
Transfer-Modal Extraction of Surface EMG Features for Upper Limb
Motor Classification
Vedant Mangrulkar and Madhav Rao
a
International Institute of Information Technology Bangalore, Bangalore, India
{vinit.mangrulkar, mr}@iiitb.ac.in
Keywords:
Accelerometer, EMG, Classifier, ML, Features, Upper Limb, Synthetic Features, Random Forest.
Abstract:
Surface Electromyography (sEMG) signals provide critical insights into muscular activity, aiding action clas-
sification and monitoring muscular disorders. However, their reliability is hindered by noise and unstructured
data. Despite the advancements in machine learning, large datasets are essential to address these challenges
and enhance decoding accuracy for further development. Hence, this work attempts to predict the sEMG fea-
tures from the accelerometer signals in a view to generate synthetic data which is useful for further develop-
ments around this physiological signal. This work examines the correlation between accelerometer-generated
sEMG features and those from original sEMG signals for four upper limb actions wrist flexion, wrist exten-
sion, wrist closing and wrist vibration focusing on the flexor carpi ulnaris and extensor carpi radialis muscles.
Synthesized features are augmented with original features to train an ML model, achieving 91% accuracy
on unseen original sEMG features. This work showcases a viable solution to generate more sEMG features
corresponding to the actions under test from an altogether different modality. This work is a step towards
synthesizing EMG signals and features for human limb movements which offers a strong platform to design
imitation learning for rehabilitation systems in the future.
1 INTRODUCTION
The human hand is one of the most important part
of our body. It plays a crucial role in our daily life
helping us perform a wide range of activities start-
ing from basic tasks to complex exercises (Schreuders
et al., 2019). The wrist, a key joint in our hand move-
ment, allows for essential movements such as flex-
ion, extension and rotation, which are very important
for various activities involving gripping, lifting and
many other fine motor skills (Eschweiler et al., 2022).
Unfortunately, some unexpected events or accidents
leads to the partial or complete impairments in upper
limb functions. Hence the patients are prescribed with
specific upper limb physiotherapy based exercises
towards effective rehabilitation (Jonna et al., 2024;
Jonna and Rao, 2022; P et al., 2023). The recovery
process although is extremely slow, but is considered
effective owing to continuous improvement (Vinay
et al., 2021). Over the years, the researchers have
explored ways of monitoring progress and effective-
ness of the therapy sessions through dedicated care-
takers or paid physicians at home or clinics and cen-
a
https://orcid.org/0000-0003-2278-9148
ters. The visual evaluation is always marred by hu-
man bias and remains inconsistent across individu-
als undergoing therapy and also along the evaluators.
Various methods around sensory systems to automate
and nullify the human bias is explored in the past, but
a more simplified and robust variant which is reliable
for practising is yet to be found.
The motion classification for human limb re-
habilitation presents an opportunity to develop au-
tomation in the personalized therapy program with
real-time feedback and assessment systems to en-
sure that the routine therapy are performed accu-
rately and effectively (Novak et al., 2012; Kwakkel
et al., 2008). Automation without much support from
care-takers helps the former to build their confidence
and self-belief, besides giving much relief to the lat-
ter. Automated features aids in progress-tracking
and continuous-monitoring, effectively improving the
therapy sessions (Hiengkaew et al., 2012). Another
distinct and radical approach of employing dynami-
cally adaptive training system is introduced and fur-
ther discussed in (Loureiro and Harwin, 2007) which
works on self-tuning the therapy based on the out-
come.
Hence an automated classification of patient
Mangrulkar, V. and Rao, M.
Transfer-Modal Extraction of Surface EMG Features for Upper Limb Motor Classification.
DOI: 10.5220/0013157800003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 721-728
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
721

limb movements is therefore extremely crucial in
the scheme of rehabilitation process (Chandrasekhar
et al., 2020a).
Electromyography (EMG) signals capture the
muscular activity of the human limb, and same is re-
ported in many literature’s for recognizing the upper
limb actions (Vinay et al., 2022; Reddy et al., 2023).
More macro-level evaluation pertains to the actions
performed by the individuals under rehabilitation, and
in-conjunction with EMG signal is a useful parameter
to analysis. Hence EMG signal based upper and lower
limb rehabilitation designs are found most suitable for
evaluating the recovery process.
The invasive variant of acquiring EMG signals is
more accurate, but does not fit this requirement of
continuous evaluation. Hence surface based EMG
signals also referred to as sEMG signals are tapped
at the surface of the human body. Although these sig-
nals when probed at the surface is conducive to noise,
but are processed with some success to find the signa-
tures mapped to the limb movements (Chandrasekhar
et al., 2020b). The sEMG signals are found to carry
critical information of limb movements and hence are
aimed toward the rehabilitation setups. However the
usage of sEMG signals for the classification of the
motor movements has its own share of problems. The
sEMG signals are marred with huge baseline noise in
addition to the distortion effects which leads to a low
SNR (Campanini et al., 2020).
The occupancy of baseline noise signal makes it
extremely difficult to extract discriminative features,
while the
distortion alters the temporal and phase compo-
nents of the signal (Merletti and Cerone, 2020). The
sEMG signals are also sensitive to several other fac-
tors including human anatomy, electrode placements
and muscle fatigues (Merletti and Cerone, 2020).
Other sensory systems including motion capture
systems, IMU, Electromagnetic Tracking Sensor and
pressure sensors fall short in reliably detecting mo-
tor imagery due to several reasons (Mason and Birch,
2003; Chizari et al., 2020). Additionally, the mo-
tion capture systems are cost ineffective when com-
pared to sEMG or MPU(motion processing unit) sen-
sors, and are predominantly built on camera subsys-
tem, which invades privacy. The video grab is typi-
cally of high bandwidth and demand high computer
power to process which makes it unviable for clinical
trials (Vitali and Perkins, 2020).
