
Deep Learning for Frailty Classification Using Raw Inertial Sensor
Gait Data
Arslan Amjad
1a
, Agnieszka Szczęsna
1b
and Monika Błaszczyszyn
2c
1
Department of Computer Graphics, Vision and Digital Systems, Faculty of Automatic Control, Electronics and Computer
Science, Silesian University of Technology, Akademicka 16, 44-100 Gliwice, Poland
2
Department of Physical Education and Sport, Faculty of Physical Education and Physiotherapy,
Opole University of Technology, Prószkowska 76, 45-758 Opole, Poland
Keywords: Deep Learning, Frailty, Gait, Healthcare, IMU Sensor.
Abstract: The Frailty is a significant health issue in older adults that increases the risk of disability, decline in
physiologic reserve and function, hospitalization, and even death. The social and economic impact of frailty
increased due to the higher healthcare costs and the medical resources. The intervention of early frailty
detection can prevent its progression and delay the disability, ultimately improving the quality of life in the
elderly population. This study aims to propose a frailty classification system based on gait data collected from
an Inertial Measurement Unit (IMU) sensor with the utilization of the Deep Learning (DL) approach. The
individual’s frailty status is classified as robust, pre-frail, or frail. A publicly available dataset of 163
participants was utilized to analyze the raw gait signals and find the most effective DL for extracting gait
patterns for frailty classification. DeepConvLSTM model has shown effective performance on raw IMU gait
data with a balanced accuracy, precision, recall, and F1-score of 91%. The results show that the proposed
methodology successfully classifies the pre-frail individuals, which demonstrate its potential to enhance
frailty detection and intervention in clinical settings. This ultimately provides an improved healthcare system
and a quality of life in elderly populations.
1 INTRODUCTION
The number of elderly individuals is increasing
dramatically as the world's population grows
(Hernigou et al., 2024). World Health Organization
data show this demographic trend: among the 8.1
billion population, people aged 60 years and older
will become 1.4 billion by 2030 and 2.1 billion by
2050 (Sun et al., 2024; United Nation, 2024; World
Health, 2024). Frailty is one of the most common and
fatal disorders in the elderly population (Hakeem et
al., 2023). Physical frailty is a multidimensional
condition that is defined as a decline in physiological
reserves. This makes older persons more vulnerable
to stresses and increases the possibility of negative
health effects (Kojima et al., 2018). Considering its
consequences link to increased illness, disability, and
death, this raises a significant public health concern
a
https://orcid.org/0000-0002-6711-4382
b
https://orcid.org/0000-0002-4354-8258
c
https://orcid.org/0000-0002-1723-4001
(Pasieczna et al., 2023). To reduce the burden of
frailty on the healthcare system and enhance the
quality of life for the aging population, it is essential
to address it through early identification, precise
assessment, and effective management.
In order to reduce the risk of frailty among older
adults, it is essential to develop an objective
healthcare solution. Traditional clinical frailty
assessment methods are time-consuming and need
specialized equipment and experienced healthcare
personnel (Obbia et al., 2020). To solve this issue,
wearable technology and advanced Machine
Learning (ML) algorithms have emerged as a
potential solution (Fan et al., 2023; Minici et al.,
2022). These technologies provide continuous, real-
time remote monitoring, allowing for early
identification and classification of frailty stages.
Amjad, A., Szcze¸sna, A. and Błaszczyszyn, M.
Deep Learning for Frailty Classification Using Raw Inertial Sensor Gait Data.
DOI: 10.5220/0013127300003890
In Proceedings of the 17th International Conference on Agents and Artificial Intelligence (ICAART 2025) - Volume 3, pages 311-317
ISBN: 978-989-758-737-5; ISSN: 2184-433X
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
311
This study offers a smart stack of an Inertial
Measurement Unit (IMU) sensor and Deep Learning
(DL) technologies as a promising solution for frailty
classification. An IMU sensor was worn by each
participant to collect their raw IMU gait signals.
These signals were then pre-processed and converted
to the frequency domain in order to capture the
underlying patterns. Following this, a Deep Learning
(DL) algorithm was used to extract intrinsic gait
parameters for frailty classification into frail, pre-
frail, or robust stages.
This study has two main objectives: 1) analyzing
the raw IMU gait signals for frailty classification and
2) finding the most effective DL algorithm for frailty
classification using raw IMU gait data. The ultimate
goal of this research is to develop an early frailty
detection system that will detect the frailty stage
timely and prevent the frailty from progressing in
older adults. Early detection of frailty allows
individuals to seek medical advice and take
appropriate measures, which lowers the total cost of
healthcare for society. This study proposed an
intelligent frailty assessment system that will be
expanded into a real-time application, increasing its
use and impact in clinical settings.
