
Modulating Cerebral Rhythms in Parkinson's Disease: Insights on
the Role of Auditory Stimulation
Pablo García-Peña
1a
, Juan M. López
2b
, Milagros Ramos
1,3,4 c
, Daniel González-Nieto
1,3,4 d
and Guillermo de Arcas
2,5,6 e
1
Center for Biomedical Technology (CTB), Universidad Politécnica de Madrid, Madrid, Spain
2
Instrumentation and Applied Acoustics Research Group (I2A2), Universidad Politécnica de Madrid, Madrid, Spain
3
Departamento de Tecnología Fotónica y Bioingeniería, ETSI Telecomunicaciones, Universidad Politécnica de Madrid,
Madrid, Spain
4
Biomedical Research Networking Center in Bioengineering Biomaterials and Nanomedicine (CIBER-BBN), Madrid, Spain
5
Departamento de Ingeniería Mecánica, ETSI Industriales, Universidad Politécnica de Madrid, Madrid, Spain
6
Laboratorio de Neuroacústica, Universidad Politécnica de Madrid, Madrid, Spain
Keywords: 40 Hz, Gamma Entrainment, C57BL/6, Electroencephalography, Slowing EEG.
Abstract: Parkinson's disease (PD) is a neurodegenerative disease characterized by a slowing of brain rhythms, leading
to cognitive and motor deficits. Auditory stimulation (AS) has proven to have great potential as a non-invasive
approach to modulate brain activity. Nevertheless, despite promising clinical and preclinical findings, optimal
AS parameters for its use in PD remain unclear. To investigate the potential therapeutic effects of AS in PD,
we aimed to establish an optimal preclinical model and stimulation protocol. Two mouse strains were
compared, CD1 and C57BL/6, and assessed their auditory sensitivity. 3-months C57BL/6 mice was selected
as the most suitable model for auditory studies. Two literature-based AS protocols were applied, a 10 kHz
carrier tone modulated with 40 Hz pulses and a 40 Hz amplitude-modulated tone. Our results demonstrate
that comparing pre- and post-stimulation periods, the 10 kHz/40 Hz protocol consistently induced a reduction
in delta power and an increase in gamma relative power, with persistent effects of the latter 24 hours post-
stimulation. These findings suggest that this specific AS protocol holds promise for targeting abnormal brain
rhythms associated with PD and may have potential therapeutic implications. Further research is needed to
explore the underlying mechanisms and optimize AS parameters for clinical translation.
1 INTRODUCTION
Parkinson's disease (PD), a neurodegenerative
disorder characterized by motor impairments
(tremors, rigidity and akinesia), hyposmia and
cognitive decline with sleep disorders, is a significant
global health burden. PD has been identified as the
fastest growing neurological condition affecting 11.8
million people globally in 2021, a 273.9% increase
since 1990 (Steinmetz et al., 2024). The pathological
hallmark of PD is the loss of dopaminergic neurons
a
https://orcid.org/0000-0002-4928-0213
b
https://orcid.org/0000-0001-7847-8707
c
https://orcid.org/0000-0001-5798-9508
d
https://orcid.org/0000-0003-2972-729X
e
https://orcid.org/0000-0003-1699-7389
in the substantia nigra, leading to dysregulation of
neuronal circuits in the basal ganglia and impairment
of nigrostriatal pathway. This disruption results in
abnormal brain activity, including altered neuronal
oscillations.
Electroencephalography (EEG) studies in PD
patients have revealed a characteristic pattern of
abnormal brain waves, characterized by an early
increase in delta band activity (Caviness et al., 2015;
Chu et al., 2021). Additionally, there is a reduction in
gamma band activity (Ahveninen et al., 2000), which
752
García-Peña, P., López, J. M., Ramos, M., González-Nieto, D. and de Arcas, G.
Modulating Cerebral Rhythms in Parkinson’s Disease: Insights on the Role of Auditory Stimulation.