The pressure sensors on the other hand are sen-
sitive to their placements and alignments, which
proves to be challenging for rehabilitation purposes
(Lawrence et al., 2014).
Overall, the sEMG based systems are found to be
an apt solution for the domestic and clinical setups
where affordability is a major factor, however build-
ing the whole system requires more reliable data to-
wards training the classifier. One way to deal with this
challenge is to acquire large sEMG dataset for train-
ing, thereby making the model resilient to high degree
of variability among the individuals and also along the
acquisition factors. However, compiling sEMG sig-
nals requires comprehensive effort and hence for most
of the custom applications, sEMG signals remain un-
favourable. A synthesized set of sEMG dataset for
building classifier is one of the solution towards fill-
ing the aforementioned void. An electrical model
based sEMG features are reported in the past (Vinay
et al., 2022), however these remains non-reliable for
the real-world applications even with the addition of
Gaussian modeled noise component to the synthe-
sized features. A suitable alternative is to derive syn-
thetic features from a different set of physical sensors
which preferably is less sensitive to motion artifacts.
Accelerometers are found reliable for motion classi-
fier both for upper and lower limb, however these
are not equipped to offer distal information instead
offers proximal information when utilized in the hu-
man body. For instance, the muscle activity for wrist
movement is captured by sEMG signal even at the dis-
tal end of the upper arm, whereas MPUs are likely to
be positioned near the wrist joint to capture reliable
signal. Consequently the proximal accelerometer sen-
sory signal carries the necessary limb movement in-
formation which can be extracted to the correspond-
ing synthetic distal sEMG signal features. These syn-
thetic features supplement to build large dataset for
realizing ML classifier model to work for real-world
automated rehabilitation tasks. This paper demon-
strates the principle working of the proposed transfer
of modality for training model and evaluates the same
using original sEMG signal features and also for the
mixed (original and synthetic) dataset. The main con-
tributions of the paper are: i) Prediction of sEMG fea-
tures from the extracted real time accelerometer data,
and further validating the same with ensemble model
to achieve reliable results, and
ii) Extracted sEMG features show comparable
performance with respect to other features extracted
from original sEMG signal. The dataset and model
files are made freely available at (Mod, ) for further
usage to the researchers and designers community.
2 PROPOSED METHOD
The proposed framework as shown in the Figure 1
comprises of three primary systems. The first system
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
722
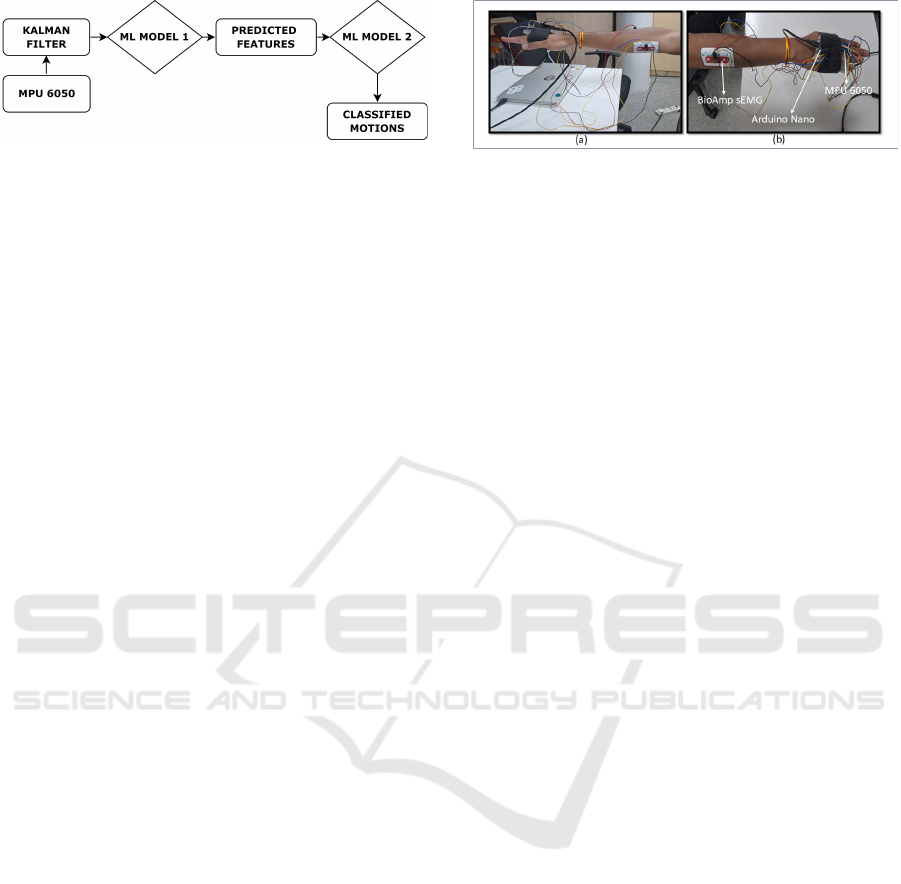
Figure 1: Proposed framework#1 comprising of Ensemble
model to extract sEMG features from MPU signals and fur-
ther supplying to a Classifier model. Here Model#1 is En-
semble model, and Model#2 is a Classifier model.
focuses on data acquisition from MPU sensors.
The second system involves prediction of the
sEMG features from the filtered accelerometer data.
The IMU data for the specific movements is taken to
label the data appropriately and further train the ex-
traction model. The last system engages in the classi-
fication of four identified upper limb movements us-
ing these synthetic features extracted from IMU data.
ML MODEL 1 is the prediction model which predicts
the features from the filtered data and ML MODEL 2
is the classifier model which classifies the four mo-
tions.
