Extraction of Conductivity and
Permittivity
The conductivity and relative permittivity of a sample
were evaluated through impedance measurements
conducted in a controlled geometry. These data were
compared with existing literature values for living
tissue (see Figure 4).
Figure 4: Electrical properties of 4 living tissue - Adapted
from Gabriel et al. 1996 (Gabriel, 1996).
To extract the values of conductivity (σ) and
relative permittivity (ε
r
) from bioimpedance
measurements (Z), admittance ( Y ) was calculated
according to the methodology proposed by A. Ivorra
(A.Ivorra, 2002):
𝑌
1
𝑍
and 𝑌𝐺𝑖𝐵 (2)
where G is conductance and B is susceptance. The
values of conductivity and relative permittivity are
expressed as follows:
𝜎
𝐺
𝐾
and 𝜀
𝐵
𝐾2𝜋𝑓𝜀
(3)
with the cell coefficient 𝐾𝐴/𝑑, 𝐴 is the cross-
sectional area and 𝑑 is the cylinder length (see Figure
3). In this document, the term “relative permittivity”
will be used interchangeably with “permittivity”.
3 RESULTS
3.1 PVA-C Electrical Properties as a
Function of Number of Cycles and
Molecular Weight
The evolution of the electrical properties of two
PVA-C samples with different molecular weights
over multiple cycles is shown in Figure 5. In the case
of the 60 kDa sample, the first cycle produced a
hydrogel that lacked sufficient robustness for
handling and measurement.
Figure 5: Electrical properties of PVA-C samples over
multiple cycles.
Electrical Properties of Filled PVA-C for Bioelectrical Impedance Spectroscopy Phantoms
29

The molecular weight significantly influences the
conductivity of the samples and the permittivity at
low frequencies. Conductivity increases with the
number of freeze-thaw cycles, but higher molecular
weight correlates with lower conductivity. For both
PVA-C samples, permittivity tends to converge at
high frequencies.
3.2 PVA-C Electrical Properties as a
Function of Concentration
Figure 6 illustrates the electrical properties of two
PVA-C samples with varying concentrations of PVA
in solution.
Figure 6: Electrical properties of PVA-C samples with
different concentrations of PVA – 10% w/w and 15% w/w.
As the concentration of PVA in solution
increases, both conductivity and permittivity show a
corresponding rise for samples of a given molecular
weight.
3.3 PVA-C Electrical Properties over
Time
Figure 7 illustrates the stability of the electrical
properties of a PVA-C sample stored in a plastic film
at room temperature over a four-day period.
Figure 7: Electrical properties of PVA-C over time.
Both conductivity and permittivity show a notable
daily increase. This increase is likely due to water loss
from samples that are not completely hermetically
sealed.
3.4 Hydrogel Based on Filled PVA-C
This section examines the impact of varying the
proportion of fillers in a solution of PVA, with a
molecular weight of 145 kDa and a concentration of
10% w/w, on the electrical properties. All samples
were subjected to a single freeze-thaw cycle.
3.4.1 Agar
The comparative electrical properties of PVA-C filled
with different proportions of agar, along with a pure
PVA-C sample, are presented in Figure 8.
Figure 8: Electrical properties of PVA-C filled with various
proportions of agar.
The addition of agar increases both conductivity
and permittivity, aligning with its ionic nature, which
enhances ion mobility within the hydrogel matrix.
This behaviour suggests that agar is a promising
candidate for mimicking the electrical properties of
biological tissues.
3.4.2 Corn Flour
Figure 9 illustrates the comparative electrical
properties of PVA-C filled with varying proportions
of corn flour, alongside a pure PVA-C sample.
Figure 9: Electrical properties of PVA-C filled with various
proportions of corn flour.
The addition of corn flour reduces conductivity
without significantly affecting permittivity,
suggesting that its primary effect is to obstruct ion
flow rather than alter dielectric properties. This
characteristic makes it suitable for applications where
reduced conductivity is desired.
3.4.3 Glycerine
Figure 10 shows the electrical properties of PVA-C
filled with various amounts of glycerine, compared to
a pure PVA-C sample.
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
30

Figure 10: Electrical properties of PVA-C filled with
various proportions of glycerine.
