
Machine Learning and Deep Learning Approaches for Early
Alzheimer’s Detection in Patients with Subjective Cognitive Decline:
A Systematic Literature Review
Zyad Taouil
a
, Nourhène Ben Rabah and Bénédicte Le Grand
Centre de Recherche en Informatique, Université Paris 1 Panthéon-Sorbonne, Paris, France
Keywords: Machine Learning, Alzheimer's Disease, Subjective Cognitive Decline, Detection, Biomarker, Classification.
Abstract: This paper investigates the application of machine learning and deep learning techniques for the early
detection of Alzheimer’s Disease (AD) in patients with Subjective Cognitive Decline (SCD), a preclinical
AD stage. Traditional diagnosis methods struggle to detect AD at this stage, making ML a promising
alternative for early intervention. A systematic literature review (SLR) was conducted to identify and analyze
the most effective ML models, data types, and preprocessing techniques for early AD detection. This review
highlights that Convolutional Neural Network (CNN), Random Forest, and logistic regression models,
particularly when applied to multimodal data (e.g., neuroimaging, genetic, and vocal features), showing high
diagnosis accuracy. Data preprocessing steps such as feature engineering and data augmentation significantly
enhance model performance. This paper also explores the practical implications of implementing ML models
in clinical settings and discusses system integration, clinician training, and ethical considerations surrounding
patient data. This research emphasizes the potential of ML to enhance early AD diagnosis.
1 INTRODUCTION
Alzheimer’s Disease (AD) is a progressive
neurodegenerative disorder that currently affects
millions of people worldwide, representing one of the
most significant public health challenges as
populations age. Traditionally, AD diagnosis relies
on clinical assessments and neuroimaging, but these
methods show limitations regarding the detection of
the disease at its earliest stages, particularly during
the preclinical phase known as Subjective Cognitive
Decline (SCD). SCD is characterized by self-reported
memory or cognitive issues (RABIN, 2017), and has
been recognized as a precursor to Mild Cognitive
Impairment and full-blown AD.
Despite the gravity of this global public health
challenge, the early diagnosis of AD remains
difficult. Many clinical tests are insufficiently
sensitive to mild changes in cognition, and advanced
imaging or biomarker analyses may not be accessible
in all healthcare settings. Consequently, there is a
critical unmet need for more cost-effective, scalable,
and accurate diagnostic methods to identify at-risk
a
https://orcid.org/0009-0008-1607-9872
individuals before irreversible neuronal damage
occurs. The potential benefits of such research are
substantial: earlier interventions may slow disease
progression, reduce healthcare costs, and improve
patients’ quality of life.
Moreover, detecting AD at this early stage could
be crucial for preventive treatments, thereby
mitigating the disease’s progression. In recent years,
Innovative applications of Machine Learning (ML)
and Deep Learning has shown great potential for
transforming medical diagnosis, particularly in areas
involving complex data such as neuroimaging,
genetic information, and cognitive assessments.
However, despite the progress made, the application
of ML to AD diagnosis in the preclinical stage,
specifically for individuals showing signs of SCD,
remains underexplored.
The central problem addressed in this study is how
machine learning and deep learning models can be
used to improve the early detection of AD in patients
with SCD, enhancing detection accuracy and
providing opportunities for earlier, more effective
interventions. Early diagnosis through ML and deep
598
Taouil, Z., Ben Rabah, N. and Grand, B. L.
Machine Learning and Deep Learning Approaches for Early Alzheimer’s Detection in Patients with Subjective Cognitive Decline: A Systematic Literature Review.
DOI: 10.5220/0013247600003890
In Proceedings of the 17th International Conference on Agents and Artificial Intelligence (ICAART 2025) - Volume 2, pages 598-610
ISBN: 978-989-758-737-5; ISSN: 2184-433X
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)

learning not only has the potential to improve the
identification of at-risk individuals based on slight
variations in biomarkers such as neuroimaging,
genetic markers, or behavioral data but also opens the
door to more targeted inclusion of individuals in
clinical trials aimed at slowing or preventing the
progression of the disease.
This paper aims to answer three key research
questions:
RQ1. What are the most effective ML models for
diagnosing AD at the preclinical stage?
RQ2. How do different types of data and
preprocessing techniques affect the performance
of these models?
RQ3. What are the practical implications of
integrating ML models into clinical settings for
early AD diagnosis?
To address these research questions, we
conducted a systematic literature review, focusing on
the application of ML techniques in the context of
Subjective Cognitive Decline and Alzheimer’s
Disease diagnosis. Then we examined the
performance of various ML models, the impact of
different data types and preparation techniques, and
the challenges involved in bringing these models into
clinical practice.
This paper is organized as follows: Section 2
provides the background of the subject and critically
reviews prior literature reviews that have addressed
it. Section 3 describes in detail the methodology used
to conduct the systematic literature review. Section 4
presents the results, addressing the different research
questions: (RQ1) focuses on analyzing the most
effective ML models, (RQ2) explores the types of
data and preprocessing techniques identified, and
(RQ3) examines the practical implications of
integrating machine learning models into clinical
settings for the early diagnosis of Alzheimer's
disease. Section 5 discusses the challenges
encountered and the research gaps identified. Finally,
Section 6 concludes the study and suggests directions
for future work.
2 BACKGROUND AND
RELATED WORKS
In this section, we present the background of the
subject and review the literature reviews that have
addressed this topic in previous years. This
comparative analysis allows us to highlight the
originality of our systematic literature review by
showing how our approach differs from previous work
and providing new perspectives on the field of study.
2.1 Background
At the forefront of AD research and clinical practice
lies the preclinical stage of the disease. This stage is
characterized by “no impairment in cognition on
standard assessments and biomarker evidence for
AD” (JESSEN, 2014). Detecting AD at this stage
provides a critical opportunity for intervention, as
therapeutic treatments applied before significant
cognitive decline may delay or even prevent the
progression to symptomatic stages such as Mild
Cognitive Impairment (MCI) and full dementia.
This approach reflects a significant shift in AD
research, moving the focus from treating advanced
stages of the disease to identifying and intervening at
its earliest, asymptomatic phase.
Subjective Cognitive Decline as a Key
Indicator: Within the preclinical phase, Subjective
Cognitive Decline has emerged as a critical focus
area. Studies have shown that individuals with SCD
are at higher risk of developing AD-related cognitive
impairments in the future, as many of them already
exhibit biological changes associated with AD, such
as elevated levels of amyloid-beta and tau proteins,
two key biomarkers of the disease (RABIN, 2017).
Given the association between SCD and these
biomarkers, SCD represents a valuable early indicator
for AD research. Individuals reporting SCD may
serve as an ideal target population for preclinical
screening, as detecting biological markers before the
appearance of clinical symptoms could provide a
crucial window for therapeutic intervention.
Moreover, SCD provides a practical and cost-
effective approach to identifying at-risk individuals,
helping streamline clinical trials and the development
of targeted treatment strategies.
Machine Learning as a Detection Tool:
Traditional detection tools for AD, such as
neuroimaging and biomarker tests, often require
advanced medical facilities, making them costly and
inaccessible to a broader population. In response,
Machine Learning has emerged as a promising
solution. Indeed, ML algorithms excel in identifying
subtle patterns within complex datasets, such as those
generated from neuroimaging or biomarker analysis.