2.1 Dataset
Surface EMG signals are captured using the Mus-
cle BioAmp Patchy sensor, which offers an input
impedance of 10
12
Ω and includes a bandpass filter
with a cutoff frequency of 72-720 Hz (Upside Down
Labs, 2024). Motion data of the wrist is measured us-
ing an Inertial Measurement Unit (IMU), specifically
the MPU 6050, which provides the accelerometer
and gyroscope data across all three axes (InvenSense,
2013). The MPU 6050 sensor and the sEMG sen-
sors are interfaced to the Arduino Nano board via I2C
communication and analog pins, respectively. The ac-
celerometer data offers the vector and movement in-
formation along the 3 axis and are preferred over gy-
roscopes which are more useful for rotational actions.
Hence in this work, only accelerometer data is em-
ployed to augment the sEMG features.
Figure 2(a and b) shows the setup to acquire the
sEMG signal from the targetted muscle group, and
IMU for the same upper limb actions. The IMU setup
was designed to
be wearable, and user-friendly, while sEMG elec-
trodes are positioned appropriately to ensure effective
data sampling during wrist movements. Two Bioamp
sEMG sensors were employed to collect the signals
from the targeted muscles at the same time, while the
IMU data is also captured for the four identified upper
limb movements. Necessary informed ethical consent
was taken from 8 healthy subjects with a declaration
Figure 2: Snapshot illustrating the setup to acquire signals
from IMU and sEMG for the defined upper limb move-
ments. IMU sensors enclosed in a black band is wrapped
around the palm of the hand, whereas the sEMG signals are
acquired from the electrodes positioned at the forearm. (a)
shows the position of electrodes on the flexor carpi ulnaris
muscles, and (b) shows the position of electrodes on the ex-
tensor carpi radialis muscles.
approving that the subjects had no prior weakness or
injury, nor any prior surgery performed in the past on
any part of the upper or lower limb.
These participants were evenly distributed be-
tween 4 females and 4 males to present generic re-
sults. Participants’ characteristics included an aver-
age age of 19.5 ± 0.87 years and an average weight
of 62.32 ± 16.71 kg. All participants provided writ-
ten informed consent before participating in the ex-
periments. The data was collected in accordance
with the Helsinki Declaration of 1975, as revised in
2000 (Ashcroft et al., 2008). The subjects were in-
structed to perform four distinct wrist motions: wrist
flexion, wrist extension, wrist closing(extension and
contraction of fingers), and wrist vibration(shaking of
hand). The dataset collected for training the model
comprised a total of 108,656 samples. Figure 3 shows
one such instance of the real-time sEMG signal along
with the corresponding accelerometer and gyroscope
data in all 3 axes for all the four aforementioned
wrist actions. To ensure data randomness, subjects
performed wrist motions in a randomized sequence.
This method mitigates potential biases and enhances
the robustness of the dataset for better analysis (Is-
mail Fawaz et al., 2018). Electrodes were positioned
on the flexor carpi ulnaris and extensor carpi radialis
muscles, on the skin. Real-time signal data was cap-
tured and stored in readable files prior to feature ex-
traction from sEMG signals and further labelling the
signal for the corresponding actions.
2.2 EMG Feature Extraction from IMU
Data
The signals were uniformly collected at a sampling
rate of 500 Hz, as proven efficient in the litera-
ture (Chen et al., 2017). Accelerometer data under-
went Kalman filtering (Process Noise Covariance(Q)
: 0.01*eye(3), Measurement Noise Covariance(R) :
0.1*eye(3), Initial Estimation Error Covariance(P) :
Transfer-Modal Extraction of Surface EMG Features for Upper Limb Motor Classification
723
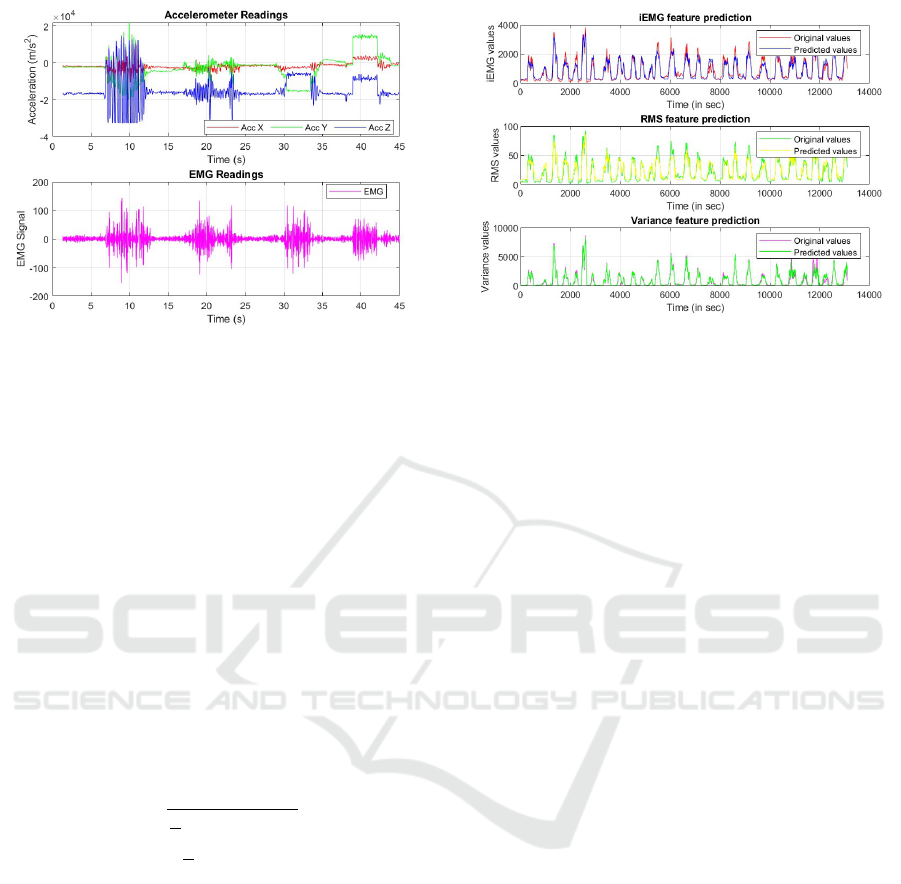
Figure 3: Pre-processed sEMG signal with the correspond-
ing Accelerometer signals for the four upper limb wrist ac-
tions - flexion, extension, closing of wrist, and vibrations.