Glycerine notably decreases conductivity while
leaving permittivity relatively unchanged. This may
be due to its insulating properties and ability to reduce
free ion concentration. Its inclusion could be
beneficial for simulating tissues with low
conductivity, but it may require reinforcement to
maintain mechanical strength.
3.4.4 NaCl
Figure 11 illustrates the comparative electrical
properties of PVA-C filled with varying proportions
of NaCl, compared to a pure PVA-C sample.
Figure 11: Electrical properties of PVA-C filled with
various proportions of NaCl.
NaCl significantly increases both conductivity
and permittivity, consistent with its role as an ionic
conductor. This makes it highly effective for
muscle-mimicking phantoms. The observed linear
relationship between NaCl concentration and
conductivity supports its controlled application.
3.4.5 Graphite
The comparative electrical properties of PVA-C filled
with different proportions of graphite, compared to a
pure PVA-C sample, are presented in Figure 12.
The conductivity and permittivity curves exhibit
significant changes. When the graphite proportion is
less than 1%, conductivity remains lower than that of
the pure PVA-C sample. In contrast, for proportions
above 2%, the conductivity curve increases beyond
that of PVA-C alone at higher frequencies. The
permittivity curve is primarily affected at
concentrations above 0.5%.
Figure 12: Electrical properties of PVA-C filled with
various proportions of graphite.
3.4.6 Lignin
Figure 13 illustrates the comparative electrical
properties of PVA-C filled with varying proportions
of lignin, compared to a pure PVA-C sample.
Figure 13: Electrical properties of PVA-C filled with
various proportions of lignin.
The incorporation of lignin into the hydrogel
leads to a notable enhancement in both conductivity
and permittivity, reaching levels comparable to those
observed with NaCl. The electrical properties show a
rapid increase at low lignin concentrations and appear
to plateau at concentrations above 10% lignin.
3.4.7 1,2-Propanediol
Figure 14 shows the electrical properties of PVA-C
filled with various amounts of 1,2-propanediol,
compared to a pure PVA-C sample.
Figure 14: Electrical properties of PVA-C filled with
various proportions of 1,2-propanediol.
The reduction in both conductivity and
permittivity suggests its use as a dielectric filler for
applications requiring a low electrical response.
However, its concentration must be carefully
controlled to prevent adverse effects on mechanical
properties.
Electrical Properties of Filled PVA-C for Bioelectrical Impedance Spectroscopy Phantoms
31
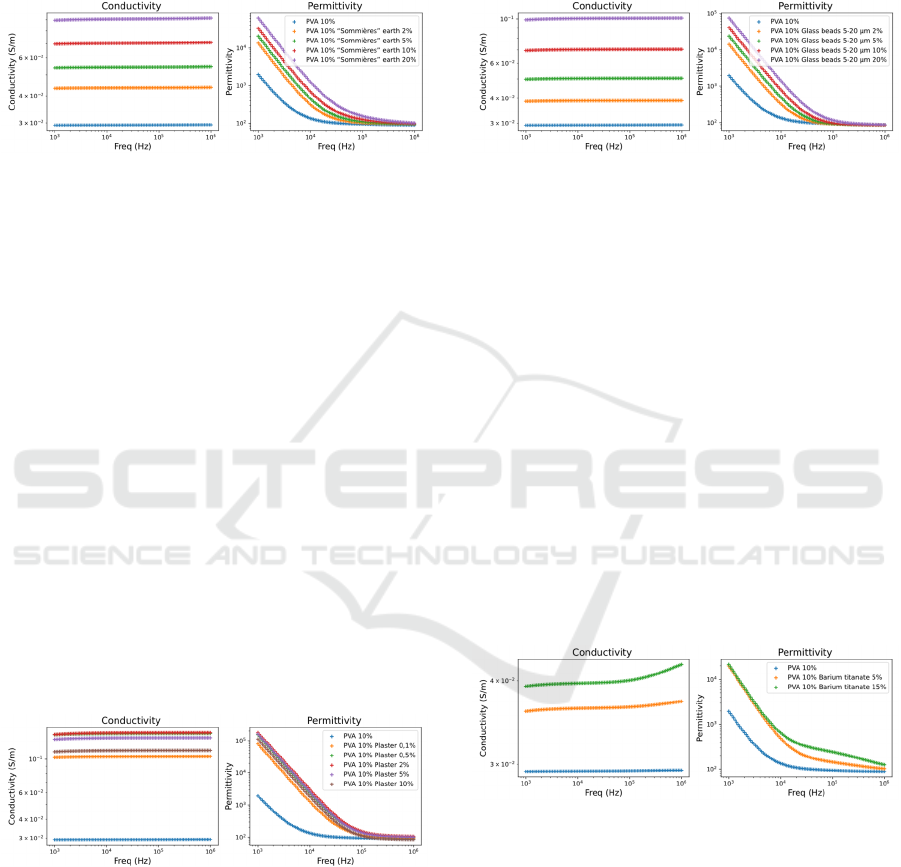
3.4.8 “Sommières” Earth
The comparative electrical properties of PVA-C filled
with different proportions of “Sommières” earth,
compared to a pure PVA-C sample, are presented in
Figure 15.
