
On the Detection of Retinal Image Synthesis Obtained Through
Generative Adversarial Network
Marcello Di Giammarco
1,2
, Antonella Santone
3
,
Mario Cesarelli
4
, Fabio Martinelli
5
and Francesco Mercaldo
1,3
1
Institute for Informatics and Telematics (IIT), National Research Council of Italy (CNR), Pisa, Italy
2
Department of Information Engineering, University of Pisa, Pisa, Italy
3
Department of Medicine and Health Sciences “Vincenzo Tiberio”, University of Molise, Campobasso, Italy
4
Department of Engineering, University of Sannio, Benevento, Italy
5
Institute for High Performance Computing and Networking (ICAR), National Research Council of Italy (CNR), Rende
(CS), Italy
{marcello.digiammarco, francesco.mercaldo}@iit.cnr.it, {antonella.santone, francesco mercaldo}@unimol.it,
Keywords:
Adversarial Machine Learning, Generative Adversarial Networks, Deep Learning, Retinal Imaging,
Robustness, Security.
Abstract:
Adversarial machine learning on medical imaging is one of the many applications for which the evaluation
of Generative Adversarial Networks in the medical field has demonstrated remarkable interest. This paper
proposes a method in which Convolutional neural Networks are trained and tested on the binary classification
of real and fake images, generated through generative adversarial networks. In this paper, the considered
experiments are on the RGB fundus retina images of the human eye. Results highlight networks with optimal
performance, and completely recognize real/fake classification; however, on the other hand, other networks
mis-classify the images, enhancing security and reliability problems.
1 INTRODUCTION
Generative Adversaries Networks (GANs) are an ex-
citing recent innovation in machine learning. GANs
are generative models: they create new instances of
data similar to your training data. The number of
novel GAN concepts, methods, and applications has
increased significantly the interest to these systems
This huge success is attributed to the high similar-
ity of the generated images to the real ones (Good-
fellow et al., 2020). For the diagnosis and identifi-
cation of diseases, medical imaging is crucial (Pan
and Xin, 2024), (Huang et al., 2024), (Zhou et al.,
2023), (Brunese et al., 2022b). Furthermore, research
on artificial intelligence using such images could po-
tentially have a beneficial effect on improvements in
healthcare. One of the challenges of machine learn-
ing in biomedical imaging regards the discrimination
ability between real and fake images, which can al-
ter the performance and invalidate the results. To ad-
dress this challenge, the fake images can be generated
with GANs. Moreover, GANs reveal their features in
many applications in healthcare and biomedical do-
mains, from data augmentation to anomaly detection
passing through medical image synthesis.
In this paper we propose a method able to rec-
ognize the real/fake classification. Fake images are
generated at different epochs of the GAN consider-
ing RGB fundus retina image datasets. In the ex-
periments, resistance and mis-classifications of the
models in the real/fake discriminations are obtained.
Moreover, the paper analyzed also trends of the out-
put model increasing the epochs of the GAN, showing
interesting results.
In the next section, is reported the related works;
while a brief overview of the GAN and its working
principles is provided in Section 3. The proposed
method, applied in the RGB retinal images, is pre-
sented in Section 4, followed by the experimental
analysis and results in Section 5. Lastly, a conclu-
sion and future research goals are shown in the last
section.
Di Giammarco, M., Santone, A., Cesarelli, M., Martinelli, F. and Mercaldo, F.
On the Detection of Retinal Image Synthesis Obtained Through Generative Adversarial Network.
DOI: 10.5220/0013228300003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 611-618
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
611

2 RELATED WORKS
A review of the literature on GAN use in medical field
is taken advantage of in this section.
The review of Singh et al.(Singh and Raza, 2021)
reports several GAN applications in the medical field.
The most recent developments in GAN-based clinical
applications for cross-modality synthesis and medi-
cal image production are presented in this chapter.
Deep convolutional GAN (DCGAN), Laplacian GAN
(LAPGAN), pix2pix, CycleGAN, and unsupervised
image-to-image translation model (UNIT) are among
the GAN frameworks that have become popular for
medical image interpretation. They continue to en-
hance their performance by adding more hybrid ar-
chitecture.
Recent work is represented by Sai Khal et al.
(Akhil et al., 2024). In their work, authors in-
vestigated the synthesis of realistic and superior
chest X-ray pictures using DCGAN. Their DCGAN
model generated synthetic images that exhibited re-
markable visual resemblances to real chest X-ray
images, including anatomical characteristics, radio-
graphic noise, and disease patterns. They also em-
ployed a range of evaluation metrics to statistically
examine the diversity and realism of the generated
images, demonstrating that their approach produced
incredibly realistic synthetic data.