The paper is organized as follows: Section 2
outlines the relevant literature work; Section 3
explains the research methodology, including the
dataset and the application of the DL algorithm for
frailty classification; Section 4 provides the results
with discussion; and finally, Section 5 concludes the
paper and suggests directions for future work.
2 LITERATURE REVIEW
For clinical gait analysis, the most commonly used
DL algorithms in the previous studies are:
Convolutional Neural Networks (CNN), Recurrent
Neural Networks (RNN), and Auto-Encoders (AE).
These algorithms became popular due to their ability
to analyze complex time-series data and
automatically extract features from raw IMU sensor
data, making them appropriate for applications such
as gait analysis.
The studies (García et al., 2022; Kou et al., 2024),
and (Li et al., 2024) classify the fall risks using CNN-
LSTM and CNN-BiLSTM algorithms. The study
(García et al., 2022) used a 3-D IMU device placed
on a wrist and leveraged the CNN-LSTM model to
achieve an accuracy of 93.60%. Whereas the study
(Kou et al., 2024) achieved an F1-score of 95.18%
and the study (Li et al., 2024) obtained an accuracy of
98.40%.
The study (Kamran et al., 2021) explored the
utility of 1-D CNN for automatically assessing
balance using data from a single IMU worn on the
lower back. They also compared the results with
handcrafted features. DL provided significant results
with an AUROC of 0.81. Another study (Hauth et al.,
2021) utilized three IMU sensors while performing
daily activities. The BiLSTM model outperformed
with an AUROC score of 0.87.
Another approach used in the previous studies
(Butt et al., 2020; San-Segundo et al., 2019; Sánchez-
DelaCruz et al., 2019) is the transformation of raw
IMU signals into images. This structured format of
input leverages the DL algorithms to extract more
enhanced features. The overview of previous studies
that utilized raw IMU gait signals with DL algorithms
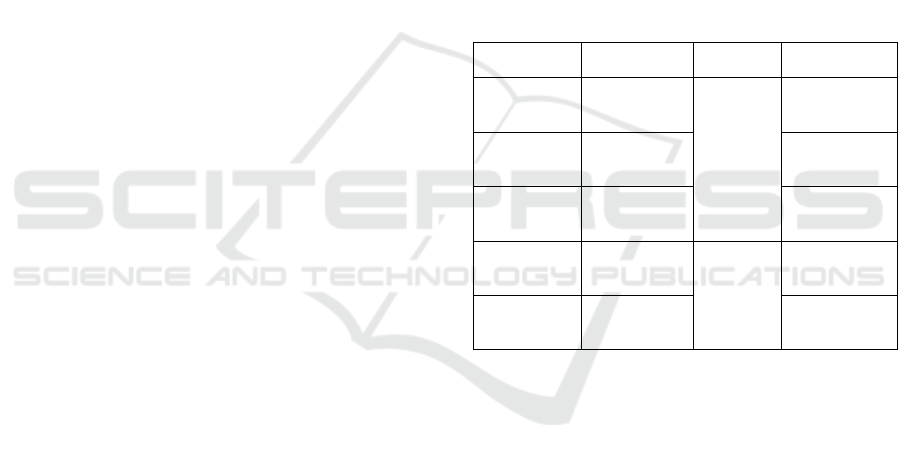
is shown in Table 1.
Table 1: Overview of relevant studies that utilized raw IMU
gait data for frailty analysis.
Ref. Algorithms Task Outcomes
(García et
al., 2022)
CNN-LSTM
Falls risks
Accuracy =
93.60%
(Kou et al.,
2024)
CNN-LSTM
F1-score =
95.18%
(Li et al.,
2024)
CNN-
BiLSTM
Accuracy =
98.40%.
(Kamran et
al., 2021)
1-D CNN
Assess
balance
AUROC =
0.81
(Hauth et al.,
2021)
BiLSTM
AUROC =
0.87
3 METHOD
The research methodology consists of key steps,
which include: 1) the analysis of raw IMU gait data
and assigning the frailty status label to each
participant; 2) pre-processing of the raw IMU data
and data formatting using sliding window technique
and wavelet transformation; and 3) implementation of
the DL algorithm to classify the frailty into frail, pre-
frail, or robust stages. The research methodology is
illustrated in Figure 1.
