
Advancing Chloride Ion Detection in Edible Oils: Enhanced
Sensitivity with NCQD/Ag Nanotriangles via Localized Surface
Plasmon Resonance
Muhammad Qayyum Othman
1
, Mohd Hafiz Abu Bakar
2,*
, Nur Hidayah Azeman
3
,
Nadhratun Naiim Mobarak
4
and Ahmad Ashrif A. Bakar
1,4,*
1
Photonics Technology Laboratory, Department of Electrical, Electronic and Systems Engineering, Faculty of Engineering
and Built Environment, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor
2
Institute of Power Engineering, Universiti Tenaga Nasional, 43000 Kajang, Selangor, Malaysia
3
Department of Chemical Sciences, Faculty of Science and Technology,
Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor
4
Institute of Microengineering and Nanoelectronics, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
Keywords: Carbon Quantum Dots, Localized Surface Plasmon Resonance, Silver Nanotriangle, Edible Oil, Chloride Ion.
Abstract: Chloride ion detection in edible oil is crucial for food safety and preventing harmful compounds like 3-MCPD
during refining. This study presents a novel method utilizing Nitrogen-Doped Carbon Quantum Dots
(NCQDs) combined with Silver Nanotriangles (AgNTs) through Localized Surface Plasmon Resonance
(LSPR) for chloride ion detection. The chemical properties of AgNT-NCQD enhance sensor performance by
improving stability and biocompatibility while providing new binding sites for chloride ions. LSPR allows
precise monitoring of the interaction between AgNT-NCQD and chloride ions, resulting in a distinct LSPR
peak for accurate detection. The synergy between surface plasmon resonance and NCQDs increases
sensitivity, with significant LSPR peak shifts upon chloride exposure. This technology offers a wider dynamic
range and lower detection limits, demonstrating excellent selectivity for chloride ions in edible oil. The
enhanced properties of NCQDs make this sensing platform vital for food quality assurance and consumer
health protection.
1 INTRODUCTION
The discovery of various contaminants in edible oils
that pose health risks has raised significant concerns
about their quality and safety. Contaminants like 3-
monochloropropane- 1,2-diol (3-MCPD) esters are
particularly concerning due to their potential
carcinogenicity (Jong-Sun et al., 2020). 3-MCPD are
byproducts formed as impurities during high-
temperature oil refining. 3- MCPD was classified as
a possible human carcinogen (Group 2B) by The
International Agency for Research on Cancer (IARC)
(Panel & Chain, 2016). These compounds are
produced during the deodorization process of oils and
have been linked to tumor development in animal
studies. According to EFSA (2016), palm oil contains
significantly higher levels of 3- MCPD than regular
fat margarine (Panel & Chain, 2016). 3-MCPD has
been known to form as a contaminant in processed
foods, including refined oils, since the 1980s (Cheng
et al., 2017). The presence of 3-MCPD has raised
global safety concerns especially in refined edible
oils. The synthesis of 3-MCPD esters is primarily
influenced by chloride, acylglycerols, pH,
temperature, and time (Kuntom et al., 2006).
Frequency depends on the nanoparticles’ material,
shape, and surrounding environment (Bakar et al.,
2022) (Abdullah et al., 2018). Moreover, gold and
silver are popular choices for these nanoparticles,
which can come in various shapes. Gold
nanoparticles (AuNPs) are favored for their stability
and biocompatibility. However, triangular silver
nanotriangles (AgNTs), with their sharp edges, are
particularly useful for enhancing electric fields in
surface-based spectroscopic techniques (Zannotti et
al., 2020)
that offer greater sensitivity. Yet, to
74
Othman, M. Q., Bakar, M. H. A., Azeman, N. H., Mobarak, N. N. and Bakar, A. A. A.
Advancing Chloride Ion Detection in Edible Oils: Enhanced Sensitivity with NCQD/Ag Nanotriangles via Localized Surface Plasmon Resonance.
DOI: 10.5220/0013131100003902
In Proceedings of the 13th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2025), pages 74-78
ISBN: 978-989-758-736-8; ISSN: 2184-4364
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
selectively detect low concentrations of target
molecules, these nanoparticles often require
additional modifications with specific materials
(Azeman et al., 2020) (Rahman et al., 2019) (Abu
Bakar et al., 2020).