Looking for the retinal imaging domain, in (Diaz-
Pinto et al., 2019) authors proposed a novel retinal im-
age synthesizer and a semi-supervised learning tech-
nique for glaucoma evaluation based on deep con-
volutional GANs was innovative. Furthermore, their
system is trained on an unparalleled quantity of pub-
licly accessible photos (86926 images), as far as we
are aware. As a result, their technology can automat-
ically offer labels in addition to producing synthetic
visuals.
In terms of security, the research of Mirsky et al.
(Mirsky et al., 2019) represents a great example of
the DCGAN application. In their article, they demon-
strated how an attacker can modify or delete medi-
cal condition evidence from volumetric (3D) medi-
cal scans using deep learning. This could be done
by an attacker to thwart a political candidate, ruin re-
search, perpetrate insurance fraud, carry out terror-
ism, or even kill someone. The authors demonstrated
how to automate the framework (CT-GAN) and used
a 3D conditional GAN to execute the assault. They
concentrated on injecting and extracting lung cancer
from CT scans to assess the attack. Additionally, they
investigated the attack surface of a contemporary radi-
ology network and illustrated one attack vector: they
used a clandestine penetration test to intercept and al-
ter CT scans on an operational hospital network.
In the work of Nagaraju et al. (Nagaraju and
Stamp, 2022), authors presented a method that em-
ployed GANs to create fake malware images and eval-
uate how well different classification methods worked
for these images. Their results demonstrated that
while the resulting multiclass classification problem
is challenging, they were able to obtain convincing
results when they restricted the problem to differenti-
ating between authentic and fraudulent samples. The
paper’s main finding is that, from a deep learning
standpoint, GAN-generated images fall short of deep
fake malware images, even if they may resemble real
malware images quite a bit.
3 GAN BACKGROUND
Since the proposed method focuses on the GAN, Fig-
ure 1 reports a block schematization of the GAN op-
eration.
A GAN main working principle is based on a
framework for competitive learning that combines
two neural networks: the discriminator and the gen-
erator. By learning to map the latent space to the data
space, the generator creates synthetic data samples
that resemble the real data distribution given random
noise (latent vectors) as input. It produces low-quality
samples at first, but with training, it improves its abil-
ity to produce more realistic samples. In order to
differentiate between actual and GAN-generated data