Figure 15: Electrical properties of PVA-C filled with
various proportions of “Sommières” earth.
The addition of “Sommières” resulted in a
moderate enhancement in conductivity and
permittivity relative to NaCl or lignin. This
improvement is likely due to its high surface area and
ionic exchange capacity. Its effect on mechanical
properties should be further explored to determine its
practical applicability.
3.4.9 Diatomaceous Earth
Diatomaceous earth is too dense to remain suspended
in the PVA mixture during freezing, causing it to
settle at the bottom of the sample due to gravity. As a
result, the samples exhibit inhomogeneity, and their
electrical properties have not been characterised.
3.4.10 Plaster
Figure 16 shows the electrical properties of PVA-C
filled with various amounts of plaster, compared to a
pure PVA-C sample.
Figure 16: Electrical properties of PVA-C filled with
various proportions of plaster.
Plaster exhibited a marked increase in both
conductivity and permittivity. Its ease of handling and
low cost make it a strong candidate for large-scale
phantom development.
3.4.11 5-20 µm Glass Beads
The comparative electrical properties of PVA-C filled
with different proportions of 5-20 µM glass beads,
compared to a pure PVA-C sample, are presented in
Figure 17.
Figure 17: Electrical properties of PVA-C filled with
various proportions of 5-20 µm glass beads.
The incorporation of glass beads into the hydrogel
matrix results in a significant increase in both
electrical conductivity and permittivity.
3.4.12 Glass Beads 150-200 µm
The 150-200 µM glass beads are too dense for the
viscosity of the PVA mixture, causing them to settle
at the bottom of the sample due to gravity. As a result,
the samples lack homogeneity, and their electrical
properties have not been measured.
3.4.13 Barium Titanate
Figure 18 illustrates the comparative electrical
properties of PVA-C filled with varying proportions
of barium titanate, compared to a pure PVA-C
sample.
Figure 18: Electrical properties of PVA-C filled with
various proportions of barium titanate.
The incorporation of barium titanate into the
hydrogel resulted in an increase in both electrical
conductivity and permittivity. The addition of 15%
barium titanate led to a significant increase in
permittivity, with a notable rise observed in the
frequency range 10
4
to 10
6
Hz compared to the 5%
barium titanate sample. The significant enhancement
in permittivity at higher concentrations highlights
barium titanate's suitability for applications requiring
high dielectric constants.
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
32

3.5 Comparison with Literature Data
A comparative graph of the conductivity and
permittivity of the samples is presented in Figure 19,
alongside literature data from living muscle and fat
tissue for reference.
Figure 19: Electrical properties of samples compared with
literature data for muscle and fat tissue.
The comparison with literature data confirms the
effectiveness of the fillers in replicating the electrical
properties of muscle and fat. For instance, NaCl and
lignin closely mimic the high conductivity and
permittivity of muscle tissue, while agar and glass
beads align more closely with the lower values typical
of fat tissue. The study highlights the importance of
selecting appropriate filler combinations to fine-tune
phantoms for specific tissues.
4 DISCUSSION
The influence of fillers on the electrical properties of
PVA-C hydrogel are summarised in Table 4.
Table 4: Summary of the principal effects of fillers on the
electrical properties of PVA-C hydrogel.