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
312
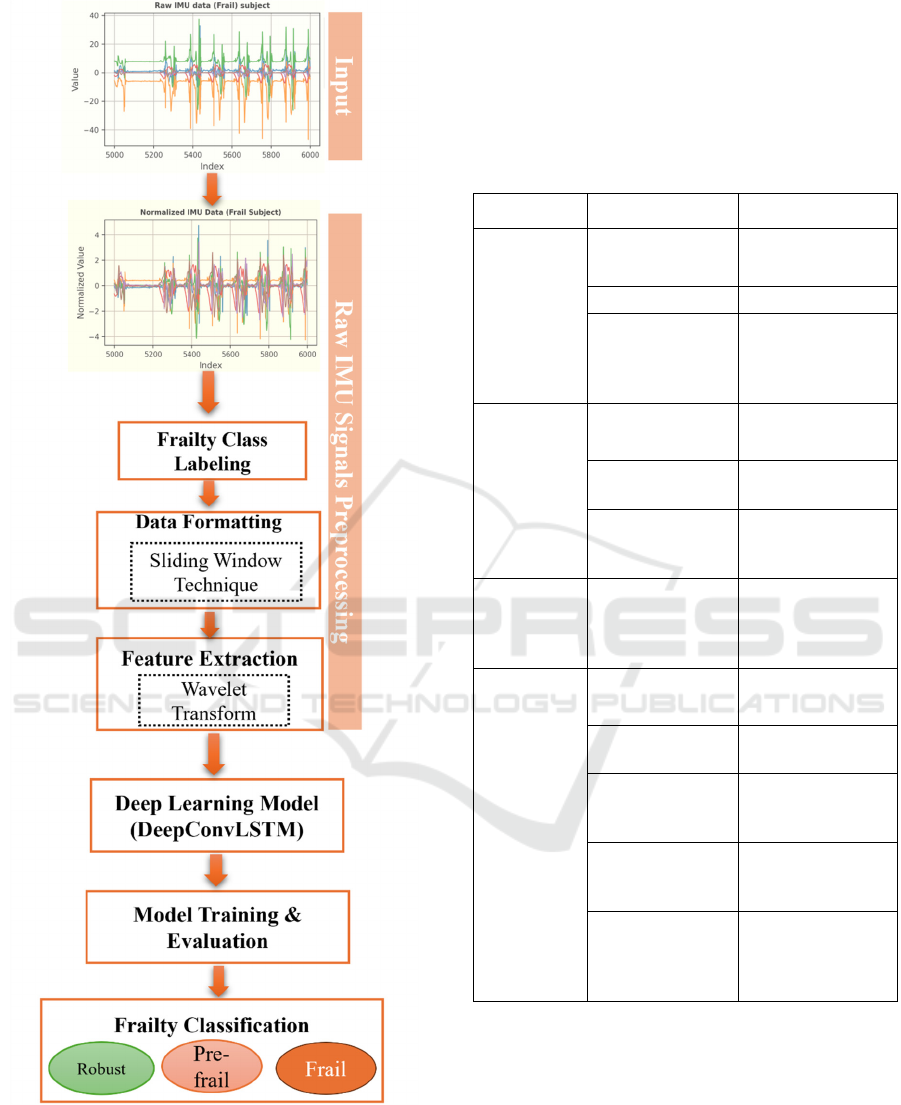
Figure 1: Research methodology.
3.1 Dataset
The GSTRIDE (García-de-Villa et al., 2023) dataset
was utilized in this study. It’s a publicly available
dataset that consists of 163 (45 mean and 118 women)
older adults. Their ages range from 70 to 98 years and
an average weight of 64.2 to 77.3 kg. The list of
parameters available in the GSTRIDE database is
shown in Table 2.
Table 2: List of Parameters available in GSTRIDE
database.
Category Parameter Description
Socio
Demo-
graphic
Age
Average age of the
subjects (years)
Gender Male/Female
Living
Environment
Type of living
environment (e.g.,
Home, Assisted
Living)
Anatomical
Weight
Average weight of
the subjects (kg)
Height
Average height of
the subjects (cm)
Body Mass Index
(BMI)
Average BMI of
the subjects
(kg/m²)
Cognitive
Global
Deterioration
Scale (GDS)
Index
Average GDS
index of the
subjects (scale 1-
7)
Functional
4-metre Gait
Speed Test
Average time
taken (seconds)
Hand Grip
Strength
Average hand grip
strength (kg)
Timed Up and
Go (TUG)
Average time
taken for TUG test
(seconds)
Short Physical
Performance
Battery (SPPB)
Average SPPB
score
Short Falls
Efficacy Scale
International
(FES-I)
Average FES-I
score
For raw IMU signal acquisition, two IMU sensors
(CSIC and Gaitup) were used, with only one sensor
worn on the foot of each participant during 15
minutes of gait (García-de-Villa et al., 2023). The
reason for using two different sensors with varying
frequencies was to assess the effect of varying
configurations of sensors on the spatio-temporal
estimation. The authors reported a minimal effect of
these varying configurations of sensors on spatio-
temporal estimation, although there was a slight
Deep Learning for Frailty Classification Using Raw Inertial Sensor Gait Data
313
variation in the accuracy of estimation (García-
Villamil et al., 2021).