samples, the discriminator works as a binary classi-
fier. It learns to give real samples high probability and
fraudulent samples low probabilities. The discrimi-
nator may function randomly at first, but it learns to
distinguish between real and fraudulent samples with
practice. The discriminator and generator are simulta-
neously and competitively trained during the training
procedure. The generator’s main objective is to trick
the discriminator by producing samples that are indis-
tinguishable from actual data. The discriminator, on
the other hand, seeks to distinguish between authen-
tic and fraudulent samples correctly. Both networks
gradually improve during the training: the discrimina-
tor gets better at distinguishing real samples from fake
ones, and the generator enhances at producing real
examples. The discriminator and generator engage
in an adversarial interaction in which the discrimi-
nator aims to maximize its ability to distinguish real
from fake samples, while the generator tries to mini-
mize the discriminator’s ability to distinguish real and
synthetic samples by producing increasingly authen-
tic samples. When the generator generates samples
that are statistically comparable to actual data and the
BIOINFORMATICS 2025 - 16th International Conference on Bioinformatics Models, Methods and Algorithms
612
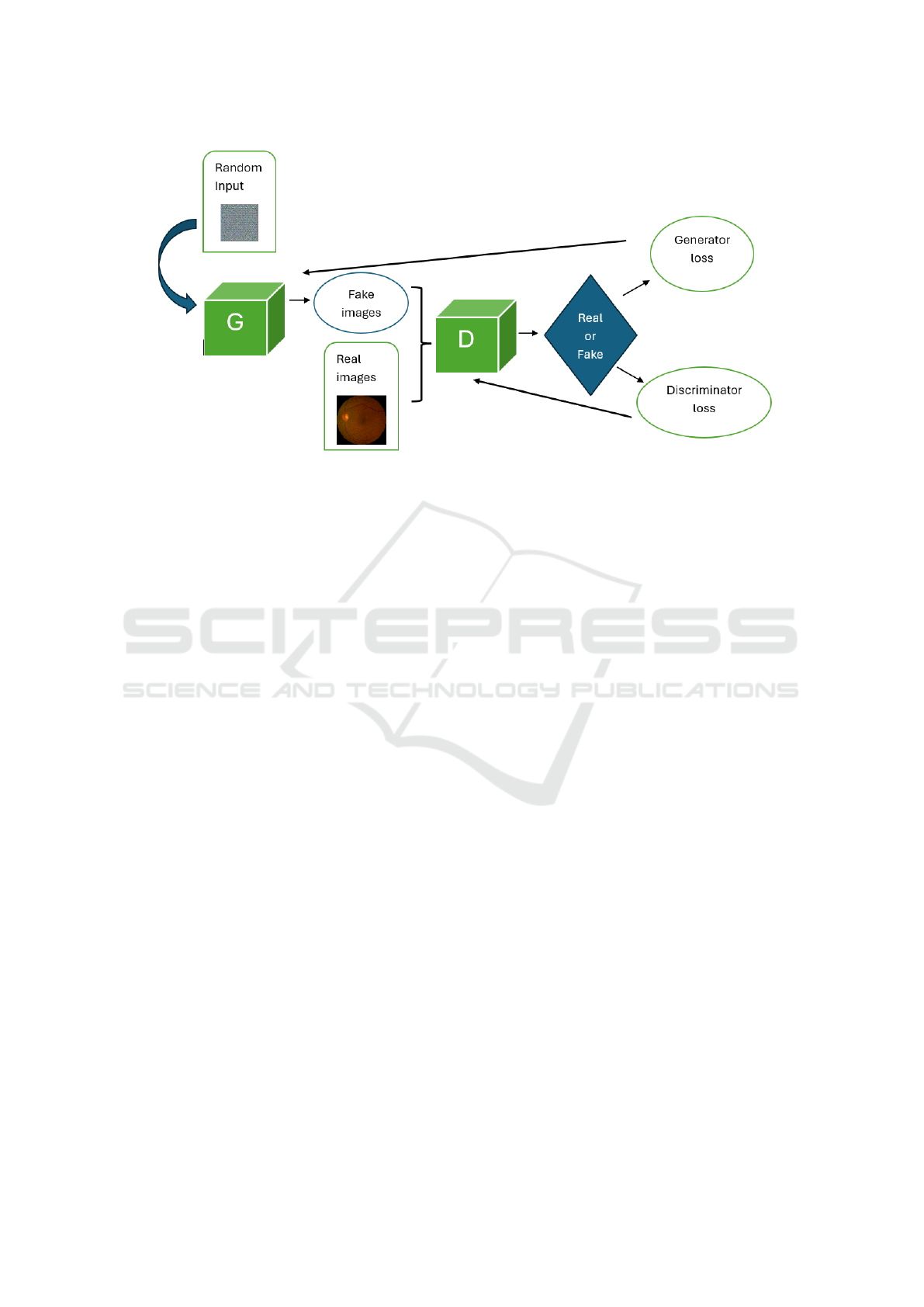
Figure 1: Block schematization of GAN.
discriminator finds it difficult to consistently distin-
guish between the original and GAN-generated sam-
ples, this adversarial process results in a Nash equi-
librium. When the discriminator is unable to differen-
tiate between synthetic and genuine samples more ac-
curately than chance, and the generator produces sam-
ples that are exactly like real data, the GAN has ide-
ally reached convergence. Convergence can be diffi-
cult to achieve and requires careful tweaking of train-
ing procedures, network architectures, and hyperpa-
rameters. The adversarial training dynamics between
the discriminator and generator, which result in the
creation of high-quality synthetic data, are essentially
the fundamental working principle of a GAN (You
et al., 2022; Wang et al., 2023; Singh and Raza, 2020).
This behavior determines a metric for how well
the generator is producing accurate data with gener-
ator loss. The difference between the generated and
actual data is used to calculate this loss. Rather, dis-
criminator loss is a metric that quantifies the discrimi-
nator’s ability to distinguish between created and gen-
uine data. It is calculated using the discriminator’s ac-
curacy in identifying authentic or fraudulent samples.