By analyzing vast amounts of multimodal data, ML
algorithms have demonstrated remarkable potential
in distinguishing early-stage AD from healthy aging
with high accuracy. This has the potential to
revolutionize early diagnosis and treatment by
Machine Learning and Deep Learning Approaches for Early Alzheimer’s Detection in Patients with Subjective Cognitive Decline: A
Systematic Literature Review
599
enabling personalized interventions that are more
precise and timelier.
ML models typically used in AD research include
classification and regression algorithms.
Classification models are designed to categorize data
into predefined classes, such as distinguishing
between individuals with AD and cognitively
unimpaired individuals (Kingsmore, 2021).
Regression models, on the other hand, analyze the
relationship between a dependent variable and one or
more independent variables and are used to predict
continuous outcomes (Horenko, 2023), such as the
progression of cognitive decline or biomarker levels.
Both types of models play a critical role in developing
more accurate detections.
2.1.1 Key Definitions
Preclinical Stage: The phase of Alzheimer’s Disease
where there is biomarker evidence for AD but no
detectable cognitive decline in standard clinical tests
(Jessen, 2014).
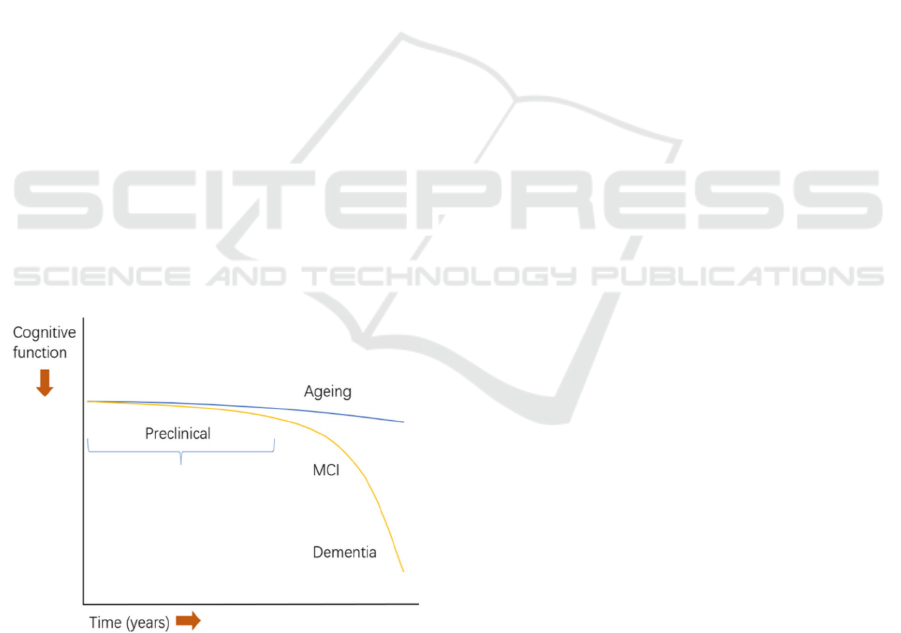
This curve illustrates the typical progression of
cognitive function over time about aging and the
onset of Alzheimer's Disease. AD is depicted with the
yellow line where it starts with the preclinical stage
which occurs before the MCI stage, “the symptomatic
predementia phase of AD” (Rabin, 2017), before
evolving, with a quick cognitive decline to dementia,
“a chronic and progressive deterioration disease
characterized by cognitive dysfunction and abnormal
mental behavior” (Shen, 2018).
Figure 1: Model of the cognitive function decline trajectory
of AD vs normal ageing (Huang, 2023).
Biomarkers: Biological indicators, such as
amyloid-beta and tau proteins, found in blood, brain
images, or cerebrospinal fluid, which provide
evidence of Alzheimer’s pathology before clinical
symptoms manifest. A “large number of clinical
studies very consistently show that these biomarkers
contribute with diagnostically relevant information,
also in the early disease stages”. (Blennow, 2018)
Single-Modal vs. Multimodal ML Approaches:
A key distinction in ML approaches for AD diagnosis
is between single-modal and multimodal data
analysis. Single-modal models analyze data from one
source, such as MRI scans, while multimodal models
integrate data from multiple sources (e.g.,
neuroimaging, biomarkers, and cognitive tests)
(REN, 2022).
2.2 Previous Literature Reviews
We identified two review papers that addressed
Alzheimer's disease (AD) diagnosis using machine
learning (ML) and deep learning techniques. We
presented these studies and highlighted the
contribution of our work in comparison to them.
2.2.1 Alzheimer’s Disease Diagnosis Using
Machine Learning: A Survey
(Dara, 2023)
This extensive survey reviews over 80 publications
from 2017 onwards, with a focus on "fundamental
machine learning architectures such as support vector
machines, decision trees, and ensemble models." The
study provides an overview of traditional ML models,
such as Support Vector Machines (SVMs), decision
trees, and ensemble methods, all of which have been
widely used in diagnosing AD by analyzing
neuroimaging and non-imaging biomarkers.
It highlights that deep learning models,
particularly CNN, have demonstrated superior
performance in handling complex neuroimaging data,
extracting features, and classifying AD with high
accuracy. Moreover, this survey highlights the need
for improved model interpretability, particularly for
deep learning models like CNN, which often function
as a "black box" in clinical contexts. The lack of
transparency in these models poses a significant
barrier to their widespread clinical adoption,
especially in the diagnosis of early-stage AD where
explainability is critical for clinician trust and
decision-making.
While this survey provides a broad overview of
ML technologies in AD diagnosis, it lacks a specific
focus on the preclinical stage of Alzheimer’s Disease.
The majority of the reviewed studies focus on
later stages of AD, such as MCI and fully developed
AD, which are symptomatic phases of the disease. As
a result, this survey does not fully capture the
potential of ML to detect AD at the preclinical stage,
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
600

when interventions could have the most significant
impact. Additionally, the review does not delve into
the technical steps of ML implementations, such as
data preprocessing, hyperparameter tuning, or the
challenges of working with different types of data.
These elements are crucial for understanding how ML
models can be optimized for early-stage AD
detection.
2.2.2 Systematic Review on Machine
Learning and Deep Learning
Techniques in the Effective Diagnosis
of Alzheimer’s Disease (Arya, 2023)
This systematic review focuses on the use of machine
learning methods, such as Random Forest (RF),
SVMs, and Logistic Regression, to classify patients
as cognitively normal or suffering from AD.
This review puts significant emphasis on imaging
modalities, particularly Positron Emission
Tomography (PET) and Magnetic Resonance
Imaging (MRI), for detecting AD-related changes in
the brain. The authors argue that deep learning
methods for feature extraction, combined with
traditional ML models like SVMs for classification,
are highly efficient in diagnosing AD.
Though the study provides valuable insights into
the application of ML for AD diagnosis, its focus
remains largely on symptomatic patients rather than
those in the preclinical stage. The omission of SCD
as a critical marker for early detection leaves a gap in
understanding how ML can be applied to detect AD
before a significant cognitive decline occurs.
Furthermore, the study is heavily focused on
neuroimaging, particularly PET and MRI scans,
which, while important, do not fully capture the range
of potential detection tools and data types. Other non-
invasive biomarkers, such as vocal features, genetic
data, or cognitive test results, are underexplored in
this review.