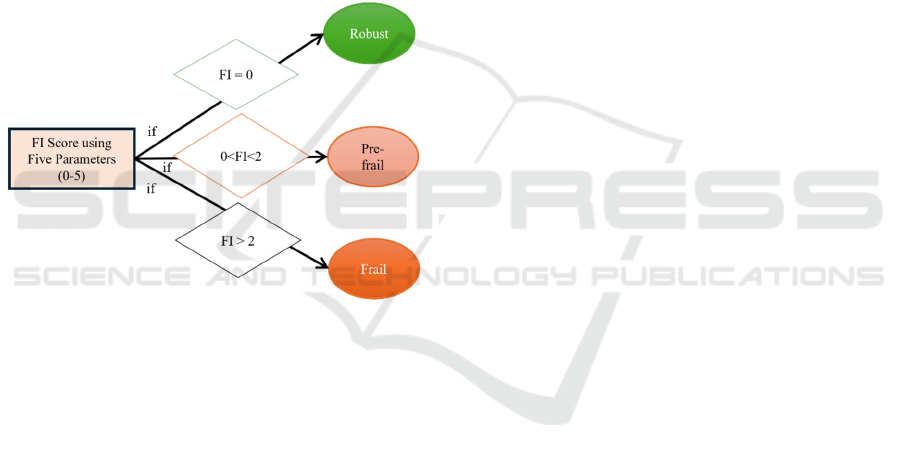
3.2 Class Labelling of Participants
The Standardized Fried's phenotype (Fried et al.,
2001) test was adopted to label the frailty status of
each participant. In this test, the Frailty Index (FI)
score is calculated using the five parameters. Each
parameter’s score is assigned a score of 0 or 1. The
final FI score is calculated by summing the score of
all parameters (ranges from 0 to 5) (García-de-Villa
et al., 2023). The class label is assigned to each
participant based on FI score. If the FI score is 0, then
the frailty class label is “Robust”. If the FI score is 1
or 2, then the frailty class label is “Pre-frail”,
otherwise the frailty label is “Frail”, as shown in
Figure 2.
Figure 2: Criteria for assigning the frailty label to each
participant.
3.3 Data Pre-Processing
After raw IMU signal data acquisition and labelling,
pre-processing became a critical step for further
analysis. In this stage, outliers were removed from
raw IMU signals, and the signals were normalized
using the “StandardScaler” function. The data was
then segmented into smaller chunks using the
“Sliding Window” technique (Jaén-Vargas et al.,
2022), which allows the extraction of spatio-temporal
features from the time-series IMU signals. The
window size set in this study was 200, with a stride of
50. Next, a wavelet transformation is applied to each
segment to capture both time and frequency domain
features. The “pywt.wavedec” function was used for
Daubechies wavelet of order 1 (“db1”). This
frequency transformation is suitable for raw IMU
signals to capture the sharp changes in the signals
(Chakraborty et al., 2020; Kuduz et al., 2023; Michau
et al., 2022).
At the end, the segmented windows were divided
into 75% training set. The remaining segments were
divided into equal sets for validation and testing. The
code is implemented in Python, version 3.5, using
Spyder as the development environment.
3.4 Deep Learning (DL) Algorithm
Architecture
The input data is ready after pre-processing steps. It
can be input to a DL algorithm for frailty
classification. The DeepConvLSTM (Ordóñez et al.,
2016) algorithm was utilized for this purpose. The
model consists of a convolutional layer with Long
Short-Term Memory (LSTM) layers to capture both
the spatial and temporal (spatio-temporal) features in
raw IMU signals, which makes it an effective
algorithm for frailty classification.
Two different DeepConvLSTM models were
created and trained on the training dataset. The best
model was selected based on high accuracy and
minimum losses on both training and validation
datasets. After finalizing the training process, the best
model’s hyperparameters were saved and tested on
the test dataset. The models were created using an
open-source Python’s library, McFly (van Kuppevelt
et al., 2020).
In this study, the architecture of the best
DeepConvLSTM model was initialized with a
“BatchNormalization” layer followed by a reshape
operation. Following this, a 2D convolutional layer
with 54 filters was applied, followed by normalized
and activated layers. After convolution, the resulting
tensor is reshaped to prepare it for recurrent
processing. Mathematically, the convolutional
process is defined as: for an input 𝒙∈ℝ
(where 𝑇 is time, 𝑊 is width, H is height, and 𝐶 is
channels), the convolutional process is depicted in
(1).