Increase in both conductivity
and permittivity
Agar, NaCl, lignin,
“Sommières” earth,
plaster, glass beads,
b
arium titanate
Decrease in both conductivity
and
p
ermittivit
y
1,2-propanediol
Decrease in conductivity
Corn flour,
glycerine
Change in conductivity and
increase in
p
ermittivit
y
Graphite
It is observed that certain fillers are more effective
in modifying the electrical properties of hydrogels.
Depending on the objectives of the developed
phantom, a combination of different fillers can be
used to efficiently mimic the electrical properties of
living tissues (see Figure 19). In previous works, the
authors developed a muscle phantom by
incorporating fillers such as agar, graphite, and NaCl
into a PVA-C matrix. This formulation was
specifically designed to mimic the electrical
properties of living muscle tissue while exhibiting
excellent mechanical properties. As a result, it
provides a reliable model for bioimpedance studies
(Bublex et al., 2024).
The measurements performed on all samples
demonstrated a plateau in permittivity at high
frequencies (1 MHz). This plateau correlates with the
relative permittivity of water (ε
r
= 80), which can be
attributed to the water-based composition of the
samples. This behaviour is consistent with the
expected properties of hydrogels and confirms the
significant influence of water content on their
electrical response.
PVA-C-based phantoms are predominantly
investigated for their mechanical properties.
However, it is essential to recognise that the
incorporation of any filler inevitably impacts the
hydrogel’s mechanical properties. For instance, while
1,2-propanediol effectively reduce conductivity,
concentrations exceeding 10% result in a notable
decline in mechanical strength, rendering the
hydrogel difficult to handle.
The study of PVA-C revealed shrinkage and
alterations in mechanical properties across
freeze-thaw cycles. Further investigations are needed
to assess these properties. Additionally, storage
remains a significant concern; plastic film is not a
viable option for maintaining electrical properties
over an extended period. Vacuum packing should be
investigated to prevent water loss.
5 CONCLUSIONS
This study comprehensively examined the impact of
various fillers on the electrical properties of PVA-C
hydrogels to enhance their suitability for
bioimpedance spectroscopy (BIS) applications.
Through systematic experimentation, the effects of
fillers on electrical conductivity and permittivity were
quantified and compared with the electrical properties
of living tissues such as muscle and fat.
The results underscore the potential of specific
fillers, such as NaCl and lignin, to closely mimic the
electrical properties of muscle, while other fillers, like
agar and glass beads, show promise for developing fat
phantoms.
Furthermore, the study revealed that while fillers
are essential for achieving the desired electrical
characteristics, their incorporation also affects the
mechanical properties of the hydrogel. This
highlights the importance of optimizing filler
Electrical Properties of Filled PVA-C for Bioelectrical Impedance Spectroscopy Phantoms
33

composition to balance both electrical and
mechanical performance. The observed correlation
between water content and permittivity emphasizes
the need for improved storage solutions, such as
vacuum packing, to maintain the long-term stability
of electrical properties.
In conclusion, the findings of this research
provide a robust foundation for developing advanced
PVA-C-based electrical phantoms, offering reliable
and stable alternatives for BIS studies and other
biomedical applications.
ACKNOWLEDGEMENTS
This work is part of the Symphonies project,
receiving funding from BPI France for the France
2030 program, supported by the French government.
REFERENCES
Adelnia, H., Ensandoost, R., Shebbrin Moonshi, S., Gavgani,
J. N., Vasafi, E. I., & Ta, H. T. (2022). Freeze/thawed
polyvinyl alcohol hydrogels : Present, past and future.
European Polymer Journal, 164, 110974.
A.Ivorra. (2002). Bioimpedance Monitoring for
physicians : An overview.
Anand, G., Lowe, A., & Al-Jumaily, A. (2019). Tissue
phantoms to mimic the dielectric properties of human
forearm section for multi-frequency bioimpedance
analysis at low frequencies. Materials Science and
Engineering: C, 96, 496‑508.
Belay, M. (2023). Review on Physicochemical Modification
of Biodegradable Plastic : Focus on Agar and
Polyvinyl Alcohol (PVA). Advances in Materials
Science and Engineering, 2023, 1‑11.
Bera, T. K. (2014). Bioelectrical Impedance Methods for
Noninvasive Health Monitoring : A Review. Journal of
Medical Engineering, 2014, 1‑28.