Carbon quantum dots (CQDs) are tiny (less than
10 nm) fluorescent particles made of carbon with
unique properties. Their core is carbon, but their
surface has elements such as oxygen, hydrogen, and
nitrogen. CQDs can be improve by attaching specific
chemical groups (amino, hydroxyl, carboxyl) to their
surface, making them more reactive and water-
soluble (Nazri et al., 2022). Different materials such
as chitosan or branched polyethyleneimine can be
used for this. By attaching certain groups like
polyamines, CQDs can be made to selectively bind to
specific ions (Yoo et al., 2019). For instance, Nazri et
al. in 2022, used CQDs with amino groups and silver
nanoparticles to detect chlorophyll in water. The
author’s study showed that improved CQDs
(NCQDs) were much better at detecting chlorophyll
(Nazri, 2022).
In this study, we evaluate the potential of
functionalized carbon quantum dots (CQDs) for
chloride ion detection using a localized surface
plasmon resonance (LSPR)-based optical sensor.
Amino-functionalized CQDs (NCQDs) were
synthesized via a two-step hydrothermal method
employing polyethyleneimine as the amino group
precursor. The composite film comprised of these
functionalized CQDs and triangular silver
nanotriangles (AgNTs) served to enhance the
sensitivity of the LSPR sensor. The introduction of
amine groups on the CQD surface facilitated
improved chloride ion interaction through
electrostatic interactions. The performance of the
sensor was assessed by monitoring the wavelength
shift of the LSPR spectrum across varying chloride
ion concentrations. This analysis aimed to establish
the sensor's linearity, range, sensitivity, and detection
limit
.
2 EXPERIMENTAL SECTION
2.1 Materials
Citric acid, polyethyleneimine, silver nitrate, sulfuric
acid (98%), hydrogen peroxide (H
2
O
2
), trisodium
citrate, sodium borohydride (NaBH
4
), 3-
aminopropyltrimethoxysilane (97%) (APTES),
polyvinyl alcohol, ethanol, and acetone were
purchased from Sigma Aldrich. Ethylenediamine,
heavy metals, iso-propyl, and ammonia solution
(30%) were purchased from R&M Chemicals, and
edible oil was purchased from the local market
.
2.2 Experimental Setup
Building on prior research, this work utilizes
triangular silver nanoparticles (AgNT) synthesized
via a room- temperature chemical reduction process.
The synthesis utilizes readily available chemicals
such as silver nitrate, trisodium citrate, sodium
borohydride, and water. The composite sensing
material AgNT-NCQD morphology was
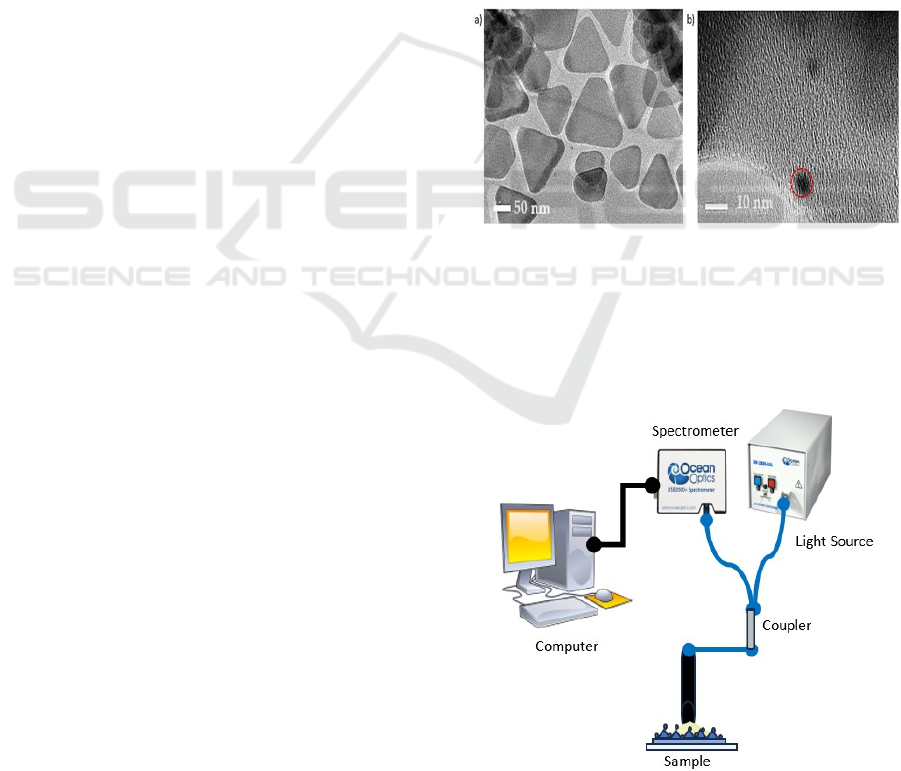
characterized by HRTEM and FESEM. Figure 1(a)
shows triangular-shaped nanoparticles with a scale
bar for 50nm. Conversely, Figure 1(b) obtained using
FESEM demonstrates that the NCQDs possess a
spherical morphology and are smaller than 10
nanometers
.
