
Comparative Analysis of Generalized Multiscale Entropy Methods for
Coarse-Grained Time Series Construction in Assessing Autonomic
Balance in Peripheral Arterial Disease Patients
O. Barquero-P
´
erez
1 a
, R. Goya-Esteban
1 b
, E. Sarabia-Cachadi
˜
na
2 c
and J. Naranjo-Orellana
3 d
1
Dept. Signal Theory and Communications, Universidad Rey Juan Carlos, Fuenlabrada, Madrid, Spain
2
Centro de Estudios Universitarios Cardenal Sp
´
ınola CEU, Sevilla, Spain
3
Departamento Deporte e Inform
´
atica, Universidad Pablo de Olavide, Sevilla, Spain
{oscar.barquero, rebeca.goyaesteban}@urjc.es, esarabia@ceu.es, jnarore@upo.es
Keywords:
Autonomic Nervous Systems, Heart Rate Variability, Generalized Multiscale Entropy, Peripheral Arterial
Disease.
Abstract:
Peripheral Arterial Disease (PAD) is a chronic condition that significantly impacts autonomic balance, as
reflected in Heart Rate Variability (HRV). However, the characterization of autonomic balance in PAD pa-
tients using HRV is still unclear. Generalized Multiscale Entropy (GMSE) is a nonlinear method capable of
characterizing the complexity of HRV across multiple time scales, offering a more nuanced understanding
of autonomic dysfunction in PAD patients. 14 healthy male subjects (60±5 years) and 14 male intermittent
claudication patients (64±6 years) underwent 10 minutes of ECG recording from which RR interval time se-
ries were obtained. This study provides a comparative analysis of different GMSE methods for constructing
coarse-grained time series, specifically using the mean, mean absolute deviation (MAD), standard deviation
(σ), and variance (σ
2
) approaches. By applying these methods, we investigate their efficacy in differentiat-
ing between healthy individuals and PAD patients. Our results demonstrate that the variance coarse-grained
method offers superior discriminatory power, revealing statistically significant differences. These findings
suggest that the variance-based GMSE method is the most effective approach for assessing autonomic imbal-
ance in PAD patients, with potential applications in improving diagnostic tools and treatment strategies.
1 INTRODUCTION
Peripheral Arterial Disease (PAD) is an atheroscle-
rotic condition characterized by the occlusion of ar-
teries located distal to the aortic bifurcation (Ramos
et al., 2009). This arterial blockage reduces the oxy-
gen supply to the lower limb muscles during physi-
cal activity, leading to pain and forcing individuals to
stop walking. This condition, known as intermittent
claudication, often results in significant limitations in
daily physical activities and negatively impacts the
health-related quality of life of those patients (Fein-
glass et al., 1996; Crowther et al., 2007; Celis et al.,
2009). PAD is a chronic and progressive disorder that
can severely restrict an individual’s mobility and inde-
pendence, making it an important public health con-
a
https://orcid.org/0000-0002-7235-3986
b
https://orcid.org/0000-0002-0402-8487
c
https://orcid.org/0000-0002-4444-6755
d
https://orcid.org/0000-0001-9180-1732
cern, particularly among the elderly population.
Atherosclerosis, and consequently PAD, is partic-
ularly prevalent among the elderly population. This
condition is closely associated with several risk fac-
tors, including diabetes mellitus, hypertension, el-
evated body mass index, and dyslipidemia (Ramos
et al., 2009; Diehm et al., 2009). Furthermore, the
risk of developing PAD is further increased by both
current and past smoking habits (Diehm et al., 2009).
Heart Rate Variability (HRV) is a fluctuation in
the time intervals between consecutive heartbeats. It
allows to non-invasively measure the autonomic ner-
vous system regulation of the cardiovascular system.
It provides valuable insight into the balance between
sympathetic and parasympathetic nervous activity,
making it a crucial tool for assessing autonomic func-
tion (Camm et al., 1996; Shaffer and Ginsberg, 2017).