(Mercaldo et al., 2024b)
Following this broad overview, we consider a par-
ticular kind of GAN known as DCGAN (Deep Convo-
lutional Generative Adversarial Network (Fang et al.,
2018; Liu et al., 2022)) in our work. By adding
deep convolutional neural networks, DCGAN is an
expansion of the GAN concept, although the working
principles are the same as previously shown. Com-
pared to previous GAN architectures, DCGANs can
generate high-quality images, which has led to their
widespread application for tasks like image produc-
tion, super-resolution, and style transfer. By utilizing
convolutional layers (Brunese et al., 2022a), (Mar-
tinelli et al., 2022),(Mercaldo et al., 2024a), (Di Gi-
ammarco et al., 2024a), (Mercaldo et al., 2022), hier-
archical feature learning, and architectural improve-
ments like batch normalization and Leaky ReLU, the
DCGAN architecture strongly improves the quality of
generated images.
4 THE METHOD
In this section, our method is presented and exploited.
The method is based on the discrimination between
GAN-generated images and real ones in the biomed-
ical environment and aims to obtain an output model
able to distinguish those biomedical images.
RGB images of the fundus retina represent the
case study, but the concept behind is available for
any biomedical imaging. The main steps are shown
in Figure 2.
The approach starts from the dataset. The dataset
consists of an RGB fundus retina and is called the
original dataset. This dataset is the ”real dataset”
cited in Figure 1 and several generations of fake im-
ages, varying the number of epochs, are taken into
account for the evaluation goal of the CNNs. In Fig-
ure 2 are reported the epochs, from 250 to 500; and
the GAN-generated fake images samples more and
more similar to the original ones as the epochs in-
crease. 2,000 images are obtained at the end of the
adversarial image generation, beginning with the first
epoch where the distortion is fully superimposed on
the input image and ending with the last epoch where
the distortion is undetectable to medics. In this sense,
On the Detection of Retinal Image Synthesis Obtained Through Generative Adversarial Network
613

Figure 2: Main steps of the case study on RGB retinal images.
the main focus of our study is on the results of the
DL network classification, specifically how well these
networks discriminate between real and fake images.
The selected epochs for the CNNs evaluation are:
250, 300, 350, 400, 450 and 500 Each of these sets
of fake images and the original samples are the in-
puts of the CNNs, which aim to distinguish real and
fake retinal images. The networks under analysis are
the following: Standard CNN (Di Giammarco et al.,
2022a), (Di Giammarco et al., 2022b), InceptionV3
(Xia et al., 2017), Resnet50 (He et al., 2016), and the
MobileNet (Howard et al., 2017). The networks are
evaluated on the main metrics: accuracy, precision,
recall, and loss. The main attention regards the ac-
curacy trends over the GAN-epochs. In other words,
for the previous networks, the CNNs evaluation con-
cerns whether the accuracy was reduced or not after
the classifications of the set of fake images at different
GAN-epochs.
5 RESULTS AND DISCUSSION
In this section, the cited experiments on the dataset
are conducted and reported.
The dataset under analysis is the RetinaMNIST
reported at the following link:
1
. Going into detail,
this dataset consists of 2.000 images of RGB fundus
retina.
The mentioned networks are trained and evaluated
in a binary classification throughout several DCGAN
epochs generation in the experimental analysis sec-
tion to differentiate real from fake retinal images.
For the training, validation, and testing sets, the
two classes—the real and the fake—are split at an
80-10-10 rate following each GAN image genera-
tion. The following hyper-parameter combinations
are used to train and test the datasets on the four net-
works listed above 50 epochs, 32 batch size, 0.0001
learning rate, and the input size as an image, or
64x64x3. This combination is the average combina-
tion for all networks, based on multiple testing and
outcomes. Tables 1, 2, 3 and 4 report the evaluation
1
https://medmnist.com/
BIOINFORMATICS 2025 - 16th International Conference on Bioinformatics Models, Methods and Algorithms
614

of the metrics in terms of accuracy precision, recall,
F-Measure and Area Under the Curve (AUC) for the
four CNNs, respectevely.
A list of considerations through the evaluation of
the results is reported below:
• Standard CNN, network developed by the authors
(Di Giammarco et al., 2022b) shows the best re-
sult. Metrics such as accuracy, precision and re-
call maintain the maximum values in all the DC-
GAN epochs, meaning that this network com-
pletely recognizes the real and the fake images.
Also in the RGB case, the Standard CNN obtains
the most reliable output model, such as the previ-
ous work with the grayscale retinal images (Di Gi-
ammarco et al., 2024b).
• Different behavior is for the MobileNet. The net-
work indeed distinguishes between the real and
the GAN-generated images, but it is possible to
observe that the loss gradually enhances accord-
ing to the number of DCGAN epochs. This be-
havior will produce misclassification when the
DCGAN epochs are enhanced. However, in our
study (until 500 DCGAN epochs), the MobileNet
model correctly recognized real and fake images.