eye(3), State Transition and Covariance : eye(3))
to significantly enhance accuracy by reducing noise
and improving the reliability of the IMU data (Farag,
2020). Surface electromyography (sEMG) signals
were processed with the help of a Butterworth 4
th
order band-pass filter for enhanced efficiency (Shi,
2012). Following data acquisition, feature extrac-
tion was conducted for a window size of 50 sam-
ples (100 ms) with a step size of 1 sample (2 ms)
to retain higher order of dataset. Time-domain fea-
tures including Integrated EMG (iEMG), Root Mean
Square (RMS), and Variance were extracted and an-
alyzed for each motion. These features serve as the
most discriminative inputs for further motion classifi-
cation tasks (Phinyomark et al., 2012).
iEMG =
R
T
0
|EMG(t)|dt
RMS =
q
1
N
∑
N
i=1
(EMG
i
)
2
Variance =
1
N
∑
N
i=1
(EMG
i
− µ)
2
(1)
2.3 Model Feature Prediction
Filtered accelerometer data and extracted sEMG fea-
tures serve as input and output, respectively, for pre-
dicting sEMG features. Each feature (iEMG, RMS,
Variance) is predicted independently. To predict
iEMG and RMS features, we employed an ensemble
model combining a Convolutional Neural Network
(CNN) and a Random Forest (RF) to enhance predic-
tive performance. CNN was employed for its abil-
ity to capture spatial hierarchies in the data through
convolutional layers (LeCun et al., 2015). Random
Forest was used for its robustness and ability to han-
dle nonlinear relationships (Khalilia et al., 2011). For
predicting Variance, an ensemble model combining
Figure 4: Original features versus trained ML Predicted fea-
tures for all four selected upper limb actions.
Random Forest (estimators=500, depth=30, MinSam-
plesSplit=3, MinSamplesLeaf=2, RandomState=42)
and LightGBM(estimators=500, LearningRate=0.05,
depth=30, leaves=40, MinchildSamples=20, Ran-
domState=42) was utilized for improved accuracy.
LightGBM is particularly effective for time series
feature prediction due to its fast training speed, low
memory usage, and ability to manage large datasets
and complex feature interactions efficiently (Ke et al.,
2017). The outputs from these models were com-
bined to achieve a more robust and accurate pre-
dictions. The CNN architecture consisted of mul-
tiple convolutional layers followed by pooling lay-
ers, batch-normalization, and dropout layers to pre-
vent over-fitting, and dense layers for the final output
(Krizhevsky et al., 2012; Ioffe and Szegedy, 2015).
The RF model was tuned with hyperparameters such
as the number of trees, maximum depth, and mini-
mum samples split to achieve optimal performance.
For feature engineering, rolling statistics : mean and
standard deviation are calculated with a window size
of 10 to enhance the input feature set.
The predictions from both models were then en-
sembled using a weighted average approach. The
weights were determined based on the individual per-
formance of each model on a validation set. This en-
semble approach leveraged the strengths of both mod-
els, leading to improved overall performance com-
pared to using each model individually (Zhou, 2012).
The prediction model was trained for 70% of the
dataset, 10% for validation, and remaining 20% for
testing. The ensemble model achieved R
2
value (co-
efficient of determination) of 0.789 and 0.775 for the
prediction of iEMG and RMS features with respect to
the original extracted features respectively. The en-
semble model for the prediction of Variance showed
R
2
value of 0.715. Figure 4 showcases the plot of all
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
724
the three predicted features in contrast with the orig-
inal features. The R
2
value is a crucial metric in re-
gression analysis as it quantifies the proportion of the
variance in the dependent variable that is predictable
from the independent variables. R
2
is a key indica-
tor of model performance and reliability in predictive
analytics (Frost, 2019).
2.4 Motion Classifier Model
Post the prediction of the sEMG features, the data
was labelled for the classification of the wrist move-
ments. The classifier model used 70% of the dataset
for training, 10% for validation, and remaining 20%
of the dataset for testing. A grid search was con-
ducted to optimize hyperparameters, using a 5-fold
cross-validation for all models. The 70-10-20 split
for training, validating and testing dataset is proved
to be efficient due to its balance between training,
and evaluation (Arlot and Celisse, 2009). Between
the various models trained, RF offered the best results
for the classification of the wrist movements with pa-
rameters(estimators = 200, depth = 20, samplesSplit
= 5, MinSamplesLeaf = 2, RandomState = 42, Cri-
terion = ’gini’, bootstrap = True, features = ’sqrt’)
RF is widely recognized as an effective classifier for
movement recognition tasks due to its robustness and
ability to handle complex, non-linear relationships
in data. It combines multiple decision trees to im-
prove predictive accuracy and reduce the risk of over-
fitting, making it particularly suitable for classifying
movements from sensor data (Pal, 2005). Amongst
the other classification models attempted were SVM
model with RBF and poly kernel, and Gradient Boost-
ing model (Van Messem, 2020; Stoean and Stoean,
2013; Friedman, 2006).
3 RESULTS AND DISCUSSIONS
The proposed pipeline framework (#1) extracts sEMG
features from accelerometer data which is further
supplied to classifier model. The proposed pipeline
framework which transfers the modality of sensors
data is benchmarked with two staright-forward meth-
ods. One where the original sEMG features are ex-
tracted from the original sEMG signals and further
classified for upper limb movements also referred to
as Benchmark framework#1. The other one which
employs accelerometer data to classify the motions,
which is referred to as Benchmark framework#2.