HRV analysis can reveal subtle abnormalities in the
autonomic regulation of the heart, which may have
important implications for understanding the physio-
Barquero-Pérez, O., Goya-Esteban, R., Sarabia-Cachadiña, E. and Naranjo-Orellana, J.
Comparative Analysis of Generalized Multiscale Entropy Methods for Coarse-Grained Time Series Construction in Assessing Autonomic Balance in Peripheral Arterial Disease Patients.
DOI: 10.5220/0013165000003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 893-898
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
893

logical mechanisms underlying various cardiovascu-
lar disorders, including peripheral arterial disease.
While HRV has been extensively studied in var-
ious cardiovascular diseases, and some studies have
examined HRV in peripheral arterial disease patients,
the role of nonlinear characterization of HRV in pre-
dicting intermittent claudication in PAD patients re-
mains underexplored. Traditional HRV analysis of-
ten focuses on linear methods, which may not fully
capture the complex, nonlinear dynamics of the HRV.
Therefore, a nonlinear approach may be required to
reveal more subtle autonomic dysfunctions in PAD
patients and potentially improve our understanding of
the relationship between HRV and symptoms like in-
termittent claudication.
Some previous studies have not found a signif-
icant relationship between heart rate variability and
improvements in walking ability in peripheral arte-
rial disease patients (Leicht et al., 2011; Sandercock
et al., 2007), other research has revealed a positive
association between HRV indices and maximal walk-
ing distance, though not with claudication distance,
in symptomatic PAD individuals (Lima et al., 2016).
These mixed findings suggest that further investiga-
tion is needed to better understand the capacity of
HRV to characterize autonomic dysfunction in PAD
patients and its potential relationship to clinical out-
comes like walking performance.
The primary objective of this study is to in-
vestigate whether resting HRV differs between pa-
tients with intermittent claudication and healthy con-
trol subjects. To achieve this, we will employ Gen-
eralized Multiscale Entropy (GMSE), a nonlinear
analysis method that characterizes the complexity
of physiological time series over multiple temporal
scales (Costa and Goldberger, 2015). We hypothe-
size that GMSE will detect significant differences in
HRV patterns between PAD patients with intermittent
claudication and healthy individuals. This could po-
tentially offer a new strategy for understanding au-
tonomic imbalance in this patient population, which
may have important implications for managing and
monitoring this chronic and debilitating condition.
The structure of the paper is as follows. In Sec-
tion 2, the dataset is explained. In Section 3, GMSE
is explained. In Section 4, the statistical analysis is
explained. In Section 5, results are reported. Finally,
in Section 6, conclusions are presented.
2 DATASET
Fourteen male control individuals (60±5 years old,
90±12 kg, 174±7 cm) and 14 male PAD patients
(64±6 years old, 83±17 kg, 168±7 cm) exhibiting in-
termittent claudication were recruited from two hos-
pitals in Seville, Spain. The control subjects were se-
lected based on the following criteria: absence of car-
diovascular disease, no ongoing medical treatment,
and an ankle-brachial index greater than 1. In con-
trast, the PAD patient group consisted of individuals
referred by the vascular surgery departments of the
participating hospitals, with a confirmed PAD diag-
nosis, no prior surgical interventions, and an ankle-
brachial index less than 0.9 (Schroll and Munck,
1981; Aboyans et al., 2012). All participants, in-
cluding both the control and PAD groups, were non-
smokers and had not taken any cardiovascular-related
medications for at least three months prior to the
study. The participants reported to the laboratory in
the morning, two hours after breakfast, having ab-
stained from caffeine consumption and exercise for
24 hours before the data recording. The RR interval
time series were recorded for 15 minutes at rest in the
supine position using a Firstbeat Bodyguard recorder.
The initial five minutes of each recording were ex-
cluded to allow for participant relaxation (Chidean
et al., 2018; Cachadi
˜
na et al., 2018).
3 GENERALIZED MULTISCALE
ENTROPY FOR HEART RATE
VARIABILITY
GMSE is an advanced nonlinear method used to quan-
tify the complexity of physiological time series across
multiple temporal scales. Traditional entropy mea-
sures, such as Sample Entropy (SampEn), quantify
the unpredictability or irregularity of a time series on
a single scale (Richman and Moorman, 2000). Al-
though effective, these measures may not fully cap-
ture the multiscale nature of physiological signals.