• Finally, the ResNet50 and the InceptionV3
strongly decrease their performance as the DC-
GAN epochs increase. In particular, these de-
creasing trends are evident from 400 to 500
epochs, within this latter situation the networks
fail the classification, and are not able to distin-
guish real and fake images.
A better view of these two trends for the ResNet50
and InceptionV3 is reported in Figures 3 and 4.
The two figures show the accuracy-epochs trend
of the ResNet50 and the InceptionV3. The plot 3 re-
veals that the accuracy quickly drops from 450 to 500
DCGAN epochs, while the int the InceptionV3 plot,
shows in Figure 4 the main decreasing is between 400
and 450, and report the 0,5 at 500 DCGAN epochs.
In the output, networks such as Standard CNN
and MobileNet correctly recognize the images, im-
possible to distinguish with a human eye analysis. In
this way, the output model can be applied to medi-
cal imaging instruments with deep learning prediction
implementation. These models represent, for any dis-
ease retinal image classification, the best solution to
prevent possible attacks and to improve reliability and
credibility. On the other hand, CNNs like ResNet50
and InceptionV3 with high values of DCGAN epochs,
which means fake images are very similar to the orig-
inal ones, not recognize the real and the fake images.
This behavior does not guarantee reliability on possi-
ble predictions, with high risk on the diagnosis proce-
dure and, consequentially, on the patient’s health.
The optimal response of the Standard CNN with
the retinal images is confirmed also in the diabetic
retinopathy classifications reported in the paper (Mer-
caldo et al., 2023).
6 CONCLUSIONS AND FUTURE
WORKS
In conclusion, in this work, we present a general
method to discriminate between real and fake images
generated with the DCGAN to enhance data security
and reliability. Being extendable on each biomedical
imaging technique, the authors decide to analyze the
RGB retinal images case. From the four CNNs in the
experiment, two of these i.e. Standard
CNN and Mo-
bileNet, distinguish the real/fake task, and generate an
output model optimal for automated AI-based imag-
ing devices. The other two networks represent a bad
choice for the same imaging devices, with the pos-
sibility of altering results including GAN-generated
images in the testing folder, drastically reducing the
reliability.
For future works, authors will exploit additional
GANs for testing the CNNs. Another future work is
related to the application of federated machine learn-
ing for data privacy reasons.
ACKNOWLEDGEMENTS
This work has been partially supported by EU DUCA,
EU CyberSecPro, SYNAPSE, PTR 22-24 P2.01 (Cy-
bersecurity) and SERICS (PE00000014) under the
MUR National Recovery and Resilience Plan funded
by the EU - NextGenerationEU projects, by MUR -
REASONING: foRmal mEthods for computAtional
analySis for diagnOsis and progNosis in imagING -
PRIN, e-DAI (Digital ecosystem for integrated anal-
ysis of heterogeneous health data related to high-
impact diseases: innovative model of care and re-
search), Health Operational Plan, FSC 2014-2020,
PRIN-MUR-Ministry of Health, the National Plan for
NRRP Complementary Investments D
∧
3 4 Health:
Digital Driven Diagnostics, prognostics and therapeu-
tics for sustainable Health care, Progetto MolisCTe,
Ministero delle Imprese e del Made in Italy, Italy,
CUP: D33B22000060001, FORESEEN: FORmal
mEthodS for attack dEtEction in autonomous driv-
iNg systems CUP N.P2022WYAEW and ALOHA: a
framework for monitoring the physical and psycho-
logical health status of the Worker through Object de-
On the Detection of Retinal Image Synthesis Obtained Through Generative Adversarial Network
615

Table 1: Metrics evaluation for the Standard CNN.
Metrics 250 Epochs 300 Epochs 350 Epochs 400 Epochs 450 Epochs 500 Epochs
Accuracy 1.0 1.0 1.0 1.0 1.0 1.0
Precision 1.0 1.0 1.0 1.0 1.0 1.0
Recall 1.0 1.0 1.0 1.0 1.0 1.0
F-Measure 1.0 1.0 1.0 1.0 1.0 1.0
AUC 1.0 1.0 1.0 1.0 1.0 1.0
Loss 0.0 0.0 0.0 0.0 0.0 0.0
Table 2: Metrics evaluation for the MobileNet.