𝒙
𝐶𝑜𝑛𝑣
(
𝒙
(1)
Conv
2D
represents the 2D convolutional operation
in the model; the overall equation of the
convolutional process with filters F is:
𝒙
[
𝑡,𝑤,ℎ,𝑐
]
𝒙
[
𝑡,𝑤 𝑖,ℎ 𝑗,𝑘
]
∙𝑭
[
𝑖,𝑗,𝑘,𝑐
]
𝑏
[
𝑐
]
(2)
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
314
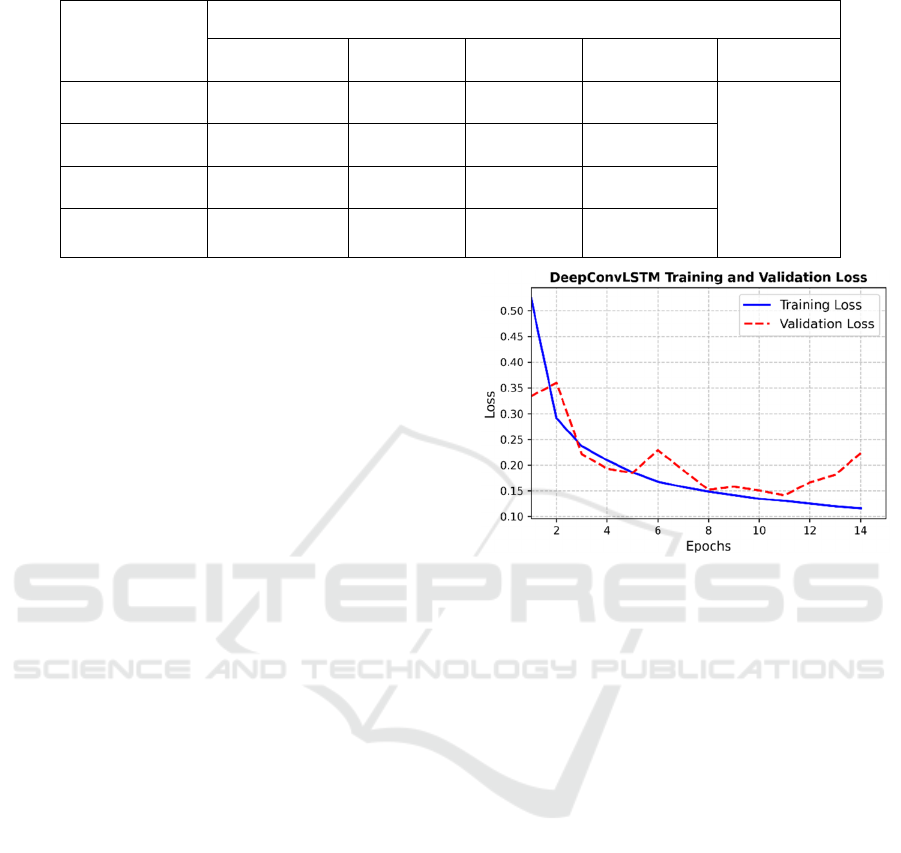
Table 3: DeepConvLSTM model performance on test dataset.
DeepConvLSTM performance (Test Set)
Precision Recall F1-score Support Accuracy
Robust 0.94 0.93 0.93 39063
0.91
Pre-frail 0.92 0.90 0.91 38269
Frail 0.67 0.85 0.75 6541
Weighted Avg. 0.91 0.91 0.91 83873
In (2), the F
W
and F
H
are filter weight and height,
respectively. Whereas the F
C
represents input
channels and bias is represented with b[c].
The convolutional operation results were input
into the stack of LSTM layers. Four LSTM layers
were used in the model with 29, 95, 94, and 46 units,
respectively. After that, the dropout layer was added
to prevent the model overfitting. The model
concluded with the “TimeDistributed” layer with 3
units followed by “softmax” activation for the
classification task. The final description of a model
can be represented as:
Conv(54)−BN−LSTM(29)−LSTM(95)−LSTM(94)−
LSTM(46)−D−TD(3)−S.
4 RESULTS AND DISCUSSION
The evaluation criteria in this study consist of two
phases. In the first phase, the two created
DeepConvLSTM models were evaluated in the
training process based on their best training and
validation accuracy and minimum losses,
respectively. The second phase evaluated the best
selected DeepConvLSTM model on the testing
dataset using metrics such as accuracy, precision,
recall, and F1-score (Wasikowski et al., 2010).
In the training phase, the hyperparameters of
DeepConvLSTM models were fine-tuned using 25
epochs with a batch size of 64 and a stopping patience
of 3. The hyperparameters of the best model were
reported as a learning rate of 0.0268, a regularization
rate of 0.0004, and convolutional filters and LSTM
dimensions as 54 and (29, 95, 94, 46), respectively.
The model achieved training and validation accuracy
of 95.18% and 94.14% with corresponding losses of
0.1163 and 0.1415, respectively, as shown in Figure
3.
Figure 3: Training and validation losses of
DeepConvLSTM in training phase.