Multiscale Entropy (MSE) extends this approach by
evaluating entropy over a range of scales, providing
a more comprehensive characterization of the under-
lying dynamics (Costa et al., 2005). GMSE further
enhances MSE by allowing for flexible scaling pa-
rameters, which can be adjusted to fit specific types
of data or clinical contexts (Costa and Goldberger,
2015). This type of methods has been used with suc-
cess to characterize long-range correlated cardiovas-
cular an respiratory signals (Martins et al., 2020; Silva
et al., 2015)
In MSE definition, {y
(τ)
j
} where each coarse-
grained time series at scale τ is constructed by av-
eraging non-overlapping blocks of τ consecutive data
points from the original time series {x
i
}. The GMSE
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
894

modifications use different statistical moments to
build the coarse-grained time-series. In this work we
are going to compare the following four approaches:
• Averaging: Each coarse-grained time series is
constructed by averaging non-overlapping blocks
of τ consecutive data points from the original time
series {x
i
}:
y
(τ)
j
=
1
τ
jτ
∑
i=( j−1)τ+1
x
i
, j = 1, 2, . . . ,
N
τ
• Mean Absolute Deviation (MAD): The coarse-
grained time series can also be computed using
the mean absolute deviation of τ consecutive data
points:
y
(τ)
j
=
1
τ
jτ
∑
i=( j−1)τ+1
|x
i
− ˆµ|
where ˆµ is the sample mean of the τ data points in
each block.
• Standard Deviation, σ: Alternatively, each block
of τ data points can be used to compute the stan-
dard deviation:
y
(τ)
j
=
v
u
u
t
1
τ
jτ
∑
i=( j−1)τ+1
(x
i
− ˆµ)
2
• Variance, σ
2
: Finally, the coarse-grained series
can be based on the variance of the data points
within each block:
y
(τ)
j
=
1
τ
jτ
∑
i=( j−1)τ+1
(x
i
− ˆµ)
2
Where N is the length of the original time series,
and τ is the scale factor.
Once the coarse-grained time series are generated,
the entropy of each is calculated using a method such
as Sample Entropy (SampEn):
SampEn(m, r, N) = − ln
A(m + 1)
B(m)
where m is the embedding dimension, r is the toler-
ance (typically a percentage of the time series stan-
dard deviation), and A(m +1) and B(m) represent the
number of matching template vectors of length m + 1
and m, respectively.
GMSE is defined as the entropy measure com-
puted across all scales τ, producing a profile that re-
flects the time series complexity at various temporal
resolutions. This approach is particularly effective in
HRV analysis, where entropy at different scales can
reveal the balance between sympathetic and parasym-
pathetic influences on the heart (Costa and Gold-
berger, 2015).
4 STATISTICAL ANALYSIS
To statistically analyze the differences between the
control and PAD patients groups, we will employ a
combination of bootstrap resampling and exponential
curve fitting to the GMSE curve. This approach will
allow us model the complexity and autonomic bal-
ance of heart rate variability measured by GMSE.
First, we will perform bootstrap resampling with
replacement on the GMSE curves for both the con-
trol and PAD groups. This will generate multiple re-
sampled GMSE curves for each group, allowing us
to compute the average GMSE curve and assess the
variability within each group.
For each average GMSE curve from the bootstrap
resampling, we will then fit an exponential curve us-
ing the equation:
GMSE(τ) = C · e
−κτ
where C and κ are constants of the model deter-
mined by optimization methods. Whereas τ repre-
sents the different time scales.
This exponential curve fitting will allow us to ex-
tract the values of C
c
and C
pad
as well as κ
c
and
κ
pad
for the control and PAD patient groups, respec-
tively, from the bootstrap resampling process. We can
then assess whether there are significant differences
in these exponential model parameters between the
two groups by comparing the distributions of the fitted
model parameters obtained from the bootstrap resam-
pling procedure.