Metrics 250 Epochs 300 Epochs 350 Epochs 400 Epochs 450 Epochs 500 Epochs
Accuracy 1.0 1.0 1.0 1.0 1.0 1.0
Precision 1.0 1.0 1.0 1.0 1.0 1.0
Recall 1.0 1.0 1.0 1.0 1.0 1.0
F-Measure 1.0 1.0 1.0 1.0 1.0 1.0
AUC 1.0 1.0 1.0 1.0 1.0 1.0
Loss 1.24x10
−7
4.91x10
−7
1.18x10
−6
5.43x10
−6
9.85x10
−6
2.23x10
−5
Table 3: Metrics evaluation for the ResNet50.
Metrics 250 Epochs 300 Epochs 350 Epochs 400 Epochs 450 Epochs 500 Epochs
Accuracy 1.0 1.0 1.0 0.970 0.997 0.5
Precision 1.0 1.0 1.0 0.970 0.997 0.5
Recall 1.0 1.0 1.0 0.970 0.997 0.5
F-Measure 1.0 1.0 1.0 0.970 0.997 0.5
AUC 1.0 1.0 1.0 0.982 0.999 0.497
Loss 1.24x10
−7
1.35x10
−5
6.0x10
−4
3.74x10
−3
2.358 4.389
Table 4: Metrics evaluation for the InceptionV3.
Metrics 250 Epochs 300 Epochs 350 Epochs 400 Epochs 450 Epochs 500 Epochs
Accuracy 1.0 1.0 0.995 0.950 0.600 0.5
Precision 1.0 1.0 0.995 0.950 0.600 0.5
Recall 1.0 1.0 0.995 0.950 0.600 0.5
F-Measure 1.0 1.0 0.995 0.950 0.600 0.5
AUC 1.0 1.0 0.999 0.965 0.624 0.5
Loss 7.28x10
−3
1.02x10
−2
4.22x10
−2
7.81x10
−2
0.673 0.693
tection and federated machine learning, Call for Col-
laborative Research BRiC -2024, INAIL.
REFERENCES
Akhil, M. S., Sharma, B. S., Kodipalli, A., and Rao, T.
(2024). Medical image synthesis using dcgan for chest
x-ray images. In 2024 International Conference on
Knowledge Engineering and Communication Systems
(ICKECS), volume 1, pages 1–8. IEEE.
Brunese, L., Brunese, M. C., Carbone, M., Ciccone, V.,
Mercaldo, F., and Santone, A. (2022a). Automatic
pi-rads assignment by means of formal methods. La
radiologia medica, pages 1–7.
Brunese, L., Mercaldo, F., Reginelli, A., and Santone, A.
(2022b). A neural network-based method for respira-
tory sound analysis and lung disease detection. Ap-
plied Sciences, 12(8):3877.
Di Giammarco, M., Dukic, B., Martinelli, F., Cesarelli, M.,
Ravelli, F., Santone, A., and Mercaldo, F. (2024a). Re-
liable leukemia diagnosis and localization through ex-
plainable deep learning. In 2024 Fifth International
Conference on Intelligent Data Science Technologies
and Applications (IDSTA), pages 68–75. IEEE.