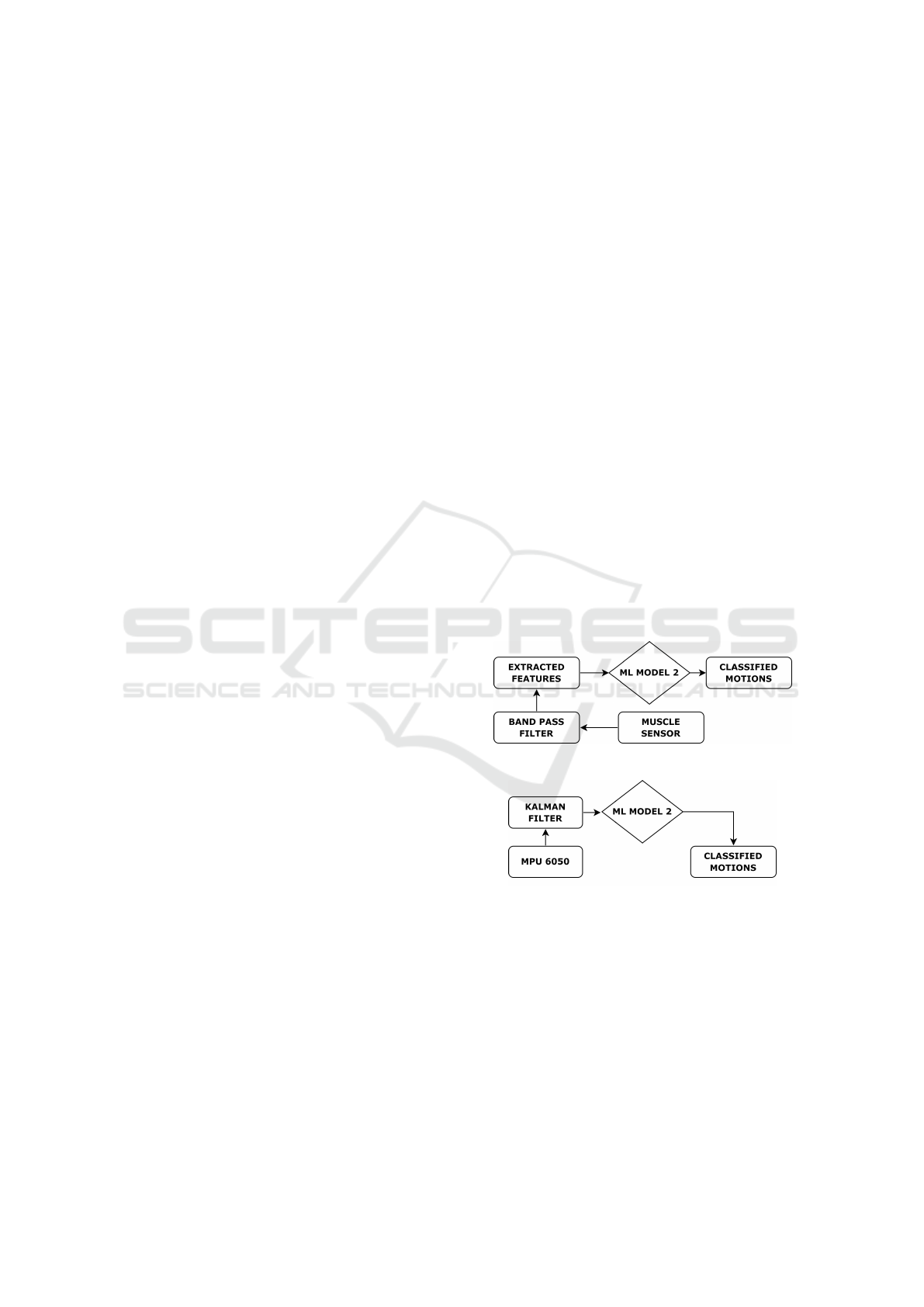
These two benchmarking frameworks are illustrated
in Figures 5 (a, b). The proposed framework (#2) is
comprised of extracted synthetic sEMG features from
the accelerometer data and further merging with the
original sEMG features. The augmented dataset con-
sists of synthetic features and original features which
is then employed to train the classifier model. The
overall process flow is illustrated in Figure 6. All the
benchmark frameworks are compared with the pro-
posed ones. While the first proposed framework ex-
tracts the sEMG features from a different modality
of sensors, the second proposed framework utilizes
the same but augments the dataset with original fea-
tures to build the classifier model. Table 1 shows
the accuracy of the classifier models including the
proposed one and the benchmark model for the four
wrist movements. The proposed synthetic features
evolved framework shows better precision than the
original features extracted proposed framework (#1)
which is expected considering the features are ex-
tracted from accelerometer data in the proposed work
which are proximally placed whereas the EMG elec-
trodes are distally placed. Additionally the proposed
framework (#2) trained with augmented synthetic and
original sEMG features shows comparable accuracy
with respect to the original ones. As expected the
accelerometer devised classifier shows high precision
over others which is attributed to the proximal place-
ment of sensors, that captures the dynamic motions
accurately.
(a)
(b)
Figure 5: (a) Benchmark framework#1 where the original
features from sEMG signals are extracted and further sup-
plied to classifier model, and (b) Benchmark framework#2
where the accelerometer are used to classify the motion
instead of extracting sEMG features from Accelerometer
data.