Specifically, this statistical analysis will provide
insights into the underlying differences in the com-
plexity and autonomic balance of heart rate variabil-
ity between the healthy control subjects and the PAD
patients. The exponential curve fitting and compari-
son of the model parameters between the groups can
shed light on the specific alterations in the cardiovas-
cular autonomic regulation associated with peripheral
arterial disease, potentially leading to a better under-
standing of the disease and improved diagnostic or
prognostic tools.
5 RESULTS
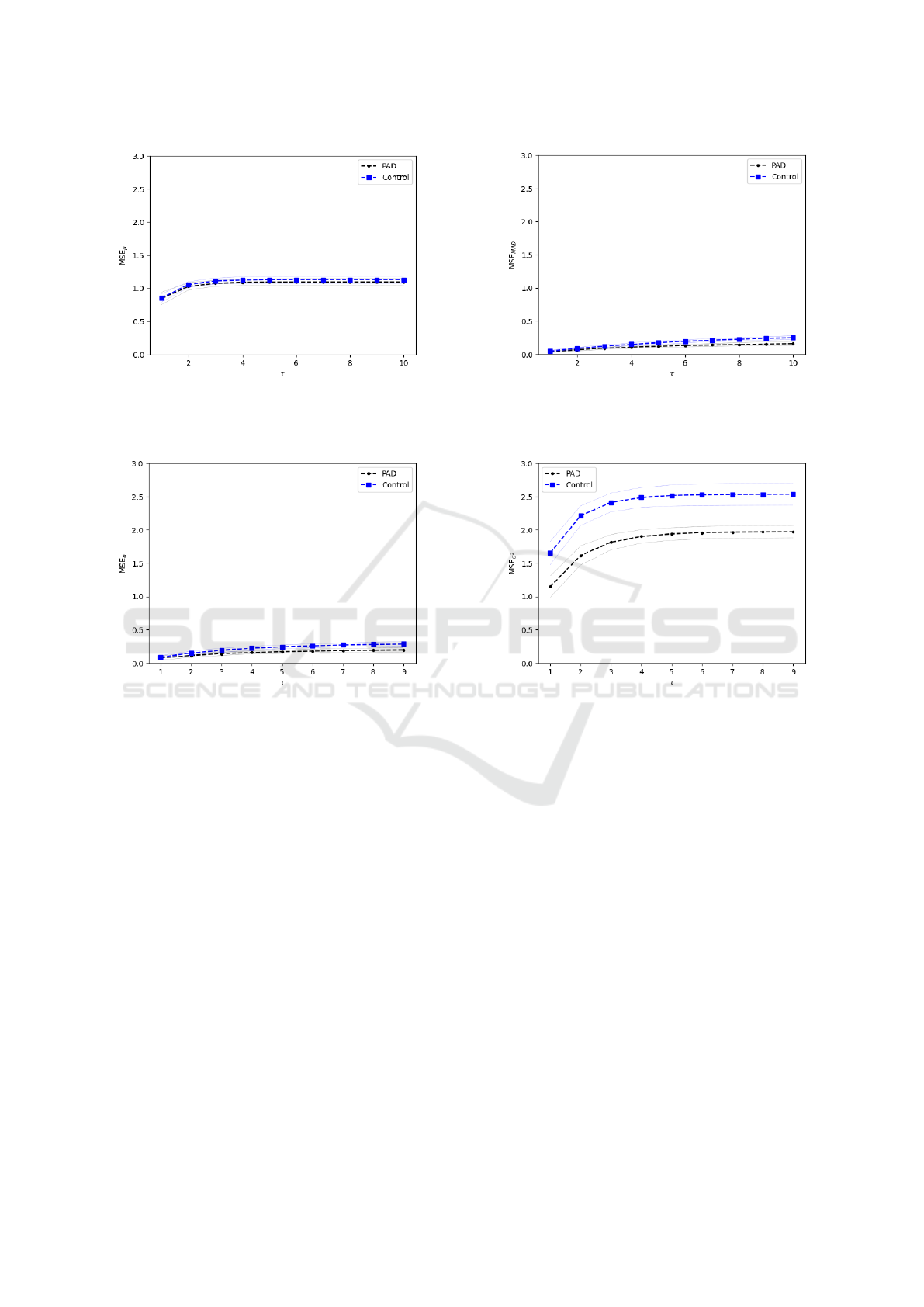
Figure 1 shows the average value and standard de-
viation of the GMSE curve obtained from the boot-
strap resamplings for the different methods to build
the coarse-grained time series, in particular, (a) us-
ing the average MSE
µ
, (b) using the MAD MSE
MAD
,
(c) using the standard deviation MSE
σ
, and (d) using
the variance MSE
σ
2
. From scale two, the curves are
statistically different, showing that GMSE, with vari-
ance, is able to distinguish control subjects from PAD
Comparative Analysis of Generalized Multiscale Entropy Methods for Coarse-Grained Time Series Construction in Assessing Autonomic
Balance in Peripheral Arterial Disease Patients
895
(a) GMSE average values for healthy subjects and PAD
patients, using average to build coarse-grained time se-
ries.