Di Giammarco, M., Iadarola, G., Martinelli, F., Mercaldo,
BIOINFORMATICS 2025 - 16th International Conference on Bioinformatics Models, Methods and Algorithms
616
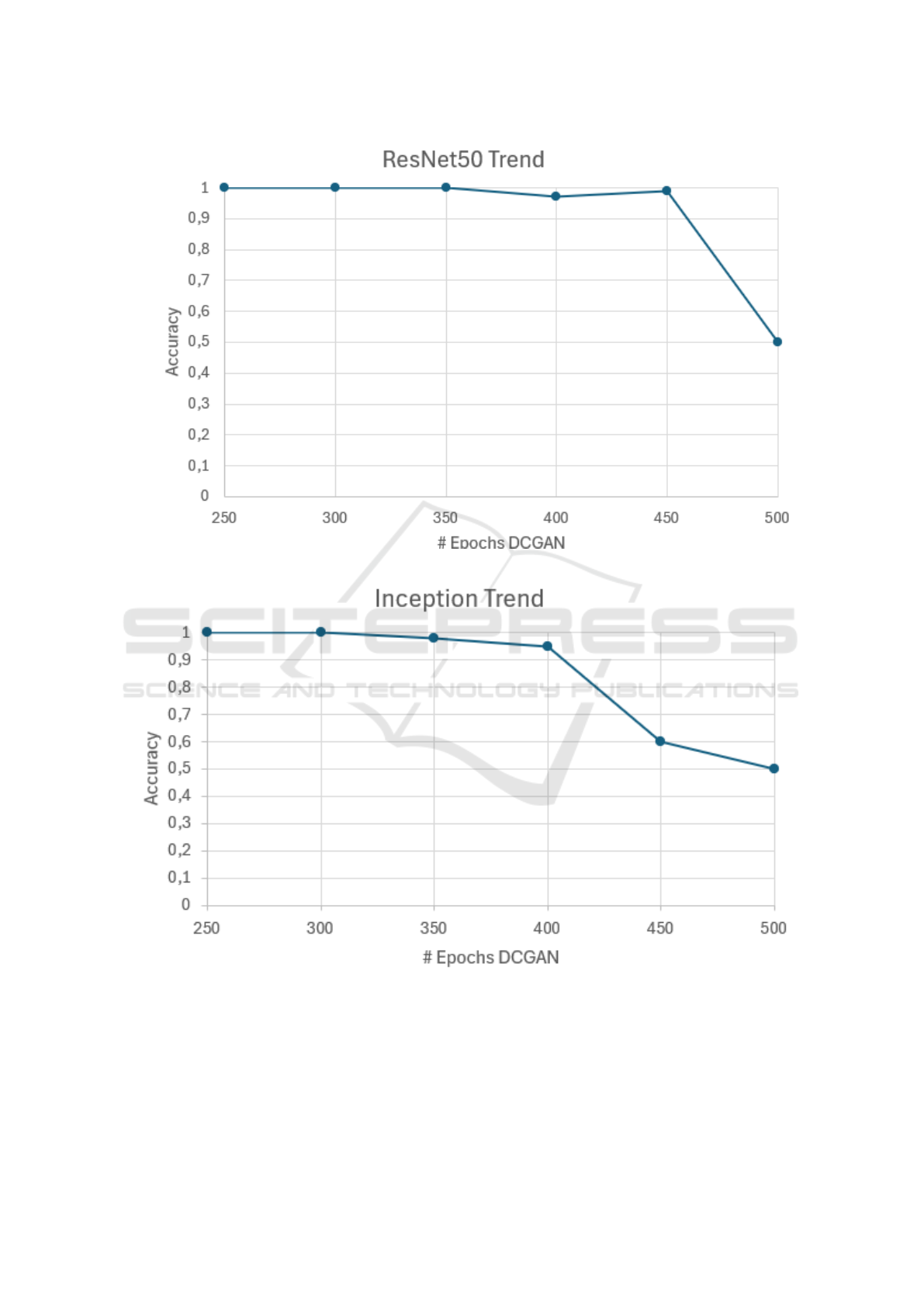
Figure 3: Accuracy-epochs trends of the ResNet50.
Figure 4: Accuracy-epochs trends of the InceptionV3.
On the Detection of Retinal Image Synthesis Obtained Through Generative Adversarial Network
617

F., Ravelli, F., and Santone, A. (2022a). Explainable
deep learning for alzheimer disease classification and
localisation. In International Conference on Applied
Intelligence and Informatics, 1-3 September, Reggio
Calabria, Italy. in press.
Di Giammarco, M., Iadarola, G., Martinelli, F., Mercaldo,
F., and Santone, A. (2022b). Explainable retinopathy
diagnosis and localisation by means of class activation
mapping. In 2022 International Joint Conference on
Neural Networks (IJCNN), pages 1–8. IEEE.
Di Giammarco, M., Santone, A., Cesarelli, M., Martinelli,
F., and Mercaldo, F. (2024b). Evaluating deep learn-
ing resilience in retinal fundus classification with gen-
erative adversarial networks generated images. Elec-
tronics, 13(13):2631.
Diaz-Pinto, A., Colomer, A., Naranjo, V., Morales, S., Xu,
Y., and Frangi, A. F. (2019). Retinal image syn-
thesis and semi-supervised learning for glaucoma as-
sessment. IEEE transactions on medical imaging,
38(9):2211–2218.
Fang, W., Zhang, F., Sheng, V. S., and Ding, Y. (2018).
A method for improving cnn-based image recogni-
tion using dcgan. Computers, Materials & Continua,
57(1).
Goodfellow, I., Pouget-Abadie, J., Mirza, M., Xu, B.,
Warde-Farley, D., Ozair, S., Courville, A., and Ben-
gio, Y. (2020). Generative adversarial networks. Com-
munications of the ACM, 63(11):139–144.