2.2.3 Contribution of Our Work
The research gaps identified in the studies underscore
the importance of focusing on the preclinical stage of
Alzheimer’s Disease AD, when early intervention
may be most effective. Unlike these broad reviews,
our research specifically targets the preclinical stage,
aiming to harness ML techniques to detect the earliest
signs of cognitive decline, particularly in individuals
reporting SCD. By focusing on this critical phase of
the disease, we aim to contribute to the growing body
of work that seeks to enable early diagnosis and
intervention through Machine Learning. Our work
also distinguishes itself by incorporating a more
detailed computer science perspective. We provide a
deeper analysis of the ML implementations, including
the specificities of different algorithms, their data
dependencies, and the importance of data
preprocessing. In fact, preprocessing techniques, such
as feature selection, data augmentation, and handling
of missing data, are often overlooked but are crucial
to the performance of ML models in medical
diagnostics. By addressing these technical aspects,
we offer a comprehensive understanding of how ML
can be effectively integrated into the early detection
process for Alzheimer’s Disease.
Moreover, our study explores the use of
multimodal data, integrating neuroimaging, genetic,
speech and linguistic data to improve the performance
of ML models. While previous studies have primarily
focused on single-modal approaches (e.g., MRI or
PET scans), our research investigates the synergistic
effects of combining multiple data types to enhance
diagnostic accuracy and reliability. This approach is
particularly important for detecting early-stage AD,
where symptoms are minimal, and a single data
source may not provide sufficient information for an
accurate diagnosis.
Furthermore, we emphasize the need for
explainable AI (XAI) models in clinical settings,
ensuring that machine learning models not only
perform well statistically but also provide actionable
insights that clinicians can trust and implement in
their decision-making processes. By focusing on the
explainability of ML models, our work aims to bridge
the gap between technological advancements and
clinical applicability, ensuring that the developed
models can be realistically integrated into healthcare
settings.
3 METHODOLOGY
To conduct this research, we followed the
Kitchenham methodology, formally known as the
"Guidelines for Performing Systematic Literature
Reviews in Software Engineering" (KITCHENHAM,
2007). This framework, originally developed for
software engineering research, is highly suitable for a
review involving ML technologies applied to medical
diagnosis. We also refer to Kitchenham's
complementary work, "Procedures for Performing
Systematic Reviews" (Kitchenham, 2004), for
detailed guidance on each step of the methodology.
Machine Learning and Deep Learning Approaches for Early Alzheimer’s Detection in Patients with Subjective Cognitive Decline: A
Systematic Literature Review
601

3.1 Planning
3.1.1 PICOC Framework
We employed the PICOC (Population, Intervention,
Comparison, Outcome, Context) criteria to formulate
the research questions:
Population (P). Cognitively unimpaired
individuals diagnosed as healthy controls (HC)
or with SCD.
Intervention (I). Application of ML and/or DL
techniques to detect early AD.
Comparison (C). Various ML models tested
Outcome (O). Diagnosis performance metrics
like accuracy, sensitivity, specificity, F1-score,
and AUC-ROC.
Context (C). Academic research environments
utilizing diverse datasets (e.g., neuroimaging,
genetic, clinical records).
3.1.2 Research Questions
Using the PICOC framework, we formulated the
three research questions outlined in the introduction.
3.1.3 Keywords and Search String
This search string allowed us to collect 81 articles in
February 2024.
“("Machine learning" OR "machine-learning" OR
"Deep learning" OR "deep-learning") AND
"Alzheimer" AND (diagnosis OR detect OR predict)
AND (preclinical OR "Subjective Cognitive Decline"
OR "Subjective Cognitive Impairment" OR
"Subjective Memory Disorder")”
3.1.4 Sources
We sourced the literature primarily from Scopus,
accessing a variety of publications, including
PubMed, IEEE Xplore, and ScienceDirect.
3.1.5 Inclusion/ Exclusion Criteria
We then applied inclusion and exclusion criteria to
retain only the relevant papers.
Inclusion criteria:
- Studies focusing on ML applications in
diagnosing AD at the preclinical stage.
- Experimental research involving diverse
populations and biomarkers.
- Studies published after 2021 to reflect the most
recent advancements.
Exclusion criteria:
- Studies not written in English.
- Studies focusing on later stages of AD (MCI or
dementia) or that did not use ML models.
3.2 Conducting
3.2.1 Study Selection
After running the query, we filtered the articles using
the inclusion/exclusion criteria resulting in 38 papers,
where at last 28 were selected, after complete reading
of the articles.
3.2.2 Data Extraction
A data extraction table was used to synthesize
relevant data across studies. Key elements included:
- ML Algorithms: Specific algorithms used (e.g.,
SVM, CNN).
- Data Types: Neuroimaging, biomarker data,
cognitive tests.
- Preprocessing: Techniques like data cleaning,
scaling, feature selection.
- Performance Metrics: Accuracy, sensitivity,
specificity, F1-score.
This structured approach provided a basis for
quantitative and qualitative analysis.
3.3 Tools
We used Parsifal for systematic review management
(Parsifal) and Zotero (Zotero) to organize articles by
tags and track citation metrics, publication dates, and
references.
By employing this structured methodology, we
ensured that our review covered the most relevant and
high-quality studies on ML applications for
diagnosing Alzheimer's Disease at the preclinical
stage, focusing particularly on SCD.
4 RESULTS
Figure 2 shows the number of papers from the SLR
that are used to answer our three research questions.
Figure 2: Usage of papers throughout SLR.
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
602
4.1 What Are the Most Effective
Machine Learning Models for
Diagnosing Alzheimer's Disease at
the Preclinical Stage? (RQ1)
All selected studies (28) contributed to answering our
first research question, highlighting the following key
ML models: Convolutional Neural Network
(CNN), Random Forest (RF), Logistic Regression
(LR), and Support Vector Machines (SVM). Each
algorithm's specific characteristics and their
performance in AD diagnosis are discussed below.