The DeepConvLSTM model’s performance on
test data is reported in Table 3. It shows that the
DeepConvLSTM achieved an accuracy of 91% on
test data. Whereas the overall frailty stage-wise
confusion matrix is depicted in Figure 4.
The results in Table 3 suggested that the model
effectively classified the pre-frail and robust
individuals but reported low precision in the case of
the frail class. This is due to the highly class-
imbalanced, as the frail class has fewer instances,
which may overlap the features with other classes.
This problem can be overcome by adding more frail
instances
utilizing data augmentation techniques to
synthetically increase the number of frail samples or
applying class-weighted loss functions and
oversampling methods like Synthetic Minority Over-
Sampling Technique (SMOTE) (Hosseini et al.,
2024) during model training. However, this study
used raw IMU sensor signals as input, keeping the
original data with its spatio-temporal properties.
This ensures the effectiveness of the DL model for
frailty classification.
Deep Learning for Frailty Classification Using Raw Inertial Sensor Gait Data
315
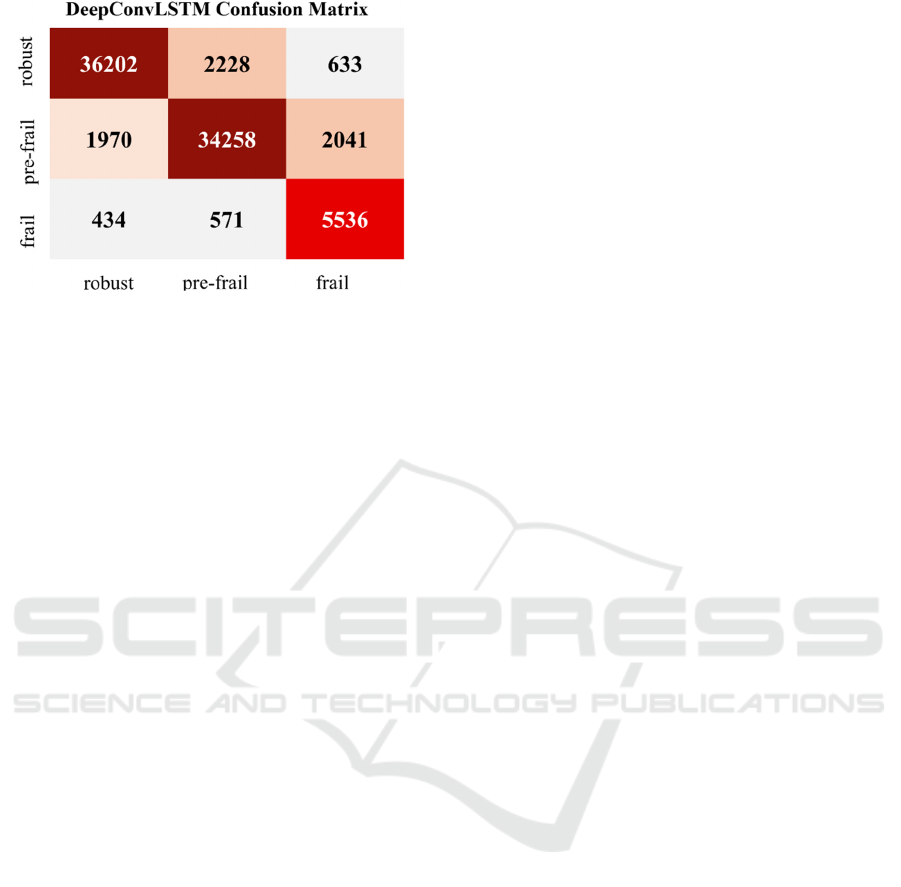
Figure 4: Confusion matrix represents the performance of
DeepConvLSTM model.
Accurate classification is a major concern in
clinical settings, as it directly influences patient care
and intervention strategies. Overall, the DL model
performed effectively; better performance on frail
individuals will enhance clinical decision-making
and personalized care.
The proposed approach may have some
challenges when applied in the clinical healthcare
system. These challenges include maintaining the
privacy of data, facilitating real-time processing with
wearable IMU sensors, and smoothly integrating into
clinical workflows. Furthermore, it is crucial to
validate the system in real-world settings and achieve
generalizability across a variety of demographics.
The method's potential for the early frailty detection
task is highlighted by its adaptability to diverse
operational circumstances and scalability to multiple
sensor configurations.
5 CONCLUSION
In the world of a growing elderly population, frailty
is an important factor in the adverse health outcomes
among elders. Early and accurate detection of frailty
can significantly enhance clinical decision-making,
leading to better patient care and management.