(b) GMSE average values for healthy subjects and PAD
patients, using MAD to build coarse-grained time se-
ries.
(c) GMSE average values for healthy subjects and PAD
patients, using σ to build coarse-grained time series.
(d) GMSE average values for healthy subjects and PAD
patients, using σ
2
to build coarse-grained time series.
Figure 1: GMSE comparison of methods to build the coarse-grained time series for healthy subjects (dots) and PAD patients
(squares) with mean and standard deviation curves obtained from the bootstrap resampling.
patients. It is clear, that using the variance to create
the coarse-grained time series allows to better distin-
guish healthy subjects and PAD patients.
Figure 2 shows the mean and standard deviation
for the C parameter of the exponential model. Among
the methods evaluated for constructing coarse-grained
time series, only the σ
2
approach demonstrates suf-
ficient discriminatory power to reliably differentiate
between healthy individuals and PAD patients, yield-
ing statistically significant differences.
6 CONCLUSIONS
The findings of this study provide significant insights
into the autonomic dysfunction associated with PAD
and its manifestation in patients with intermittent
claudication. By employing GMSE analysis of HRV,
we were able to discern notable differences in auto-
nomic regulation between PAD patients and healthy
controls. This study underscores the utility of non-
linear methods in capturing the complexity of physio-
logical signals, which traditional linear methods may
overlook. In fact, previous studies using linear meth-
ods (both time and frequency domain) and nonlin-
ear (SampEn, DFA) did not show strong differences