4.1.1 Most Used Machine Learning
Algorithms
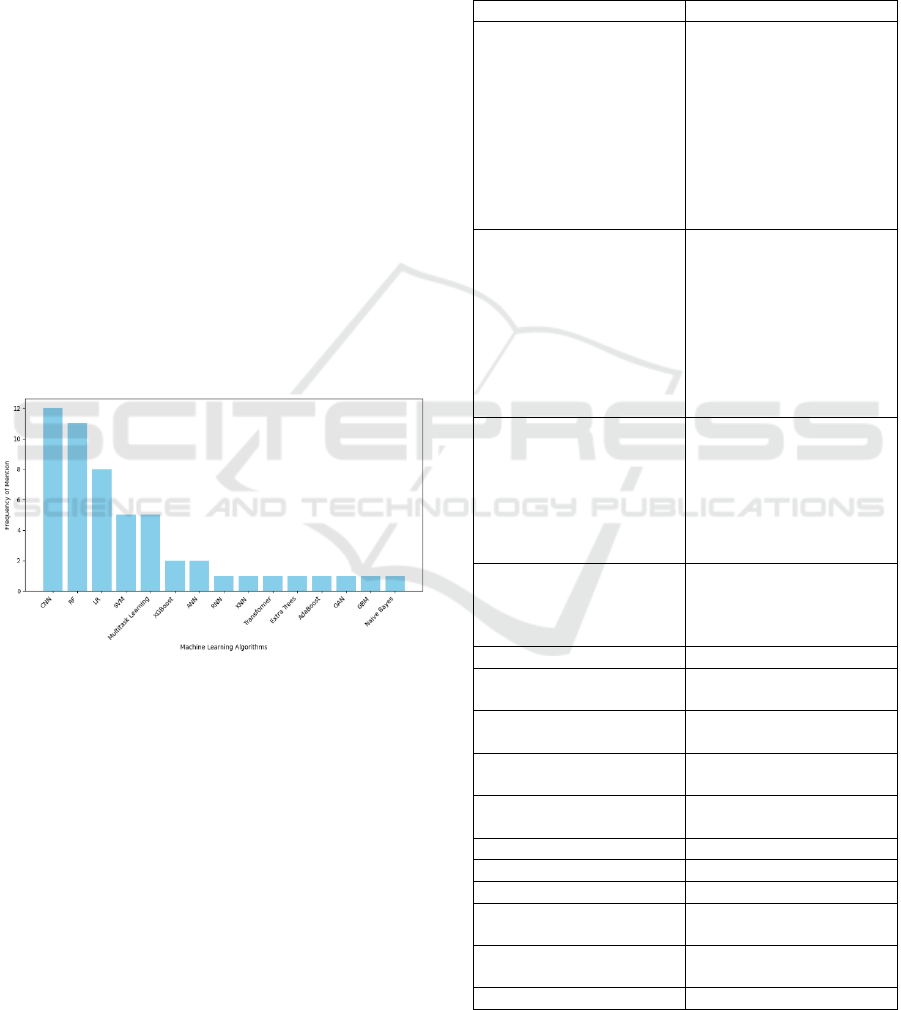
As depicted in Figure 3, CNN emerged as the most
frequently used algorithm, appearing in 12 studies.
CNN are highly effective for neuroimaging tasks
(e.g., MRI, and PET scans) due to its ability to extract
spatial features from high-dimensional image data
(SONG, 2020). However, CNN lacks explainability
(Mattia, 2021) and requires high computational
resources (Logan, 2021), which can limit its clinical
applicability.
Figure 3: Most used ML algorithms in AD classification.
RF appeared 11 times and is characterized by its
robustness to overfitting and its ability to handle
multimodal data (neuroimaging, cognitive, and
genetic) (Sarica, 2017). RF models are particularly
useful in scenarios where diverse datasets need to be
integrated. However, like CNN, RF models suffer from
low interpretability, though tools like SHAP values can
partially mitigate this issue (Avraam, 2023).
LR, used in 8 studies, is appreciated for its
simplicity and transparency, making it suitable for
binary classification tasks (e.g., disease vs. no
disease). Despite its interpretability, LR has
limitations in handling high-dimensional data and
complex relationships, which are common in AD-
related datasets (Menezes, 2017).
SVM used in 5 studies, is robust for high-
dimensional data (CHEN, 2011) and offer
explainability through kernel functions (Mandhala,
2014). However, SVM can be computationally
intensive and sensitive to parameter selection (Land,
2002).
Table 1: Used Algorithms in Literature.
Al
g
orithms References
Convolutional Neural
Network (CNN)
(MOHI UD DIN DAR,
2023), (ODUSAMI,
2022), (OKTAVIAN,
2022), (ANGKOSO,
2022), (FU’ADAH,
2021), (EBRAHIMI,
2021), (MURUGAN,
2021), (SHAMRAT,
2023),
(KIM N. H., 2023)
Random Forest (RF) (BOHN, 2023), (KIM N.
H., 2023), (CHIU, 2022),
(REN Y. S., 2023),
(SCHEIJBELER E. P.,
2022), (BAYAT, 2021),
(JANG, 2021),
(GAUBERT, 2021),
(GOUW, 2021), (KIM J.
L., 2021
)
Logistic Regression (LR) (KIM N. H., 2023),
(HAJJAR, 2023),
(JIANG, 2022), (JANG,
2021), (GAUBERT,
2021), (SHIMODA,
2021), (SCHEIJBELER
E. P., 2022
)
Support Vector Machine
(SVM)
(KIM N. H., 2023),
(CHIU, 2022), (JIANG,
2022), (GAUBERT,
2021
)
Multitask Learnin
g
(
LEI, 2021
)
XGBoost (KIM N. H., 2023),
(
SHIMODA, 2021
)
Artificial Neural Network
(ANN)
(HAJJAR, 2023)
Recurrent Neural Network
(
RNN
)
(EBRAHIMI, 2021)
K-Nearest Neighbor
(
KNN
)
(KIM N. H., 2023)
Transforme
r
(
SIBILANO, 2023
)
Extra Trees (TER HUURNE, 2023)
AdaBoost (KIM N. H., 2023)
Generative Adversial
Network
(
GAN
)
(HWANG, 2023)
Gradient Boosting
Machine
(
GBM
)
(KIM N. H., 2023)
Naïve Ba
y
es
(
KIM N. H., 2023
)
Other ensemble methods, such as AdaBoost,
Machine Learning and Deep Learning Approaches for Early Alzheimer’s Detection in Patients with Subjective Cognitive Decline: A
Systematic Literature Review
603
XGBoost, and Gradient Boosting Machine (GBM),
appeared less frequently but holds promising results
in combining multiple weak learners (Mandhala,
2014) to improve prediction accuracy.
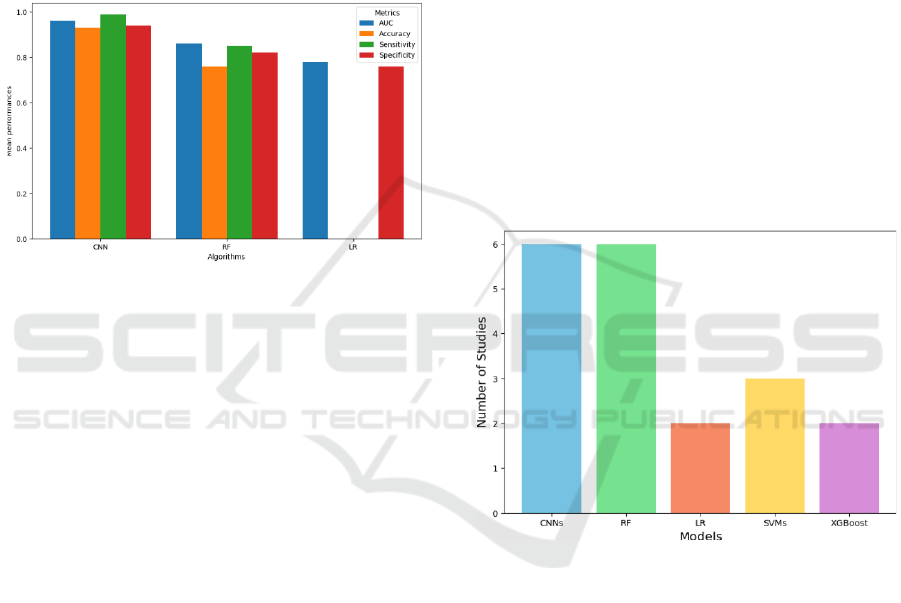
4.1.2 Global Performance of Algorithms
To evaluate the global performance of the 3 most
popular ML algorithms from the previous question,
namely CNN, RF and LR, we analyzed in figure 4
their mean metrics across studies, including AUC,
accuracy, sensitivity, and specificity.