This study proposed a sensor-based approach with
a DL algorithm to classify the frailty into robust, pre-
frail, or frail stages. The DeepConvLSTM model
demonstrated its effectiveness in frailty classification
using raw IMU sensor data, with an overall accuracy
of 91%. The performance of the DL model has shown
it's potential to develop a frailty classification system
that depicts the real-world clinical scenario.
The limitations of this research work are: 1) The
small size of the dataset limited the performance of
the DL model; and 2) A diverse dataset and the
selection of features may also affect the DL
performance. Future studies should focus on the
diverse types of sensors for the data collection. There
is also a need to develop a real-time application to
monitor the frailty status in a real-world clinical
environment.
REFERENCES
Butt, A. H., Cavallo, F., Maremmani, C., & Rovini, E.
(2020). Biomechanical parameters assessment for the
classification of Parkinson Disease using Bidirectional
Long Short-Term Memory. 2020 42nd Annual
International Conference of the IEEE Engineering in
Medicine & Biology Society (EMBC), 5761–5764. doi:
10.1109/EMBC44109.2020.9176051
Chakraborty, J., & Nandy, A. (2020). Discrete wavelet
transform based data representation in deep neural
network for gait abnormality detection. Biomedical
Signal Processing and Control, 62, 102076. doi:
10.1016/j.bspc.2020.102076
Fan, S., Ye, J., Xu, Q., Peng, R., Hu, B., Pei, Z., Yang, Z.,
& Xu, F. (2023). Digital health technology combining
wearable gait sensors and machine learning improve the
accuracy in prediction of frailty. Frontiers in Public
Health, 11. doi: 10.3389/fpubh.2023.1169083
Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B.,
Hirsch, C., Gottdiener, J., Seeman, T., Tracy, R., Kop,
W. J., Burke, G., & McBurnie, M. A. (2001). Frailty in
Older Adults: Evidence for a Phenotype. The Journals
of Gerontology Series A: Biological Sciences and
Medical Sciences, 56(3), M146–M157. doi:
10.1093/gerona/56.3.M146
García-de-Villa, S., Neira, G. G.-V., Álvarez, M. N.,
Huertas-Hoyas, E., Ruiz, L. R., Del-Ama, A. J.,
Sánchez, M. C. R., & Jiménez, A. R. (2023). A database
with frailty, functional and inertial gait metrics for the
research of fall causes in older adults. Scientific Data,
10(1), 566. doi: 10.1038/s41597-023-02428-0
García-Villamil, G., Ruiz, L., Jiménez, A. R., Granja, F. S.,
& Rodríguez-Sánchez, M. C. (2021). Influence of
IMU’s Measurement Noise on the Accuracy of Stride-
Length Estimation for Gait Analysis. IPIN-WiP.
Retrieved from https://api.semanticscholar.org/
CorpusID:247314771
García, E., Villar, M., Fáñez, M., Villar, J. R., de la Cal, E.,
& Cho, S.-B. (2022). Towards effective detection of
elderly falls with CNN-LSTM neural networks.
Neurocomputing, 500, 231–240. doi:
10.1016/j.neucom.2021.06.102
Hakeem, F. F., Maharani, A., Todd, C., & O’Neill, T. W.
(2023). Development, validation and performance of
laboratory frailty indices: A scoping review. Archives
of Gerontology and Geriatrics, 111, 104995. doi:
10.1016/j.archger.2023.104995
Hauth, J., Jabri, S., Kamran, F., Feleke, E. W., Nigusie, K.,
Ojeda, L. V., Handelzalts, S., Nyquist, L., Alexander,
N. B., Huan, X., Wiens, J., & Sienko, K. H. (2021).
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
316

Automated Loss-of-Balance Event Identification in
Older Adults at Risk of Falls during Real-World
Walking Using Wearable Inertial Measurement Units.
Sensors, 21(14), 4661. doi: 10.3390/s21144661
Hernigou, P., Bumbasirevic, M., Pecina, M., & Scarlat, M.
M. (2024). Eight billion people, sixteen billion hip
joints today: are future orthopedists prepared to treat a
world of ultra-old patients and centenarians in 2050?