The proposed frameworks along with two other
benchmark frameworks are evaluated for Precision,
Recall, and F1-score. Table 1 lists the accuracy
metrics for the proposed model (#1), two bench-
mark frameworks and proposed merged classifier
Transfer-Modal Extraction of Surface EMG Features for Upper Limb Motor Classification
725
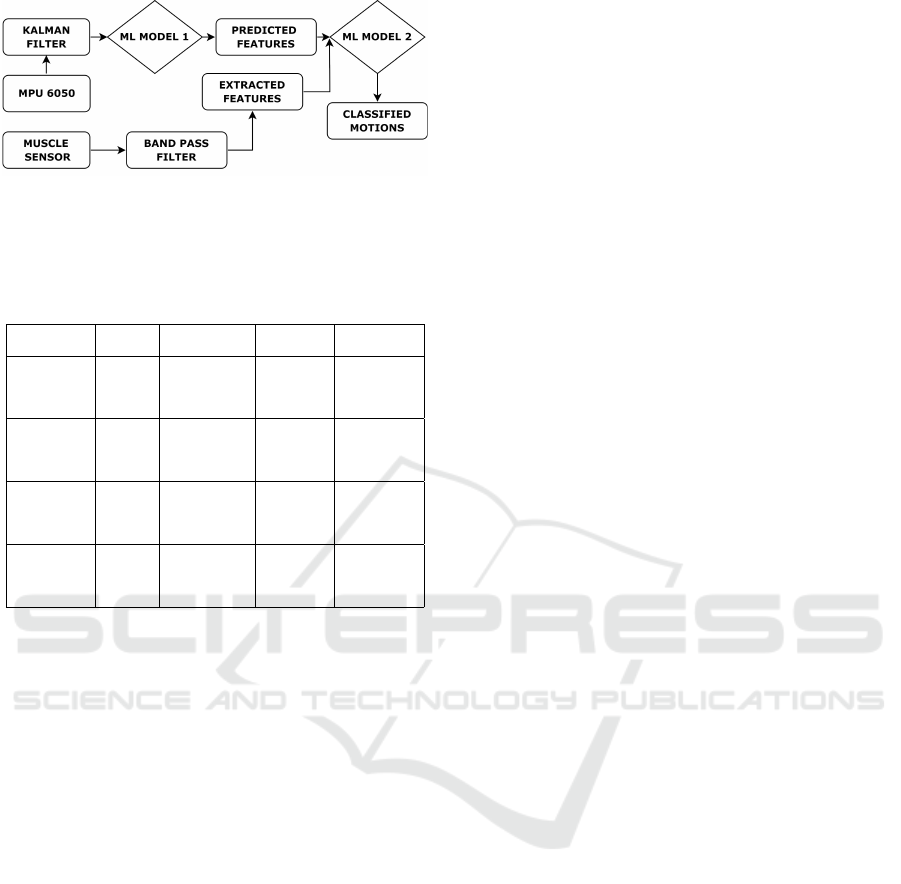
Figure 6: Proposed framework (#2) where the synthetic and
original sEMG features are supplied to train classifier mod-
els.
Table 1: Accuracy metrics for the proposed frameworks
along with other benchmark frameworks.
Models Motions Precision (%) Recall (%)
F1
SCORE (%)
Proposed
framework#1
0
1
2
3
78
88
85
81
72
88
77
73
75
88
81
77
Benchmark
framework#1
0
1
2
3
76
73
70
79
78
66
57
77
77
69
62
78
Benchmark
framework#2
0
1
2
3
99
94
91
90
94
94
92
85
96
94
92
88
Proposed
framework#2
0
1
2
3
77
80
78
77
77
70
74
71
77
74
70
74
model. The proposed framework (#1) which classi-
fies the four wrist movements using three predicted
sEMG features derived from accelerometer readings,
achieves an average precision of 83%, with average
F1 score of 80.25% This proposed framework lever-
ages the mapping of two different modality of sensors
- accelerometer and sEMG, by predicting sEMG fea-
tures from accelerometer readings before classifying
the wrist movements. The trained prediction model
tries to map the sEMG features for an accelerom-
eter signal that is generated on wrist action. This
prediction is not real, but it forsees that sEMG fea-
tures from the accelerometer signal which is gener-
ated from movement dynamics and not from muscle
activity. The mapping of muscle activity to movement
is performed by the prediction model.
The prediction model is trained to accurately map
the accelerometer signal with sEMG features for all
the four wrist movements.
In comparison, the benchmark framework#1
shown in Figure 5 (a), classifies wrist movements
through the three sEMG features directly extracted
from the original sEMG signals, achieving an average
precision of 74.5% as listed in the Table 1.
While this framework benefits from the direct
measurement of muscle activity through sEMG sig-
nals, the electrodes positioned at distal end is prone
to lose critical information and hence the drop in ac-
curacy. Multiple electrodes at the distal end is bound
to improve the accuracy by consolidating vector fea-
tures.
Direct sEMG readings offer high temporal reso-
lution and are effective for muscle activity detection,
but are limited by noise and variability in electrode
placement.
The benchmark framework#2 as portrayed in Fig-
ure 5 (b), which classifies wrist movements directly
from accelerometer readings, achieves the highest av-
erage precision of 94% among all the frameworks.
This framework’s great precision underscores the ef-
fectiveness of accelerometer data in capturing the dy-
namics of wrist movements. Accelerometers provide
detailed motion data, including acceleration and an-
gular velocity, which are directly related to the phys-
ical movements being classified. The high precision
indicates that accelerometer readings are highly reli-
able for distinguishing between different wrist move-
ments. However, this framework lack insights into the
underlying muscle activity, which is crucial in certain
applications such as rehabilitation or bio-mechanical
analysis. Besides, the accelerometers positioning is
always restricted to specific regions thereby limiting
the design space for rehabilitation and related tasks.
The proposed merged framework#2 as illustrated
in the Figure 6, combines the dataset of predicted
EMG features along with the dataset of original
sEMG features, thus creating a richer dataset. It is
then employed as input to classify the upper limb mo-
tions. The proposed merged dataset devised frame-
work achieves an average precision of 77.5% which
is comparable with the original sEMG feature trained
framework (74.5%). This strengthens the proposal
of augmenting the dataset through predicted sEMG
features from the other modality of sensors when-
ever the original sEMG signal and data acquisition is
not possible. It is also noted that pure synthetic fea-
tures present classifier (proposed variant) accuracy of
around 83%, which is vastly deviated from the origi-
nal framework (#1). Hence the augmented dataset is
much closer to the real sEMG signal generated and
devised framework.