He, K., Zhang, X., Ren, S., and Sun, J. (2016). Deep resid-
ual learning for image recognition. In Proceedings of
the IEEE conference on computer vision and pattern
recognition, pages 770–778.
Howard, A. G., Zhu, M., Chen, B., Kalenichenko, D.,
Wang, W., Weyand, T., Andreetto, M., and Adam,
H. (2017). Mobilenets: Efficient convolutional neu-
ral networks for mobile vision applications. arXiv
preprint arXiv:1704.04861.
Huang, P., Xiao, H., He, P., Li, C., Guo, X., Tian, S.,
Feng, P., Chen, H., Sun, Y., Mercaldo, F., et al.
(2024). La-vit: A network with transformers con-
strained by learned-parameter-free attention for in-
terpretable grading in a new laryngeal histopathol-
ogy image dataset. IEEE Journal of Biomedical and
Health Informatics.
Liu, B., Lv, J., Fan, X., Luo, J., and Zou, T. (2022). Ap-
plication of an improved dcgan for image generation.
Mobile information systems, 2022(1):9005552.
Martinelli, F., Mercaldo, F., and Santone, A. (2022). Wa-
ter meter reading for smart grid monitoring. Sensors,
23(1):75.
Mercaldo, F., Ciaramella, G., Iadarola, G., Storto, M.,
Martinelli, F., and Santone, A. (2022). Towards ex-
plainable quantum machine learning for mobile mal-
ware detection and classification. Applied Sciences,
12(23):12025.
Mercaldo, F., Di Giammarco, M., Apicella, A., Di Iadarola,
G., Cesarelli, M., Martinelli, F., and Santone, A.
(2023). Diabetic retinopathy detection and diagno-
sis by means of robust and explainable convolutional
neural networks. Neural Computing and Applications,
35(23):17429–17441.
Mercaldo, F., Di Giammarco, M., Ravelli, F., Martinelli, F.,
Santone, A., and Cesarelli, M. (2024a). Alzheimer’s
disease evaluation through visual explainability by
means of convolutional neural networks. Interna-
tional Journal of Neural Systems, 34(2):2450007–
2450007.
Mercaldo, F., Martinelli, F., and Santone, A. (2024b).
Deep convolutional generative adversarial networks in
image-based android malware detection. Computers,
13(6).
Mirsky, Y., Mahler, T., Shelef, I., and Elovici, Y. (2019).
{CT-GAN}: Malicious tampering of 3d medical im-
agery using deep learning. In 28th USENIX Security
Symposium (USENIX Security 19), pages 461–478.
Nagaraju, R. and Stamp, M. (2022). Auxiliary-classifier
gan for malware analysis. In Artificial Intelligence for
Cybersecurity, pages 27–68. Springer.
Pan, H. and Xin, L. (2024). Fdts: A feature disentangled
transformer for interpretable squamous cell carcinoma
grading. IEEE/CAA Journal of Automatica Sinica,
12(JAS-2024-1027).
Singh, N. K. and Raza, K. (2020). Medical image gen-
eration using generative adversarial networks. arXiv
preprint arXiv:2005.10687.
Singh, N. K. and Raza, K. (2021). Medical image genera-
tion using generative adversarial networks: A review.
Health informatics: A computational perspective in
healthcare, pages 77–96.
Wang, X., Guo, H., Hu, S., Chang, M.-C., and Lyu, S.
(2023). Gan-generated faces detection: A survey and
new perspectives. ECAI 2023, pages 2533–2542.
Xia, X., Xu, C., and Nan, B. (2017). Inception-v3 for flower
classification. In 2017 2nd international conference
on image, vision and computing (ICIVC), pages 783–
787. IEEE.
You, A., Kim, J. K., Ryu, I. H., and Yoo, T. K. (2022).
Application of generative adversarial networks (gan)
for ophthalmology image domains: a survey. Eye and
Vision, 9(1):6.
Zhou, X., Tang, C., Huang, P., Tian, S., Mercaldo, F., and
Santone, A. (2023). Asi-dbnet: an adaptive sparse
interactive resnet-vision transformer dual-branch net-
work for the grading of brain cancer histopathological
images. Interdisciplinary Sciences: Computational
Life Sciences, 15(1):15–31.
BIOINFORMATICS 2025 - 16th International Conference on Bioinformatics Models, Methods and Algorithms
618