Figure 1: Surface morphology using HRTEM and FESEM
showing the structure of a) AgNT b) NCQD.
Experimental setup comprised an HR4000CG-
UV-NIR spectrometer from Ocean Optics interfaced
with a reflection probe boasting a numerical aperture
of 0.22. This configuration facilitated the
Figure 2: Detection of chloride ion using localized surface
plasmon resonance (LSPR) setup.
Advancing Chloride Ion Detection in Edible Oils: Enhanced Sensitivity with NCQD/Ag Nanotriangles via Localized Surface Plasmon
Resonance
75
measurement of the reflectivity spectra of both
AgNTs and AgNT-NCQDs in edible oil containing
10 ppm chloride ions. The measurements
encompassed a wavelength range of 350-850 nm and
were swiftly captured following spectra acquisition.
To ensure optimal signal acquisition, the probe was
meticulously positioned above the sample as
illustrated in Figure 2.
3 RESULT AND DISCUSSION
The study evaluated the fabricated AgNT-NCQD film
sensor's ability to quantitatively detect chloride ions
in edible oil by compared its efficiency and sensitivity
to AgNT alone, using edible oil as the baseline for all
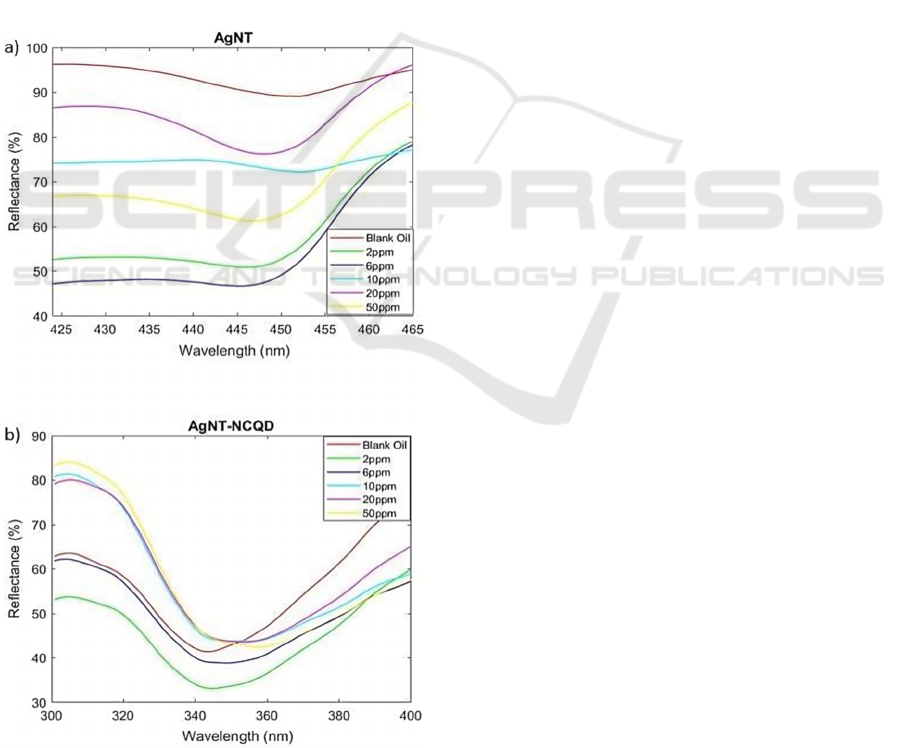
sensor materials. Figure 3(a) shows when AgNTs
were exposed to chloride ion, the wavelength value
was shifted from baseline (450 nm) to 448.37, 447.06,
Figure 3: The reflectance spectra of the LSPR sensor in the
presence of chloride ion with different concentrations
ranging from 2-50 ppm of (a) AgNT, (b) AgNT-NCQD.
453.09, 448.11, and 447.32 nm for 2, 6, 10, 20, and
50 ppm of chloride ion concentrations, respectively.