in HRV (Cachadi
˜
na et al., 2018; Leicht et al., 2011;
Sandercock et al., 2007; Lima et al., 2016).
Our results indicate that PAD patients exhibit a
reduced complexity in HRV across multiple time
scales, as evidenced by lower GMSE values com-
pared to healthy subjects. This reduction in com-
plexity suggests a diminished adaptability of the au-
tonomic nervous system in PAD patients, potentially
due to chronic ischemic conditions affecting the car-
diovascular system. Our study uniquely contributes
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
896
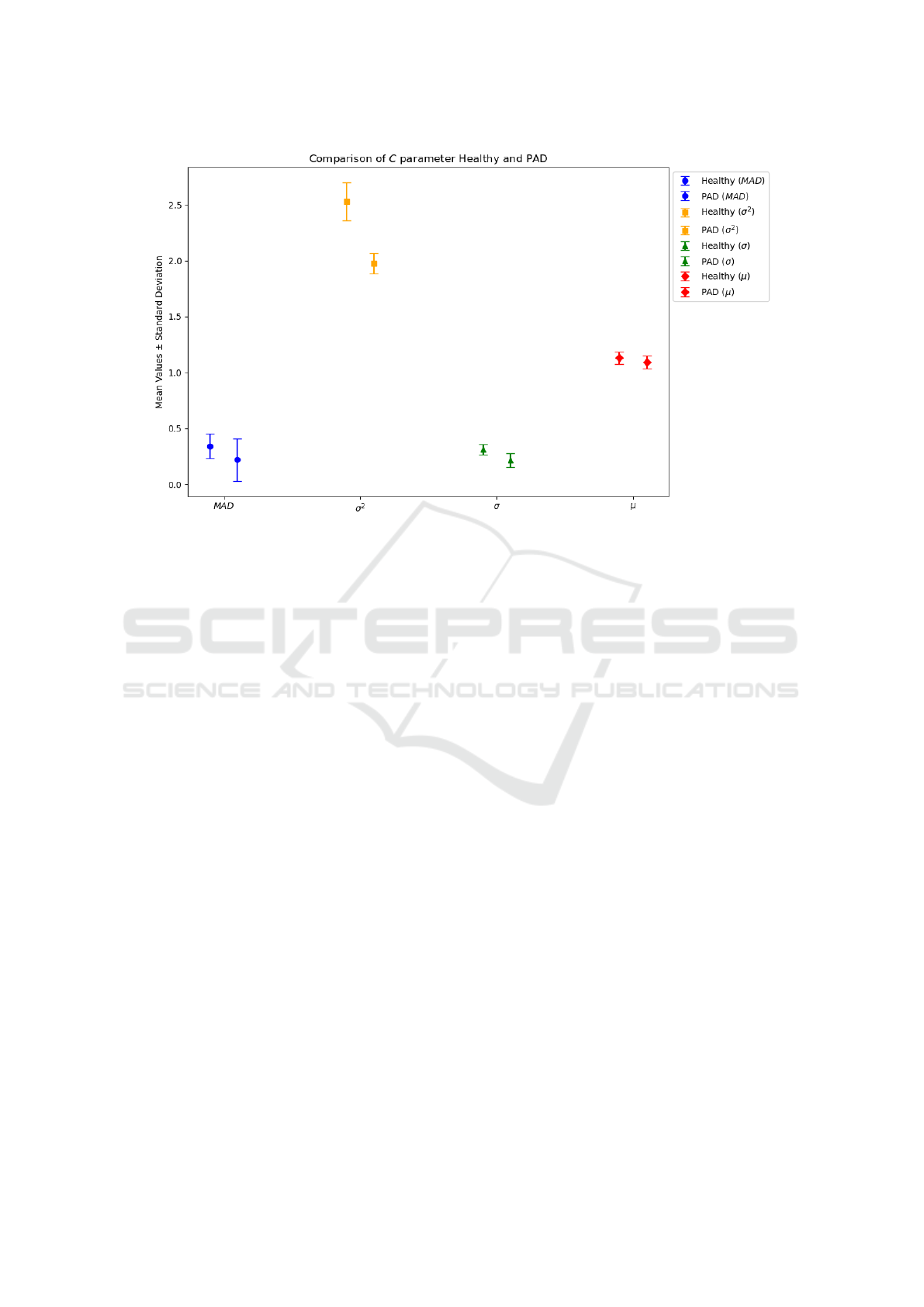
Figure 2: Mean and standard deviation for the C parameter of the exponential model for the different methods to build the
coarse-grained time series in GMSE.
by utilizing a MSE approach to provide a more strong
proof of these alterations. Moreover, among all the
possible ways to build the coarse-grained time series
in GMSE, the best option to be able to distinguish
between healthy and PAD patients is using the σ
2
.
The results indicated a reduction in the complexity of
HRV time series in PAD patients, which is in agree-
ment with previous results.
The clinical implications of these findings are
substantial. The ability to distinguish PAD patients
from healthy individuals based on HRV complexity
could help to the search for new diagnostic tools and
therapeutic strategies aimed at improving autonomic
function in this population. Furthermore, the use of
GMSE as a diagnostic marker could enhance the mon-
itoring of disease progression and the efficacy of in-
terventions aimed at restoring autonomic balance.
However, this study is not without limitations.