Bublex, A., Montalibet, A., Massot, B., & Gehin, C. (2024).
Eco-Friendly Bioimpedance Muscle Phantom : PVA-
Agar Hydrogel Mimicking Living Tissue at Low
Frequencies. 2024 46th Annual International
Conference of the IEEE Engineering in Medicine and
Biology Society (EMBC), Orlando, Florida.
Chen, S. J.-S., Hellier, P., Marchal, M., Gauvrit, J.-Y.,
Carpentier, R., Morandi, X., & Collins, D. L. (2012).
An anthropomorphic polyvinyl alcohol brain phantom
based on Colin27 for use in multimodal imaging.
Medical Physics, 39(1), 554‑561.
Duboeuf, F., Basarab, A., Liebgott, H., Brusseau, E.,
Delachartre, P., & Vray, D. (2009). Investigation of
PVA cryogel Young’s modulus stability with time,
controlled by a simple reliable technique. Medical
Physics, 36(2), 656‑661.
Dunne, E., McGinley, B., O’Halloran, M., & Porter, E.
(2018). A realistic pelvic phantom for electrical
impedance measurement. Physiological Measurement,
39(3), 034001.
Fromageau, J., Gennisson, J.-L., Schmitt, C., Maurice, R.
L., Mongrain, R., & Cloutier, G. (2007). Estimation of
polyvinyl alcohol cryogel mechanical properties with
four ultrasound elastography methods and comparison
with gold standard testings. IEEE Transactions on
Ultrasonics, Ferroelectrics and Frequency Control,
54(3), 498‑509.
Gabriel, C. (1996). Compilation of the Dielectric Properties
of Body Tissues at RF and Microwave Frequencies.:
Defense Technical Information Center.
Gautam, U. C., Pydi, Y. S., Selladurai, S., Das, C. J., Thittai,
A. K., Roy, S., & Datla, N. V. (2021). A Poly-vinyl
Alcohol (PVA)-based phantom and training tool for use
in simulated Transrectal Ultrasound (TRUS) guided
prostate needle biopsy procedures. Medical
Engineering & Physics, 96, 46‑52.
Getangama, N. N., de Bruyn, J. R., & Hutter, J. L. (2020).
Dielectric properties of PVA cryogels prepared by
freeze–thaw cycling. The Journal of Chemical Physics,
153(4), 044901.
Goyal, K., Borkholder, D. A., & Day, S. W. (2022). A
biomimetic skin phantom for characterizing wearable
electrodes in the low-frequency regime. Sensors and
Actuators A: Physical, 340, 113513.
Grimnes, S., & Martinsen, O. (2015). Bioimpedance and
bioelectricity basics. Academic Press.
Hess, A., Liu, J., & Pott, P. P. (2022). Analysis of Dielectric
Properties of Gelatin-based Tissue Phantoms. Current
Directions in Biomedical Engineering, 8(2), 340‑343.
Khaled, W., Neumann, T., Ermert, H., Reichling, S.,
Arnold, A., & Bruhns, O. T. (2007). P1C-1 Evaluation
of Material Parameters of PVA Phantoms for
Reconstructive Ultrasound Elastography. 2007 IEEE
Ultrasonics Symposium Proceedings, 1329‑1332.
Kobayashi, M., & Hyu, H. S. (2010). Development and
Evaluation of Polyvinyl Alcohol-Hydrogels as an
Artificial Atrticular Cartilage for Orthopedic Implants.
Materials, 3(4), 2753‑2771.
Mobashsher, A. T., & Abbosh, A. M. (2014). Three-
Dimensional Human Head Phantom With Realistic
Electrical Properties and Anatomy. IEEE Antennas and
Wireless Propagation Letters, 13, 1401‑1404.
Suga, R., Inoue, M., Saito, K., Takahashi, M., & Ito, K.
(2013). Development of multi-layered biological tissue-
equivalent phantom for HF band. IEICE
Communications Express, 2(12), 507‑511.
Wan, W., Bannerman, A. D., Yang, L., & Mak, H. (2014).
Poly(Vinyl Alcohol) Cryogels for Biomedical
Applications. In O. Okay (Éd.), Polymeric Cryogels
(Vol. 263, p. 283‑321). Springer International
Publishing.
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
34