Additionally, the merged dataset devised model
(proposed #2) was evaluated on original unseen
sEMG features and synthetically generated but un-
seen sEMG features individually.
Accuracy of 91% is achieved when the proposed
framework 1 (which is trained completely on the orig-
inal unseen dataset) is used and is given the aug-
mented training dataset of the proposed framework
2 for testing. Accuracy of 95% is achieved when
the proposed framework 2 (which is trained on the
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
726

augmented dataset) is used and is given the origi-
nal unseen dataset to test. This strongly supports the
use of accelerometers derived sEMG features for aug-
menting the dataset and further employing the trained
model on unseen dataset successfully. The mapping
of accelerometer data to sEMG features although does
not fully capture the electro-muscular activity but it
augments the dataset well to train the model and ex-
tract discriminative features for original and unseen
sEMG features. The augmentation of sEMG dataset
through other modality of sensors opens up several
avenues to train the model for human limb. The four
wrist actions are purely to showcase the effect of syn-
thetic features and the dataset.
4 CONCLUSION
The proposed sEMG features extracted from a dif-
ferent modality of sensor is introduced in this work
towards classifying four of the upper limb motions.
These motion classifiers are useful to design and de-
velop an adaptive rehabilitation system with predic-
tive control strategy. The proposed synthetically gen-
erated sEMG features from the ML model show high
degree of correlation with the original sEMG gener-
ated features for four of the wrist actions. The data
collected was based on the four motions which were
restricted to the flexor carpi ulnaris and extensor carpi
radialis muscles. An ensemble model was proposed
and verified for attaining maximum accuracy for ex-
traction of sEMG features. Post the prediction of the
features, RF classifier was employed for classifying
the four identified motions. The machine learning
models were optimised with appropriate hyperparam-
eters and features ensuring best possible results. The
predicted sEMG features augmented with orginal fea-
tures demonstrates comparable motion classifier ac-
curacy. Additionally for unseen original sEMG fea-
tures, the proposed augmented dataset trained model
showcased a superior accuracy of 91%. This is a step
for developing large dataset of human limb motion,
which will possibly give a much needed impetus for
designing a generic human limb rehabilitation system
that is applicable to the most of the needy ones. The
generic rehabilitation system will further reduce the
cost to the users. The dataset and model files are made
freely available at (Mod, ) for further use to the re-
searchers and scientific community.
REFERENCES
Dataset and model files are available at: https://drive.goog
le.com/drive/folders/1fEIuRkBEIlyJBbj79CEXPjqh
9B16Ibon?usp=sharing.
Arlot, S. and Celisse, A. (2009). A survey of cross vali-
dation procedures for model selection. Statistics Sur-
veys, 4.
Ashcroft, R. E. et al. (2008). The declaration of helsinki.
The Oxford textbook of clinical research ethics, pages
141–148.
Campanini, I., Disselhorst-Klug, C., Rymer, W. Z., and
Merletti, R. (2020). Surface emg in clinical assess-
ment and neurorehabilitation: Barriers limiting its use.
Frontiers in Neurology, 11.
Chandrasekhar, V., Vazhayil, V., and Rao, M. (2020a). De-
sign of a portable anthropomimetic upper limb reha-
bilitation device for patients suffering from neuromus-
cular disability. In 2020 42nd Annual International
Conference of the IEEE Engineering in Medicine &
Biology Society (EMBC), pages 4708–4712.
Chandrasekhar, V., Vazhayil, V., and Rao, M. (2020b). De-
sign of a real time portable low-cost multi-channel
surface electromyography system to aid neuromus-
cular disorder and post stroke rehabilitation patients.
In 2020 42nd Annual International Conference of the
IEEE Engineering in Medicine & Biology Society
(EMBC), pages 4138–4142.
Chen, H., Zhang, Y., Zhang, Z., Fang, Y., Liu, H., and
Yao, C. (2017). Exploring the relation between emg
sampling frequency and hand motion recognition ac-
curacy. In 2017 IEEE International Conference on
Systems, Man, and Cybernetics (SMC), pages 1139–
1144.
Chizari, A., Knop, T., Sirmacek, B., van der Heijden, F., and
Steenbergen, W. (2020). Exploration of movement
artefacts in handheld laser speckle contrast perfusion
imaging. Biomedical optics express, 11(5):2352–
2365.
Eschweiler, J., Li, J., Quack, V., Rath, B., Baroncini, A.,
Hildebrand, F., and Migliorini, F. (2022). Anatomy,
biomechanics, and loads of the wrist joint. Life, 12(2).
Farag, W. (2020). Kalman-filter-based sensor fusion applied
to road-objects detection and tracking for autonomous
vehicles. Proceedings of the Institution of Mechani-
cal Engineers, Part I: Journal of Systems and Control
Engineering, 235:095965182097552.
Friedman, J. H. (2006). Recent advances in predictive (ma-
chine) learning. Journal of classification, 23(2):175–
197.
Frost, J. (2019). How to interpret r-squared in regression
analysis. statistics by jim, making statistics intuitive.
Statistics by Jim Making Statistics Intuitive.
Hiengkaew, V., Jitaree, K., and Chaiyawat, P. (2012). Mini-
mal detectable changes of the berg balance scale, fugl-
meyer assessment scale, timed “up & go” test, gait
speeds, and 2-minute walk test in individuals with
chronic stroke with different degrees of ankle plan-
tarflexor tone. Archives of Physical Medicine and Re-
habilitation, 93(7):1201–1208.
InvenSense, I. (2013). Mpu-6000 and mpu-6050 product
specification revision 3.4. United States.
Transfer-Modal Extraction of Surface EMG Features for Upper Limb Motor Classification
727

Ioffe, S. and Szegedy, C. (2015). Batch normalization: Ac-
celerating deep network training by reducing internal
covariate shift. In International conference on ma-
chine learning, pages 448–456. pmlr.