The wavelength shift (Δλ) values change to 2.10,
3.41, 2.62, 2.36, and 3.15 nm. In contrast, Figure 3(b)
shows the response of AgNT-NCQD to different
chloride concentrations. Here, we observed a distinct
trend: a narrow peak with increasing intensity as the
chloride concentration rises
.
Figure 3(b) reveals a critical observation as the
concentration of chloride ions in edible oil increases,
the reflectance peak associated with AgNT-NCQD
film undergoes a noticeable shift. At the outset, with
edible oil as the baseline, the peak is measured at
343.45 nm and exhibits minimal reflectance.
Interestingly, a positive correlation between the
concentration of chloride ions and the peak's spectral
position were observed. In simpler terms, the higher
the chloride ion concentration, the greater the shift
towards higher wavelengths observed in the
reflectance peak. Thus, the measured Δλ values for 2,
6, 10, 20, and 50 ppm of chloride ion are 0.79, 5.30,
7.68, 11.92, and 12.98 nm, respectively. The observed
wavelength shift can conclude that it is through
plausible electrostatic interaction due to positively-
charged NCQD and negatively-charged Cl.
Traditionally, oppositely charged molecules
experience stronger attraction due to electrostatic
forces, leading to faster diffusion and interaction. The
reversibility of the AgNT-NCQD sensor is supported
by the non-covalent nature of electrostatic interactions,
where chloride ions bind to the functional groups on
the NCQD surface. These interactions are relatively
weak and temporary, allowing chloride ions to detach
when rinsed or exposed to a neutralizing environment,
and subsequently reattach during reuse. However, in
our study, the LSPR sensor using AgNTs displayed an
unexpected result where the wavelength shift wasn't
consistent. This finding challenges our understanding
of how the shape and size of AgNTs affect the sensor's
response. As reported by previous studies by Azeman
and co. in 2022, the sharp corners of triangular AgNTs
typically cause a redshift (longer wavelength) in
reflectance peaks compared to spherical shapes.
Multiple arrangements within the triangular AgNT
might weaken this effect, leading to less pronounced
redshift peaks (Bakar et al., 2022).
Figure 4 depicts the calibration plots for both
AgNT and AgNT-NCQD across three separate
experiments, all encompassing chloride ion
concentrations ranging from 2 ppm to 50 ppm. The
graph clearly illustrates that the Δλ for AgNT exhibits
a gradual rise proportional to increasing chloride ion
concentrations. In contrast, the Δλ for the AgNT-
NCQD composite shows a significantly steeper rise.
PHOTOPTICS 2025 - 13th International Conference on Photonics, Optics and Laser Technology
76

Figure 4: Calibration curve AgNT and AgNT-NCQD.
Furthermore, Figure 4 shows a graph comparing
the linear regressions of AgNT and AgNT-NCQD.
Notably, when detecting varying chloride ion
concentrations, the linear regression for AgNT-
NCQD appears to consist of two distinct lines. These
two lines correspond to concentration ranges: 0-6
ppm and 6-50 ppm. It shows a high correlation
coefficient (R2 = 0.9625) for 0-6ppm and AgNT-
NCQD range 6-50ppm correlation coefficient (R2 =
0.6793) compared to the AgNT range 0-6ppm and 6-
50ppm for R2 = 0.8992 and 0.1359, respectively. The
sensitivity of the sensor can be determined by
analyzing the slope of the lines in Figure 4. The
steeper the slope, the higher the sensitivity. The film
sensors exhibits optimal sensitivity up to 20 ppm of
chloride concentration, beyond which the sensor's
binding sites become saturated, leading to a plateau in
the response curve and reduced sensitivity. In this
case, the slope of the AgNT-NCQD composite's
calibration line is significantly steeper compared to
AgNT, indicating a greater increase in Δλ for each
increment in chloride ion concentration. This
translates to a higher detection accuracy for the
AgNT-NCQD composite, signifying its superior
performance as an LSPR sensor for chloride ion
detection in edible oil
.
The analysis confirms that the AgNT-NCQD
sensor is remarkably more sensitive than the AgNT
sensor for detecting chloride ions in edible oil. The
sensitivity of AgNT-NCQD was measured to be 0.92
nm ppm-1 (0-6ppm) and 0.21 nm ppm-1 (6-50ppm),
whereas AgNT's sensitivity was only 0.53 nm ppm-1
(0-6ppm) and 0.01 nm ppm-1 (6- 50ppm). This
significant improvement can be attributed to the
NCQDs in the composite sensor. NCQDs likely
provide more binding sites for chloride ions, allowing
for a greater response from the sensor and a more
precise measurement. The performance of AgNT-
NCQD compared to AgNT strongly suggests that
incorporating NCQDs significantly enhances the
sensitivity of LSPR sensors for chloride ion detection.