International Orthopaedics, 48(8), 1939–1944. doi:
10.1007/s00264-024-06245-x
Hosseini, I., Rojas, R. F., & Ghahramani, M. (2024). Fall
Risk Assessment Using Single IMU. 2024 IEEE
International Symposium on Medical Measurements
and Applications (MeMeA), 1–6. doi:
10.1109/MeMeA60663.2024.10596880
Jaén-Vargas, M., Reyes Leiva, K. M., Fernandes, F.,
Barroso Gonçalves, S., Tavares Silva, M., Lopes, D. S.,
& Serrano Olmedo, J. J. (2022). Effects of sliding
window variation in the performance of acceleration-
based human activity recognition using deep learning
models. PeerJ Computer Science, 8, e1052. doi:
10.7717/peerj-cs.1052
Kamran, F., Harrold, K., Zwier, J., Carender, W., Bao, T.,
Sienko, K. H., & Wiens, J. (2021). Automatically
evaluating balance using machine learning and data
from a single inertial measurement unit. Journal of
NeuroEngineering and Rehabilitation, 18(1), 114. doi:
10.1186/s12984-021-00894-4
Kojima, G., Iliffe, S., & Walters, K. (2018). Frailty index
as a predictor of mortality: a systematic review and
meta-analysis. Age and Ageing, 47(2), 193–200. doi:
10.1093/ageing/afx162
Kou, J., Xu, X., Ni, X., Ma, S., & Guo, L. (2024). Fall-risk
assessment of aged workers using wearable inertial
measurement units based on machine learning. Safety
Science, 176, 106551. doi: 10.1016/j.ssci.2024.106551
Kuduz, H., & Kaçar, F. (2023). A deep learning approach
for human gait recognition from time-frequency
analysis images of inertial measurement unit signal.
International Journal of Applied Methods in
Electronics and Computers. doi: 10.58190/
ijamec.2023.44
Li, C., Cai, Y., Li, Y., & Zhang, P. (2024). Fusion of Dual
Sensor Features for Fall Risk Assessment with
Improved Attention Mechanism. Traitement Du Signal,
41(1), 73–83. doi: 10.18280/ts.410106
Michau, G., Frusque, G., & Fink, O. (2022). Fully learnable
deep wavelet transform for unsupervised monitoring of
high-frequency time series. Proceedings of the National
Academy of Sciences, 119(8). doi: 10.1073/
pnas.2106598119
Minici, D., Cola, G., Giordano, A., Antoci, S., Girardi, E.,
Bari, M. Di, & Avvenuti, M. (2022). Towards
Automated Assessment of Frailty Status Using a Wrist-
Worn Device. IEEE Journal of Biomedical and Health
Informatics, 26
(3), 1013–1022. doi: 10.1109/JBHI.
2021.3100979
Obbia, P., Graham, C., Duffy, F. J. R., & Gobbens, R. J. J.
(2020). Preventing frailty in older people: An
exploration of primary care professionals’ experiences.
International Journal of Older People Nursing, 15(2).
doi: 10.1111/opn.12297
Ordóñez, F., & Roggen, D. (2016). Deep Convolutional and
LSTM Recurrent Neural Networks for Multimodal
Wearable Activity Recognition. Sensors, 16(1), 115.
doi: 10.3390/s16010115
Pasieczna, A. H., Szczepanowski, R., Sobecki, J.,
Katarzyniak, R., Uchmanowicz, I., Gobbens, R. J. J.,
Kahsin, A., & Dixit, A. (2023). Importance analysis of
psychosociological variables in frailty syndrome in
heart failure patients using machine learning approach.
Scientific Reports, 13(1), 7782. doi: 10.1038/s41598-
023-35037-3
San-Segundo, R., Navarro-Hellín, H., Torres-Sánchez, R.,
Hodgins, J., & De la Torre, F. (2019). Increasing
Robustness in the Detection of Freezing of Gait in
Parkinson’s Disease. Electronics, 8(2), 119. doi:
10.3390/electronics8020119
Sánchez-DelaCruz, E., Weber, R., Biswal, R. R., Mejía, J.,
Hernández-Chan, G., & Gómez-Pozos, H. (2019). Gait
Biomarkers Classification by Combining Assembled
Algorithms and Deep Learning: Results of a Local
Study. Computational and Mathematical Methods in
Medicine, 2019, 1–14. doi: 10.1155/2019/3515268
Sun, Q., Xia, X., & He, F. (2024). Longitudinal association
between Body mass index (BMI), BMI trajectories and
the risk of frailty among older adults: A systematic
review and meta-analysis of prospective cohort studies.
Archives of Gerontology and Geriatrics, 124, 105467.
doi: 10.1016/j.archger.2024.105467
United Nation. (2024). World Population Prospects 2024.
Retrieved from https://population.un.org/wpp/
van Kuppevelt, D., Meijer, C., Huber, F., van der Ploeg, A.,
Georgievska, S., & van Hees, V. T. (2020). Mcfly:
Automated deep learning on time series. SoftwareX, 12,
100548. doi: 10.1016/j.softx.2020.100548
Wasikowski, M., & Chen, X. (2010). Combating the Small
Sample Class Imbalance Problem Using Feature
Selection. IEEE Transactions on Knowledge and Data
Engineering, 22(10), 1388–1400. doi: 10.1109/
TKDE.2009.187
World Health, O. (2024). Ageing. Retrieved from
https://www.who.int/health-topics/ageing#tab=tab_1.
Deep Learning for Frailty Classification Using Raw Inertial Sensor Gait Data
317