Ismail Fawaz, H., Forestier, G., Weber, J., Idoumghar, L.,
and Muller, P.-A. (2018). Data augmentation using
synthetic data for time series classification with deep
residual networks.
Jonna, P., Madurwar, A., BK, A., and Rao, M. (2024). De-
sign of 6-dof holonomic drive-based upper and lower-
limb stroke rehabilitation system. In 2024 10th In-
ternational Conference on Automation, Robotics and
Applications (ICARA), pages 233–239.
Jonna, P. and Rao, M. (2022). Design of a 6-dof cost-
effective differential-drive based robotic system for
upper-limb stroke rehabilitation. In 2022 44th Annual
International Conference of the IEEE Engineering in
Medicine & Biology Society (EMBC), pages 1423–
1427.
Ke, G., Meng, Q., Finley, T., Wang, T., Chen, W., Ma, W.,
Ye, Q., and Liu, T.-Y. (2017). Lightgbm: A highly
efficient gradient boosting decision tree. Advances in
neural information processing systems, 30.
Khalilia, M., Chakraborty, S., and Popescu, M. (2011). Pre-
dicting disease risk from highly imbalanced data using
random forest. BMC medical informatics and decision
making, 11:51.
Krizhevsky, A., Sutskever, I., and Hinton, G. E. (2012).
Imagenet classification with deep convolutional neu-
ral networks. In Pereira, F., Burges, C., Bottou, L.,
and Weinberger, K., editors, Advances in Neural In-
formation Processing Systems, volume 25. Curran As-
sociates, Inc.
Kwakkel, G., Kollen, B. J., and Krebs, H. I. (2008). Effects
of robot-assisted therapy on upper limb recovery after
stroke: A systematic review. Neurorehabilitation and
Neural Repair, 22(2):111–121. PMID: 17876068.
Lawrence, E. L., Fassola, I., Werner, I., Leclercq, C., and
Valero-Cuevas, F. J. (2014). Quantification of dexter-
ity as the dynamical regulation of instabilities: Com-
parisons across gender, age, and disease. Frontiers in
Neurology, 5.
LeCun, Y., Bengio, Y., and Hinton, G. (2015). Deep learn-
ing. nature, 521(7553):436–444.
Loureiro, R. C. and Harwin, W. S. (2007). Reach & grasp
therapy: Design and control of a 9-dof robotic neuro-
rehabilitation system. In 2007 IEEE 10th Interna-
tional Conference on Rehabilitation Robotics, pages
757–763.
Mason, S. and Birch, G. (2003). A general framework for
brain-computer interface design. IEEE Transactions
on Neural Systems and Rehabilitation Engineering,
11(1):70–85.
Merletti, R. and Cerone, G. L. (2020). Tutorial. surface emg
detection, conditioning and pre-processing: Best prac-
tices. Journal of Electromyography and Kinesiology,
54:102440.
Novak, D., Mihelj, M., and Munih, M. (2012). A survey of
methods for data fusion and system adaptation using
autonomic nervous system responses in physiological
computing. Interacting with Computers, 24(3):154–
172.
P, J., GVK, S., Rao, M., Bapat, J., and Das, D. (2023). Xore-
hab: Iot enabled wheelchair based lower limb reha-
bilitation system. In 2023 45th Annual International
Conference of the IEEE Engineering in Medicine &
Biology Society (EMBC), pages 1–5.
Pal, M. (2005). Random forest classifier for remote sensing
classification. International journal of remote sensing,
26(1):217–222.
Phinyomark, A., Nuidod, A., Phukpattaranont, P., and Lim-
sakul, C. (2012). Feature extraction and reduction of
wavelet transform coefficients for emg pattern classifi-
cation. Elektronika ir Elektrotechnika, 122(6):27–32.
Reddy, M., Jonna, P., Perala, S., Rao, M., and Vazhiyal, V.
(2023). Automated microsurgical tool categorization
using a surface-based emg system. In 2023 45th An-
nual International Conference of the IEEE Engineer-
ing in Medicine & Biology Society (EMBC), pages 1–
5.
Schreuders, T., Brandsma, J., and Stam, H. (2019). Func-
tional Anatomy and Biomechanics of the Hand, pages
3–21.
Shi, Y. (2012). The application of the butterworth low-
pass digital filter on experimental data processing. In
2011 International Conference in Electrics, Commu-
nication and Automatic Control Proceedings, pages
225–230. Springer.
Stoean, R. and Stoean, C. (2013). Modeling medical deci-
sion making by support vector machines, explaining
by rules of evolutionary algorithms with feature selec-
tion. Expert Systems with Applications, pages 2677–
2686.
Upside Down Labs (2024). Muscle-bioamp-patchy. https:
//github.com/upsidedownlabs/Muscle-BioAmpPat
chy/tree/main/hardware. [Online; accessed 11-June-
2024].
Van Messem, A. (2020). Chapter 10 - support vector ma-
chines: A robust prediction method with applications
in bioinformatics. In Srinivasa Rao, A. S. and Rao,
C., editors, Principles and Methods for Data Science,
Handbook of Statistics, pages 391–466.
Vinay, K., Nagaraj, K., Arvinda, H. R., Vikas, V., and Rao,
M. (2021). Design of a device for lower limb pro-
phylaxis and exercise. IEEE Journal of Translational
Engineering in Health and Medicine, 9:1–7.
Vinay, K., Vazhayil, V., and Rao, M. (2022). An event
driven approximate bio-electrical model generating
surface electromyography rms features. In 2022 35th
International Conference on VLSI Design and 2022
21st International Conference on Embedded Systems
(VLSID), pages 204–209.
Vitali, R. V. and Perkins, N. C. (2020). Determin-
ing anatomical frames via inertial motion capture:
A survey of methods. Journal of Biomechanics,
106:109832.
Zhou, Z.-H. (2012). Ensemble methods: foundations and
algorithms. CRC press.
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
728