Figure 4: Mean performance metrics (AUC, accuracy,
sensitivity, specificity) for CNN, RF, and LR.
In fact, in the context of classification of AD stages,
the performance of ML algorithms is typically
assessed using various metrics such as:
Accuracy which in this context “refers to the total
percentage of participants who were correctly
classified as either CU or as belonging to the targeted
clinical cohort (i.e., the fraction of true positives and
true negatives over all model classifications)”
(BOHN, 2023).
Sensitivity (or recall) “reflects the percentage of
participants from the target clinical cohort who were
correctly classified as such (calculated as true
positives / (true positives + false negatives))”
(BOHN, 2023).
Specificity (or precision) “which represents the
percentage of participants who were correctly
classified into the target clinical cohort (calculated as
true positives / (true positives + false positives))”
(BOHN, 2023).
Area Under the Curve (AUC) is “a summary
measure of the model’s ability to distinguish between
CU and the targeted clinical cohorts” (BOHN, 2023).
In the various studies reviewed, we found that
CNN, RF, and LR are the most used models. CNN
consistently outperformed other models, with a mean
AUC of 0.964 and an accuracy of 0.931 in imaging
tasks (e.g., MRI). CNN's superior image processing
capabilities make them ideal for detecting subtle
changes in brain structure at the preclinical stage. RF
achieved a mean AUC of 0.856, with strong
performance in multimodal data settings (AUC up to
0.89 (BOHN, 2023)). Indeed, RF showed good
performance across different data types, including
neuroimaging and vocal features. LR demonstrated
lower performance, with an average AUC of 0.775.
However, its simplicity and interpretability make it a
good baseline model, particularly for studies with
smaller datasets.
4.1.3 Effect of Model Tuning
14 out of the 28 studies employed model tuning (see
repartition in Figure 5), which had significant impact
on the performance of ML algorithms, especially for
Random Forest and CNN models, showing
substantial improvements when optimized through
techniques like Grid Search used in 62.5% of articles
using model tuning, Bayesian Optimization (25%)
and Incremental tuning (12.5%).
Figure 5: Repartition of algorithms using Model Tuning.
4.2 How Do Different Data Types and
Preprocessing Techniques Impact
the Performance of Machine
Learning Models in Early
Diagnosis of Alzheimer's Disease?
(RQ2)
Different types of data have been employed in the
diagnosis of AD at the preclinical stage, including
neuroimaging biomarkers, EEG, cognitive tests, and
demographic data. In the following section, we
explore the most frequent combinations of data
types with ML algorithms, the impact of data
preparation and compare standalone and multimodal
data.
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
604
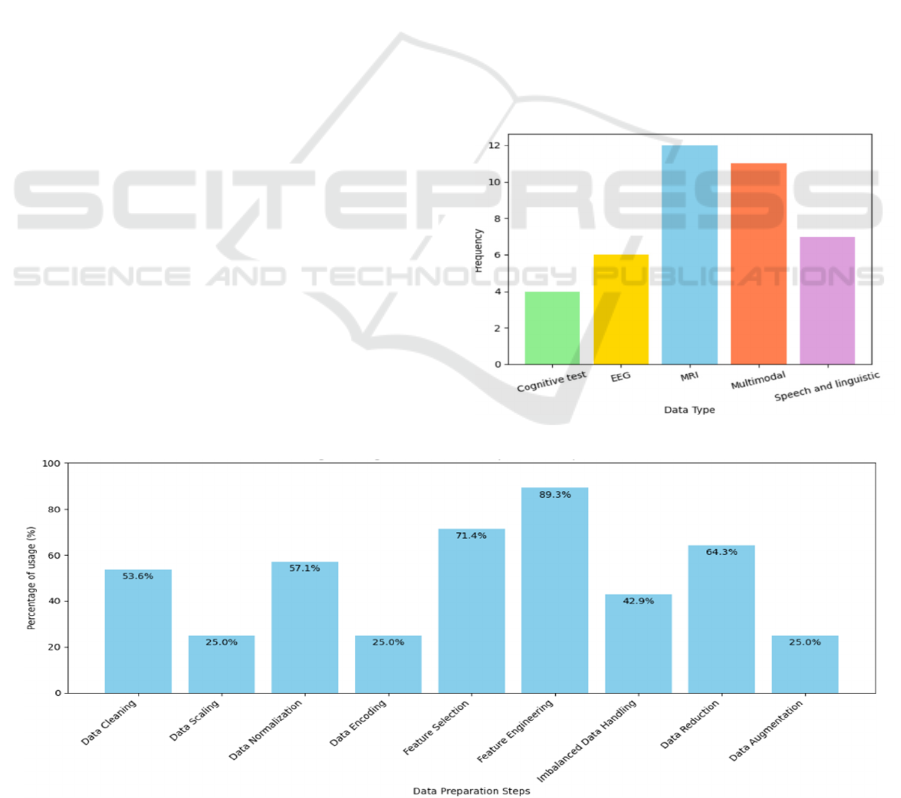
4.2.1 Evaluation of Data Types
Among the reviewed papers as shown in figure 6, MRI
data stands out as the most frequently used, appearing
in 12 studies, particularly from the ADNI dataset, the
most used dataset across the papers (9 times). This
reliance on well-curated, clinical datasets like MRI
shows a preference for high-resolution imaging despite
potential limitations in generalizability to real-world,
noisier data.
Other clinical data such as EEG data (6 times) and
cognitive tests (4 times) are also used but to a lesser
extent, suggesting that although these data types are
valuable, they may lack the detailed imaging
capabilities of MRI and available open-source
datasets such as ADNI.
Multimodal approaches, which combine both
clinical and non-clinical data types, were used in 11
studies, indicating a growing interest in integrating
diverse data sources for a more comprehensive view
of early AD indicators.
Non-clinical data such as speech and linguistic
features, though explored in a few studies (5
instances), remain less common, likely due to the
challenges in data preprocessing and standardization.
4.2.2 Impact of Data Preparation and Data
Quality on Machine Learning Model
Performance
Data preparation is a crucial step in machine learning
pipelines that involves transforming raw data into a
format suitable for model training, which can
significantly impact model performance. This process
includes cleaning the data by removing or correcting
inaccuracies, handling missing values, normalizing or
scaling features to ensure consistency across
variables, and selecting or transforming relevant
features to reduce noise.
In Figure 10, we can see that the most used data
preparation steps are feature engineering (89.3%) and
feature selection (71.4%), both of which help identify
the most relevant features for improving model
performance. Data normalization (57.1%) and data
cleaning (53.6%) are also frequently employed, to
ensure that the data is consistent and error-free.
Furthermore, a comparison of two studies using
CNN with MRI data from the ADNI dataset
illustrates the importance of comprehensive data
preparation. Indeed, in (MOHI UD DIN DAR,
2023), thorough data preparation led to an accuracy
of 0.966 for a 5-class classification task, while
(FU’ADAH, 2021), with limited data preparation
achieved a lower accuracy of 0.95 for a 4-class task.