This work obtained a high sensitivity with the
detection range (0-6ppm), primarily due to the
addition of NCQDs as the sensor layer of chloride ion
detection compared to pure AgNT. However, the
AgNT-NCQD range (6-50ppm) has lower sensitivity
than the AgNT-NCQD range (0-6ppm). The readily
available positively charged sites on the NCQD
surface might be limited at higher chloride
concentrations, hindering their interaction with the
chloride ions. This findings demonstrated that
AgNT–NCQD outperforms AgNT as a sensing
material for chloride ion detection. It is important to
note that this sensor is designed for use with vegetable
oils that have properties similar to palm oil, such as
olive oil and canola oil.
4 CONCLUSIONS
This study investigated a sensor for detecting chloride
ions using triangular silver nanoparticles (AgNTs)
film and nitrogen-doped carbon quantum dots
(NCQDs). It compared the performance of this AgNT-
NCQD film sensor to the sensors using only AgNTs.
It analyzed the sensors by measuring the shift in
reflected light wavelength as the chloride ion
concentration increased. Presence of amino in the
NCQDs creates more active sites for chloride ions to
bind, enhancing the sensor's capability. This binding
likely occurs through electrostatic interactions. The
AgNT-NCQD film sensor positively responds to
increasing chloride ion concentrations within a
specific range (potentially 0 to 6 ppm). The sensor
demonstrated a value of 0.92 nm ppm
-1
. Additionally,
it showed a strong linear relationship between the
reflected light shift and chloride concentration (R² =
0.9625). However, the Limit of Detection (LOD) was
calculated to be 4.12 ppm, suggesting room for
improvement in detecting very low chloride
concentrations. In addition, considering the potential
of LSPR, this simple and efficient detection technique
could be applied to total chlorine in edible oil where it
provides a potential real-time detection for total
chlorine utilizing AgNT-NCQD films on LSPR to
achieve easy and fast detection in the food safety
fields.
ACKNOWLEDGEMENTS
The authors would like to thank the Photonics
Advancing Chloride Ion Detection in Edible Oils: Enhanced Sensitivity with NCQD/Ag Nanotriangles via Localized Surface Plasmon
Resonance
77

Laboratory of the Department of Electrical,
Electronic & Systems Engineering, Faculty of
Engineering and Built Environment, Faculty of
Science and Technology, and the Centre for Research
and Instrument Management (CRIM), Universiti
Kebangsaan Malaysia (UKM) for all amenities
provided. The author acknowledges the Fundamental
Research Grant Scheme (FRGS), grant number
FRGS/1/2023/TK07/UKM/02/2, funded by Ministry
of Education Malaysia and Research University
Grant (GUP), grant number GUP-2023-043, funded
by the Universiti Kebangsaan Malaysia (UKM),
Malaysia.
REFERENCES
Abdullah, S., Azeman, N. H., Mobarak, N. N., Zan, M. S.
D., & Ahmad, A. A. (2018). Sensitivity enhancement of
localized SPR sensor towards Pb(II) ion detection using
natural bio-polymer based carrageenan. Optik,
168(May), 784–793. https://doi.org/10.1016/j.ijleo.
2018.05.016
Abu Bakar, M. H., Azeman, N. H., Mobarak, N. N.,
Mokhtar, M. H. H., & Bakar, A. A. A. (2020). Effect of
active site modification towards performance
enhancement in biopolymer κ-Carrageenan derivatives.
Polymers, 12(9), 1–13. https://doi.org/10.
3390/POLYM12092040
Azeman, N. H., Arsad, N., & Bakar, A. A. A. (2020).
Polysaccharides as the sensing material for metal ion
detection-based optical sensor applications. Sensors
(Switzerland), 20(14), 1–22. https://doi.org/10.
3390/s20143924
Bakar, M. H. A., Azeman, N. H., Mobarak, N. N., Nazri, N.
A. A., Abdul Aziz, T. H. T., Zain, A. R. M., Arsad, N.,
& Bakar, A. A. A. (2022). Succinyl-κ-carrageenan
Silver Nanotriangles Composite for Ammonium
Localized Surface Plasmon Resonance Sensor.