The sample size was relatively small, and the study
population was limited to male subjects, which may
affect the results. Future research should aim to in-
clude a larger and more diverse cohort to validate
these results and explore the potential sex-based dif-
ferences in the autonomic dysfunction associated with
PAD. As a future work, it would be worth to com-
pare these results with other multiscale entropy mea-
sures (Bari et al., 2014; Valencia et al., 2009)
In conclusion, this study demonstrates that GMSE
analysis, using σ
2
of HRV is a powerful tool for as-
sessing autonomic dysfunction in patients with Pe-
ripheral Arterial Disease. The significant differ-
ences in HRV complexity between PAD patients and
healthy controls highlight the potential of GMSE as a
diagnostic and monitoring tool in clinical settings.
ACKNOWLEDGEMENTS
This work was partially funded by Grant
PID2022-136887NB-I00 by MCIN/AEI/10.13039/
501100011033.
REFERENCES
Aboyans, V., Criqui, M. H., Abraham, P., Allison, M. A.,
Creager, M. A., Diehm, C., Fowkes, F. G. R., Hiatt,
W. R., J
¨
onsson, B., Lacroix, P., Marin, B., McDer-
mott, M. M., Norgren, L., Pande, R. L., Preux, P.-M.,
Stoffers, H. J., and Treat-Jacobson, D. (2012). Mea-
surement and Interpretation of the Ankle-Brachial In-
dex: A Scientific Statement From the American Heart
Association. Circulation, 126(24):2890–2909.
Bari, V., Valencia, J. F., Vallverd
´
u, M., Girardengo, G.,
Marchi, A., Bassani, T., Caminal, P., Cerutti, S.,
George, A. L., Brink, P. A., Crotti, L., Schwartz, P. J.,
and Porta, A. (2014). Multiscale Complexity Analysis
of the Cardiac Control Identifies Asymptomatic and
Symptomatic Patients in Long QT Syndrome Type 1.
PLoS ONE, 9(4):e93808.
Cachadi
˜
na, E. S., De la Cruz Torres, B., S
´
anchex Sixto,
A., Floria Mart
´
ın, P., Berral de la Rosa, F. J., and
Comparative Analysis of Generalized Multiscale Entropy Methods for Coarse-Grained Time Series Construction in Assessing Autonomic
Balance in Peripheral Arterial Disease Patients
897

Naranjo Orellana, J. (2018). Heart Rate Variability
is Lower in Patients with Intermittent Claudication: A
Preliminary Study. Archivos de medicina del deporte,
35(186):218–221.
Camm, A. J., Malik, M., Bigger, J., Breithardt, G., Cerutti,
S., Cohen, R. J., Coumel, P., Fallen, E. L., Kennedy,
H. L., Kleiger, R. E., et al. (1996). Heart Rate Variabil-
ity: Standards of Measurement, Physiological Inter-
pretation and Clinical Use. Circulation, 93(5):1043–
1065.
Celis, R., Pipinos, I. I., Scott-Pandorf, M. M., Myers, S. A.,
Stergiou, N., and Johanning, J. M. (2009). Peripheral
Arterial Disease Affects Kinematics during Walking.
Journal of Vascular Surgery, 49(1):127–132.
Chidean, M. I., Barquero-P
´
erez, O., Goya-Esteban,
R., S
´
anchez Sixto, A., De La Cruz Torres, B.,
Naranjo Orellana, J., Sarabia Cachadi
˜
na, E., and
Caama
˜
no, A. J. (2018). Full Band Spectra Analysis of
Gait Acceleration Signals for Peripheral Arterial Dis-
ease Patients. Frontiers in Physiology, 9:1061.
Costa, M. and Goldberger, A. (2015). Generalized Mul-
tiscale Entropy Analysis: Application to Quantifying
the Complex Volatility of Human Heartbeat Time Se-
ries. Entropy, 17(3):1197–1203.
Costa, M., Goldberger, A. L., and Peng, C.-K. (2005). Mul-
tiscale Entropy Analysis of Biological Signals. Phys-
ical Review E, 71(2):021906.
Crowther, R. G., Spinks, W. L., Leicht, A. S., Quigley,
F., and Golledge, J. (2007). Relationship between
Temporal-Spatial Gait Parameters, Gait Kinematics,
Walking Performance, Exercise Capacity, and Phys-
ical Activity Level in Peripheral Arterial Disease.