This demonstrates the importance of data cleaning,
normalization, and augmentation in improving
model performance.
4.2.3 Clinical, Non-Clinical vs. Mixed Data
Using mixed data sources allows machine learning
models to capture multiple dimensions of Alzheimer's
Figure 6: Frequency of data types used in the literature.
Figure 7: Percentage of usage for each data preparation step.
Machine Learning and Deep Learning Approaches for Early Alzheimer’s Detection in Patients with Subjective Cognitive Decline: A
Systematic Literature Review
605

disease, ultimately aiding in more accurate diagnoses.
For instance, integrating structural MRI and PET
scans offers anatomical information along with
amyloid deposition patterns, leading to more sensitive
and accurate identification of preclinical AD
(HWANG, 2023). Studies have demonstrated the
superior performance of multimodal data. Integrating
functional and structural neuroimaging data achieved
high diagnostic accuracy across multiple stages of
cognitive impairment (LEI, 2021). In contrast, relying
solely on unimodal data, whether clinical (e.g.,
MRI) or non-clinical (e.g., voice biomarkers), often
fails to capture AD's complex pathology, resulting in
more limited diagnostic accuracy (HWANG, 2023).
4.3 What Are the Practical
Implications of Implementing
Machine Learning Models for
Early Diagnosis of Alzheimer's
Disease in Clinical Settings? (RQ3)
Our review shows that implementing ML models for
early Alzheimer’s detection in clinical settings is
promising but presents practical and ethical
challenges. This section covers three main aspects:
the requirements for clinical integration, ethical
considerations around patient data, and the potential
for cost-effective, non-invasive screening. These
subparts highlight the primary factors impacting the
feasibility, safety, and accessibility of ML in clinical
AD diagnostics, offering insights into what is needed
for successful adoption.
4.3.1 Challenges and Requirements for
Integrating ML Models into Clinical
Workflows
Integrating ML models for AD diagnosis into clinical
settings involves addressing numerous challenges:
a. Population Diversity and Generalizability.
4 studies ((BOHN, 2023), (BAYAT, 2021), (REN Y.
S., 2023) and (HAJJAR, 2023)) suffer from limited
population diversity, focusing predominantly on non-
Hispanic White participants. This narrow
demographic scope can restrict the generalizability of
ML models, as models trained on homogenous data
may not perform well across diverse populations. In
particular, (BOHN, 2023) and (BAYAT, 2021)
emphasize the need for more inclusive datasets to
ensure broader applicability.
b. Sample Size. 12 studies ((KIM, 2023),
(HWANG, 2023), (CHIU, 2022), (REN Y. S., 2023),
(JANG, 2021), (SHIMODA, 2021), (KIM J. L.,
2021), (SCHEIJBELER E. P., 2022), (MURUGAN,
2021), (ANGKOSO, 2022), (OKTAVIAN, 2022) and
(MOHI UD DIN DAR, 2023)) had small sample
sizes. Small datasets limit the robustness of findings
and can lead to biased or unreliable predictions as
they imply overfitting (KIM, 2023).
c. Model and Data Complexity: Some ML
models, particularly deep learning approaches,
require significant computational resources to
perform well. The studies ((SIBILANO, 2023),
(JIANG, 2022), (KIM, 2023), (HWANG, 2023),
(ODUSAMI, 2022) and (ANGKOSO, 2022))
highlight the challenges raised by complex data types
and high dimensional datasets which often require
specialized hardware, making it difficult for settings
with limited resources to implement these models
effectively.
d. Data Quality and Preprocessing: The
quality of data directly impacts model performance.
Inconsistent data quality, especially in custom
datasets, can introduce noise, as seen in the studies
((KIM, 2023), (JANG, 2021), (SHIMODA, 2021),
(KIM J. L., 2021) and (MURUGAN, 2021)).
e. Cross-Validation and External Validation:
For robust performance, machine learning models
must be validated on independent datasets. (CHIU,
2022) and (SCHEIJBELER E. P., 2022) emphasize
the importance of external validation, which helps to
ensure the model's generalizability and reliability.
f. Feature Representation and Selection:
Selecting and representing relevant features is a
complex task, as highlighted by (LIU, 2022), (LEI,
2021), (SHIMODA, 2021) and (KIM J. L., 2021).
Choosing appropriate features directly impacts model
interpretability and performance, as irrelevant or
redundant features can reduce accuracy.
g. Model Interpretability: Complex models,
such as CNN, often lack transparency as
demonstrated in the studies (HWANG, 2023) and
(GAUBERT, 2021), which stress the need for
interpretable models that provide insight into their
decision-making processes, crucial for clinical
adoption.
h. Technological and Methodological
Constraints: 5 studies ((KIM, 2023), (BAYAT,
2021), (GOUW, 2021), (ANGKOSO, 2022) and
(ODUSAMI, 2022)), most of them using EEG,
underline the reliance on specific tools or platforms
limiting the model's applicability and scalability.
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
606

4.3.2 Ethical Concerns in Patient Data Usage
The integration of ML models into clinical practice
raises significant ethical concerns regarding patient
privacy, data security, and informed consent.
Compliance with regulations such as HIPAA in the
U.S. and GDPR in Europe is critical. Several studies
demonstrate rigorous adherence to ethical standards:
(BOHN, 2023) and (HAJJAR, 2023) highlight the
importance of informed consent and strict ethical
oversight to protect patient data. Similarly,
(SIBILANO, 2023) received institutional review
board (IRB) approval, ensuring ethical compliance.
Although datasets like ADNI come with
standardized ethical guidelines and transparency in
data collection, not all studies disclose their adherence
to ethical approval and data governance frameworks.
For instance, (SHAMRAT, 2023) does not mention
regulatory approval despite using ADNI, highlighting
the need for researchers to be transparent about their
specific practices for handling patient data.
4.3.3 Cost-Effective, Non-Invasive,
and Accessible Early Screening
ML models have the potential to revolutionize early
AD diagnosis by leveraging non-invasive and cost-
effective biomarkers. Several studies have explored
innovative approaches that could be integrated into
routine healthcare: (KIM, 2023) achieved high
accuracy using affordable EEG features, providing a
non-invasive screening option. Digital voice
biomarkers (HAJJAR, 2023) and eye-tracking
technologies (JANG, 2021) have demonstrated
efficacy in early AD detection, offering non-invasive
alternatives to traditional neuroimaging or
cerebrospinal fluid analysis.
Additionally, solutions like mobile health
applications and telemedicine platforms can increase
accessibility in low-resource areas. For instance, the
use of GPS driving data to monitor cognitive decline
offers a novel, non-invasive screening method that
could be implemented remotely (BAYAT, 2021).
5 DISCUSSION
5.1 Interpretation of Findings
This systematic literature review demonstrated that
CNN and RF models are the most effective ML
algorithms for diagnosing AD at the preclinical stage.
CNN excels with neuroimaging data such as MRI
(SONG, 2020), while RF models are versatile across
multimodal inputs (BOHN, 2023). Despite their high
accuracy, both face interpretability challenges and
computational demands, highlighting the need for
explainable AI methods and resource-efficient
architectures.
Key Takeaways:
• Model Tuning: Fine-tuning hyperparameters
significantly enhances diagnostic accuracy,
demonstrating that even well-performing models
need thorough optimization.