Polymers, 14(2), 1–17. https://doi.org/10.3390/
polym14020329
Blumhorst, M. R., Collison, M. W., Cantrill, R., Shiro, H.,
Masukawa, Y., Kawai, S., & Yasunaga, K. (2013).
Collaborative study for the analysis of glycidyl fatty
acid esters in edible oils using LC-MS. JAOCS, Journal
of the American Oil Chemists’ Society, 90(4), 493–500.
https://doi.org/10.1007/s11746-012-2187-7
Cheng, W. W., Liu, G. Q., Wang, L. Q., & Liu, Z. S. (2017).
Glycidyl Fatty Acid Esters in Refined Edible Oils: A
Review on Formation, Occurrence, Analysis, and
Elimination Methods. Comprehensive Reviews in Food
Science and Food Safety, 16(2), 263–281.
https://doi.org/10.1111/1541-4337.12251
EFSA. (2018). Revised safe intake for 3-MCPD in
vegetable oils and food. EUROPEAN FOOD SAFETY
AUTHORITY. https://efsa.europa.eu/en/press/news/
180110
Jędrkiewicz, R., Kupska, M., Głowacz, A., Gromadzka, J.,
& Namieśnik, J. (2016). 3-MCPD: A Worldwide
Problem of Food Chemistry. Critical Reviews in Food
Science and Nutrition, 56(14), 2268–2277.
https://doi.org/10.1080/10408398.2013.829414
Jong-Sun, L., Ji-Won, H., Munyhung, J., Kwang-Won, L.,
& Myung-Sub, C. (2020). Effects of Thawing and
Frying Methods on the Formation of Acrylamide and
Polycyclic Aromatic. Foods, 9(5), 573.
Kuntom, A., Balasundram, N., & Lin, S. W. (2006). Esters
in Refined Edible Oils and Fats. 7–10.
Mehrotra, P. (2016). Biosensors and their applications - A
review. Journal of Oral Biology and Craniofacial
Research
, 6(2), 153–159. https://doi.org/10.
1016/j.jobcr.2015.12.002
Nazri, N. A. A. (2022). Chlorophyll Detection by Localized
Surface Plasmon Resonance Using Functionalized
Carbon Quantum Dots Triangle Ag Nanoparticles.
Nanomaterials, 12(17). https://doi.org/10.3390/nano
12172999
Nazri, N. A. A., Azeman, N. H., Bakar, M. H. A., Mobarak,
N. N., Luo, Y., Arsad, N., Aziz, T. H. T. A., Zain, A. R.
M., & Bakar, A. A. A. (2022). Localized surface
plasmon resonance decorated with carbon quantum
dots and triangular ag nanoparticles for chlorophyll
detection. Nanomaterials, 12(1). https://doi.org/
10.3390/nano12010035
Panel, E., & Chain, F. (2016). Risks for human health related
to the presence of 3- and 2-monochloropropanediol
(MCPD), and their fatty acid esters, and glycidyl fatty
acid esters in food. EFSA Journal, 14(5).
https://doi.org/10.2903/j.efsa.2016.4426
Rahman, W. B. W. A., Azeman, N. H., Kamaruddin, N. H.,
Menon, P. S., Shabaneh, A. A., Mahdi, M. A., Mokhtar,
M. H. H., Arsad, N., & Bakar, A. A. A. (2019). Label-
free detection of dissolved carbon dioxide utilizing
multimode tapered optical fiber coated zinc oxide
nanorice. IEEE Access, 7(2008), 4538–4545.
https://doi.org/10.1109/ACCESS.2018.2888626
Yoo, D., Park, Y., Cheon, B., & Park, M. H. (2019). Carbon
Dots as an Effective Fluorescent Sensing Platform for
Metal Ion Detection. Nanoscale Research Letters,
14(1). https://doi.org/10.1186/s11671-019-3088-6
Zannotti, M., Vicomandi, V., Rossi, A., Minicucci, M.,
Ferraro, S., Petetta, L., & Giovannetti, R. (2020).
Tuning of hydrogen peroxide etching during the
synthesis of silver nanoparticles. An application of
triangular nanoplates as plasmon sensors for Hg2+ in
aqueous solution. Journal of Molecular Liquids, 309,
113238. https://doi.org/10.1016/j.molliq.2020.113238.
PHOTOPTICS 2025 - 13th International Conference on Photonics, Optics and Laser Technology
78