Journal of Vascular Surgery, 45(6):1172–1178.
Diehm, C., Allenberg, J. R., Pittrow, D., Mahn, M., Tepohl,
G., Haberl, R. L., Darius, H., Burghaus, I., and Tramp-
isch, H. J. (2009). Mortality and Vascular Mor-
bidity in Older Adults With Asymptomatic Versus
Symptomatic Peripheral Artery Disease. Circulation,
120(21):2053–2061.
Feinglass, J., McCarthy, W. J., Slavensky, R., Manheim,
L. M., Martin, G. J., and the Chicago Claudication
Outcomes Research Group (1996). Effect of Lower
Extremity Blood Pressure on Physical Functioning in
Patients who Have Intermittent Claudication. Journal
of Vascular Surgery, 24(4):503–512.
Leicht, A. S., Crowther, R. G., and Golledge, J. (2011). In-
fluence of Peripheral Arterial Disease and Supervised
Walking on Heart Rate Variability. Journal of Vascu-
lar Surgery, 54(5):1352–1359.
Lima, A., Soares, A., Cucato, G., Leicht, A., Franco, F.,
Wolosker, N., and Ritti-Dias, R. (2016). Walking Ca-
pacity Is Positively Related with Heart Rate Variabil-
ity in Symptomatic Peripheral Artery Disease. Euro-
pean Journal of Vascular and Endovascular Surgery,
52(1):82–89.
Martins, A., Pernice, R., Amado, C., Rocha, A. P., Silva,
M. E., Javorka, M., and Faes, L. (2020). Multivariate
and Multiscale Complexity of Long-Range Correlated
Cardiovascular and Respiratory Variability Series. En-
tropy, 22(3):315.
Ramos, R., Quesada, M., Solanas, P., Subirana, I., Sala, J.,
Vila, J., Masi
´
a, R., Cerezo, C., Elosua, R., Grau, M.,
Cord
´
on, F., Juviny
`
a, D., Fit
´
o, M., Isabel Covas, M.,
Clar
`
a, A.,
´
Angel Mu
˜
noz, M., and Marrugat, J. (2009).
Prevalence of Symptomatic and Asymptomatic Pe-
ripheral Arterial Disease and the Value of the Ankle-
brachial Index to Stratify Cardiovascular Risk. Euro-
pean Journal of Vascular and Endovascular Surgery,
38(3):305–311.
Richman, J. S. and Moorman, J. R. (2000). Physi-
ological Time-Series Analysis Using Approximate
Entropy and Sample Entropy. American Jour-
nal of Physiology-Heart and Circulatory Physiology,
278(6):H2039–H2049.
Sandercock, G. R. H., Hodges, L. D., Das, S. K., and
Brodie, D. A. (2007). The Impact of Short Term Su-
pervised and Home-Based Walking Programmes on
Heart Rate Variability in Patients with Peripheral Ar-
terial D. Journal of Sports Science and Medicine,
(6):471–476.
Schroll, M. and Munck, O. (1981). Estimation of Periph-
eral Arteriosclerotic Disease by Ankle Blood Pres-
sure Measurements in a Population Study of 60-Year-
Old Men and Women. Journal of Chronic Diseases,
34(6):261–269.
Shaffer, F. and Ginsberg, J. P. (2017). An Overview of Heart
Rate Variability Metrics and Norms. Frontiers in Pub-
lic Health, 5:258.
Silva, L. E. V., Cabella, B. C. T., Neves, U. P. D. C., and
Murta Junior, L. O. (2015). Multiscale entropy-based
methods for heart rate variability complexity analysis.
Physica A: Statistical Mechanics and its Applications,
422:143–152.
Valencia, J. F., Porta, A., Vallverdu, M., Claria, F., Bara-
nowski, R., Orlowska-Baranowska, E., and Caminal,
P. (2009). Refined Multiscale Entropy: Application to
24-h Holter Recordings of Heart Period Variability in
Healthy and Aortic Stenosis Subjects. IEEE Transac-
tions on Biomedical Engineering, 56(9):2202–2213.
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
898