• Data Types: MRI was the most used and
reliable source, yet multimodal strategies
(integrating neuroimaging, biomarkers, and
cognitive tests) typically yielded higher
accuracy and stronger robustness.
• Data Preparation: Rigorous approaches to
feature engineering, selection, and
augmentation were closely tied to improved
performance, underscoring the importance of
standardized preprocessing protocols.
Challenges:
• Clinical Integration: Barriers include model
interpretability deficits, the variability in data
quality, and the generalizability of findings to
diverse patient populations.
• Ethical and Regulatory Compliance:
Ensuring data privacy and adhering to
frameworks such as GDPR is critical for
clinician and patient trust.
• Accessibility: Cost-effective and non-invasive
methods (e.g., voice biomarkers, EEG, GPS-
driving data) show promise in democratizing
early screening to broader populations,
especially in remote or underserved areas, but
require more robust validation.
5.2 Research Gaps
Despite encouraging progress, several gaps persist in
ML-based early detection of AD:
• Population Diversity: Models used in the
literature are often trained on homogenous
cohorts, limiting generalizability. Larger, more
diverse datasets are needed to ensure equitable
performance across different ethnic and
socioeconomic groups.
• Underexplored Non-Invasive Tools: Voice
biomarkers, EEG, and other low-cost
approaches could enhance accessibility but
remain underexamined relatively compared to
expensive neuroimaging methods.
• Lack of Explainability: Neural networks,
especially CNNs, lack interpretability,
Machine Learning and Deep Learning Approaches for Early Alzheimer’s Detection in Patients with Subjective Cognitive Decline: A
Systematic Literature Review
607

hindering clinical adoption. XAI techniques
are needed to improve transparency and
clinician trust.
• Inconsistent Data Preparation: Varying
preprocessing steps reduce reproducibility,
highlighting a need for standardized
protocols and external validation strategies.
• Ethical and Privacy Concerns: As data
types diversify, stronger frameworks are
needed to protect patient confidentiality.
6 CONCLUSION AND FUTURE
WORKS
This systematic literature review contributes with a
focused analysis of machine learning and deep
learning applications specifically targeting the
preclinical stage of AD, emphasizing the early
detection of cognitive decline marked by SCD.
Unlike broader studies that address AD across
multiple stages, our work narrows in on this critical
early stage, identifying CNN and Random Forest as
top-performing models when combined with
multimodal data and rigorous data preprocessing
methods. By incorporating a computer science
perspective, we provide a detailed examination of ML
and deep learning implementation, particularly in
terms of data preprocessing and model performance,
and offer insights into how these algorithms can be
optimized for early AD diagnosis.
Future research should address several key
limitations identified in this review. First, while
promising, the current ML models lack
explainability, especially with complex models such
as CNN, posing a barrier to clinical adoption.
Integrating XAI techniques into AD diagnostic
models is essential to enhance model transparency
and build clinician trust.
Additionally, this review reveals a need for more
studies leveraging multimodal data that combines
both clinical and non-clinical sources. The integration
of data types such as neuroimaging, voice
biomarkers, and demographic information could
provide a richer, more comprehensive understanding
of early AD indicators and improve model
robustness.
While preprocessing techniques are crucial for
reliable ML outcomes, there is limited
standardization across studies. Future work should
establish consistent preprocessing protocols and
conduct rigorous external validation to ensure model
generalizability and reliability in diverse clinical
settings. By addressing these areas, future research
can advance ML-based AD diagnostics and bring
these technologies closer to practical application,
ultimately benefiting early detection and intervention
efforts in Alzheimer’s Disease.
Moreover, Longitudinal studies following
individuals over extended periods could further
clarify whether early identification of mild cognitive
deficits via ML actually delays the onset or slows the
progression of clinical AD. Such longitudinal data
would also help refine predictive models by
accounting for dynamic changes in cognition and
pathology over time.
Finally, to expedite the adoption of these
frameworks, researchers should collaborate closely
with clinicians, data scientists, ethicists, and
regulatory authorities to ensure patient safety and
meet compliance requirements. Engaging these
stakeholders early in the research cycle can align
technical development with clinical priorities and
facilitate regulatory approvals.
REFERENCES
ANGKOSO, C. V. (2022). Multiplane Convolutional
Neural Network (Mp-CNN) for Alzheimer’s Disease
Classification. In International Journal of Intelligent
Engineering & Systems , vol. 15, no 1.
ARYA, A. D. (2023). A systematic review on machine
learning and deep learning techniques in the effective
diagnosis of Alzheimer’s disease. In Brain Informatics.
vol. 10, no 1, p. 17.
AVRAAM, B. N. (2023). Local Interpretability of Random
Forests for Multi-Target Regression. arXiv preprint
arXiv:2303.16506.
BAYAT, S. B. (2021). GPS driving: a digital biomarker for
preclinical Alzheimer disease. In Alzheimer's Research
& Therapy, vol. 13, no 1, p. 115.
BLENNOW, K. e. (2018). Biomarkers for Alzheimer's
disease: current status and prospects for the future. In
Journal of internal medicine, vol. 284, no 6, p. 643-663.
BOHN, L. D. (2023). Machine learning analyses identify
multi-modal frailty factors that selectively discriminate
four cohorts in the Alzheimer’s disease spectrum: a
COMPASS-ND study. In BMC geriatrics, vol. 23, no
1, p. 837.
CHEN, S. e. (2011). A novel support vector classifier for
longitudinal high‐dimensional data and its application
to neuroimaging data. In Statistical Analysis and Data
Mining: The ASA Data Science Journal, vol. 4, no 6, p.
604-611.
CHIU, S.-I. F.-Y.-H. (2022). Machine learning-based
classification of subjective cognitive decline, mild
cognitive impairment, and Alzheimer’s dementia using
neuroimage and plasma biomarkers. In ACS Chemical
Neuroscience, vol. 13, no 23, p. 3263-3270.
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
608

DARA, O. A.-G. (2023). Alzheimer’s Disease Diagnosis
Using Machine Learning: A Survey. Applied Sciences.
vol. 13, no 14, p. 8298.
EBRAHIMI, A. L. (2021). Convolutional neural networks
for Alzheimer’s disease detection on MRI images. In
Journal of Medical Imaging, p. 024503-024503 vol. 8
no 2.
FU’ADAH, Y. N. (2021). Automated classification of
Alzheimer’s disease based on MRI image processing
using convolutional neural network (CNN) with
AlexNet architecture. In Journal of physics: conference
series. IOP Publishing, p. 012020.
GAUBERT, S. H. (2021). A machine learning approach to
screen for preclinical Alzheimer's disease. In
Neurobiology of Aging, vol. 105, p. 205-216.
GOUW, A. A. (2021). Routine magnetoencephalography in
memory clinic patients: A machine learning approach.
In Alzheimer's & Dementia: Diagnosis, Assessment &
Disease Monitoring, vol. 13, no 1, p. e12227.
HAJJAR, I. O. (2023). Development of digital voice
biomarkers and associations with cognition,
cerebrospinal biomarkers, and neural representation in
early Alzheimer's disease. In Alzheimer's & Dementia:
Diagnosis, Assessment & Disease Monitoring,, vol. 15,
no 1, p. e12393.
HORENKO, I. V. (2023). On cheap entropy-sparsified
regression learning. In Proceedings of the National
Academy of Sciences, vol. 120, no 1, p. e2214972120.
HUANG, G. L. (2023). Multimodal learning of clinically
accessible tests to aid diagnosis of neurodegenerative
disorders: a scoping review. In Health Information
Science and Systems, vol. 11, no 1, p. 32.
HWANG, U. K.-W. (2023). Real-world prediction of
preclinical Alzheimer’s disease with a deep generative
model. In Artificial Intelligence in Medicine, vol. 144,
p. 102654.
JANG, H. S. (2021). Classification of Alzheimer’s disease
leveraging multi-task machine learning analysis of
speech and eye-movement data. In Frontiers in Human
Neuroscience, vol. 15, p. 716670.
JESSEN, F. A. (2014). A conceptual framework for
research on subjective cognitive decline in preclinical
Alzheimer's disease. In Alzheimer's & dementia, vol.
10, no 6, p. 844-852.
JIANG, Z. S. (2022). Automated analysis of facial emotions
in subjects with cognitive impairment. In Plos one, vol.
17, no 1, p. e0262527.
KIM, J. L. (2021). Development of random forest algorithm
based prediction model of Alzheimer’s disease using
neurodegeneration pattern. In Psychiatry Investigation
, vol. 18, no 1, p. 69.
KIM, N. H. (2023). PET-validated EEG-machine learning
algorithm predicts brain amyloid pathology in pre-
dementia Alzheimer’s disease. In Scientific Reports,
vol. 13, no 1, p. 10299.
KINGSMORE, K. M. (2021). An introduction to machine
learning and analysis of its use in rheumatic diseases. In
Nature Reviews Rheumatology, vol. 17, no 12, p. 710-
730.
KITCHENHAM, B. (2004). Procedures for Performing
Systematic Reviews.
KITCHENHAM, B. (2007). Guidelines for performing
Systematic Literature Reviews in Software Engineering
(Kitchenham).
Land, W. A. (2002). Application of support vector
machines to breast cancer screening using mammogram
and history data. In Medical Imaging 2002: Image
Processing, SPIE, 2002. p. 636-642.
LEI, B. C. (2021). Auto-weighted centralised multi-task
learning via integrating functional and structural
connectivity for subjective cognitive decline diagnosis.
In Medical Image Analysis, vol. 74, p. 102248.
LIU, Y. Y. (2022). Assessing clinical progression from
subjective cognitive decline to mild cognitive
impairment with incomplete multi-modal neuroimages.
In Medical image analysis, vol. 75, p. 102266.
LOGAN, R. W. (2021). Deep Convolutional Neural
Networks With Ensemble Learning and Generative
Adversarial Networks for Alzheimer’s Disease Image
Data Classification, In Frontiers in aging neuroscience,
vol. 13, p. 720226.
MANDHALA, V. S. (2014). Scene classification using
support vector machines. In IEEE International
Conference on Advanced Communications, Control
and Computing Technologies, 1807-1810.
MATTIA, G. V. (2021). Neurodegenerative Traits Detected
via 3D CNNs Trained with Simulated Brain MRI:
Prediction Supported by Visualization of Discriminant
Voxels. In IEEE International Conference on
Bioinformatics and Biomedicine (BIBM) (pp. 1437-
1442).
MENEZES, F. L. (2017). Data classification with binary
response through the Boosting algorithm and logistic
regression. In Expert Syst. Appl., 69, 62-73.
MOHI UD DIN DAR, G. B. (2023). A novel framework for
classification of different Alzheimer’s disease stages
using CNN model. In Electronics, vol. 12, no 2, p. 469.
MURUGAN, S. V. (2021). DEMNET: A deep learning
model for early diagnosis of Alzheimer diseases and
dementia from MR images. In Ieee Access, vol. 9, p.
90319-90329.
ODUSAMI, M. M. (2022). An intelligent system for early
recognition of Alzheimer’s disease using
neuroimaging. In Sensors, vol. 22, no 3, p. 740.
OKTAVIAN, M. W. (2022). Classification of Alzheimer's
disease using the Convolutional Neural Network
(CNN) with transfer learning and weighted loss. arXiv
preprint arXiv:2207.01584.
PARSIFAL. (n.d.). Parsifal. https://parsif.al/about/
RABIN, L. A. (2017). Subjective cognitive decline in
preclinical Alzheimer's disease. In Annual review of
clinical psychology, vol. 13, p. 369-396.
REN, Y. S. (2023). Improving clinical efficiency in
screening for cognitive impairment due to Alzheimer's.
In Alzheimer's & Dementia: Diagnosis, Assessment &
Disease Monitoring, vol. 15, no 4, p. e12494.
REN, Y. X. (2022). Label distribution for multimodal
machine learning. In Frontiers of Computer Science,
vol. 16, p. 1-11.
Machine Learning and Deep Learning Approaches for Early Alzheimer’s Detection in Patients with Subjective Cognitive Decline: A
Systematic Literature Review
609

SÁEZ, C. R. (2021). Potential limitations in COVID-19
machine learning due to data source variability: A case
study in the nCov2019 dataset. In Journal of the
American Medical Informatics Association, vol. 28, no
2, p. 360-364.
SARICA, A. C. (2017). Random Forest Algorithm for the
Classification of Neuroimaging Data in Alzheimer's
Disease: A Systematic Review. Frontiers in Aging
Neuroscience, vol. 9, p. 329.
SCHEIJBELER, E. P. (2022). Network-level permutation
entropy of resting-state MEG recordings: A novel
biomarker for early-stage Alzheimer’s disease? In
Network Neuroscience, vol. 6 (no 2), p. 382-400.
SCHEIJBELER, E. P. (2022). Generating diagnostic
profiles of cognitive decline and dementia using
magnetoencephalography. In Neurobiology of aging,
vol. 111, p. 82-94.
SHAMRAT, F. J. (2023). AlzheimerNet: An effective deep
learning based proposition for alzheimer’s disease
stages classification from functional brain changes in
magnetic resonance images. In IEEE Access, vol. 11, p.
16376-16395.
SHEN, Y. Y. (2018). Cognitive decline, dementia,
Alzheimer’s disease and presbycusis: examination of
the possible molecular mechanism. In Frontiers in
neuroscience, vol. 12, p. 327937.
SHIMODA, A. L. (2021). Dementia risks identified by
vocal features via telephone conversations: A novel
machine learning prediction model. In PloS one, vol.
16, no 7, p. e0253988.
SIBILANO, E. B. (2023). An attention-based deep learning
approach for the classification of subjective cognitive
decline and mild cognitive impairment using resting-
state EEG. In Journal of Neural Engineering, vol. 20,
no 1, p. 016048.
SONG, T.-A. C. (2020). Super-resolution PET imaging
using convolutional neural networks. In IEEE
transactions on computational imaging, vol. 6, p. 518-
528.
TER HUURNE, D. R. (2023). The Accuracy of Speech and
Linguistic Analysis in Early Diagnostics of
Neurocognitive Disorders in a Memory Clinic Setting.
In Archives of Clinical Neuropsychology, vol. 38, no 5,
p. 667-676.
ZOTERO. (n.d.). Zotero. https://www.zotero.org/.
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
610