DOI: 10.5220/0013247800003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 752-762
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

plays a crucial role in information processing and
cognitive functions. Taken together, these findings
suggest that a characteristic hallmark of PD is a
slowing cerebral rhythms, associated with cognitive
dysfunction (Bosboom et al., 2006; Soikkeli et al.,
1991) an impairment that highly correlates with
motor decline (Miladinović et al., 2021).
Efforts to develop novel therapeutic strategies for
PD have focused on restoring normal neuronal
activity and enhancing brain plasticity. While deep
brain stimulation (DBS) has shown some success, it
carries risks and may not be suitable for all patients
due to surgery risks (Zhang et al., 2017). Non-
invasive brain stimulation techniques, such as
transcranial magnetic stimulation (TMS) and
transcranial direct current stimulation (tDCS), offer
alternative approaches to modulating brain activity,
however, further research optimizing stimulation
parameters is needed to enhance their clinical utility
(Benninger et al., 2010; Goodwill et al., 2017). There
are not pharmacological or non-pharmacological
therapies able to slow the progression of PD.
Auditory stimulation (AS) in the audible range
has emerged as a promising and non-invasive
approach for modulating neuronal activity. This
method involves using sound waves to modulate
brain activity by a mechanism called oscillatory
entrainment. This process occurs when neural
intrinsic oscillations synchronize with external
rhythmic stimulus (Henao et al., 2020; Ross & Lopez,
2020). This synchronization enhances neural activity
at the stimulus frequency by increasing signal
amplitude and/or by phase-locking, where phases of
neural oscillations align with specific phases of the
stimulus (Henao et al., 2020) By these
synchronizations, rhythmic stimuli can modulate
brain regions beyond sensory areas, reaching
supramodal regions and impacting higher-level
cognitive functions (Albouy et al., 2022) and
influence neuroprotection and neural plasticity
(Adaikkan & Tsai, 2020; Fujiki et al., 2020).
Specifically, neuronal entrainment has been
observed at different frequencies, but especially in the
beta-gamma range (13–44 Hz) (Will & Berg, 2007).
In animal models, direct microelectrode recordings
into the primary auditory cortex demonstrated an
entrainment of local field potential in response to 40-
click train stimuli (Li et al., 2018; Nakao &
Nakazawa, 2014) Several studies in mice model have
demonstrated AS potential to induce gamma
entrainment, as therapy approach in various
neurological diseases, including dementia (Chan et
al., 2022) ischemia (Zheng et al., 2020) and
Alzheimer's disease (AD) (Liu et al., 2022), were a 40
Hz audio-visual stimulation induced gamma
oscillation and reduced amyloid plaque promoting
glymphatic clearance (Iaccarino et al., 2016;
Martorell et al., 2019; Murdock et al., 2024). These
findings suggest that neuronal entrainment through
visual or auditory stimulation could not only mitigate
cognitive decline but also emerge as a potential
treatment for neurodegenerative diseases
characterized by the accumulation of pathogenic
proteins.
In PD, rhythmic auditory stimulation (RAS) is
often used in rehabilitation to engage alternative
motor networks or enhances basal ganglia function,
potentially improving gait, tremors and quality of life
(Benoit et al., 2014; Bukowska et al., 2015; Ye et al.,
2022). Our research group has previously conducted
clinical studies investigating the effects of Binaural
Beat Stimulation (BBs) in cognitive function and
brain activity in PD patients. The results suggest that
BBs can initially reduce theta power in the brain,
similar effect to the normalization seen with levodopa
treatment, which may lead to improvements in motor
function and cognition. However, this effect appears
to diminish over time due to habituation. While some
patients showed sustained benefits, others exhibited
variable responses, highlighting the need for further
research to optimize the timing and duration of BBs
to maximize therapeutic effects (Gálvez et al., 2018;
González et al., 2023).
Despite these promising findings, the optimal
parameters for AS in PD remain elusive. A lack of
standardized AS protocols hinders cross-study
comparisons and clinical translation (Ingendoh et al.,
2023). Moreover, the potential therapeutic effects of
AS observed in AD are uncertain in the context of PD.
To fill this knowledge gap, a preclinical model of AS
in PD is essential to systematically investigate the
effects of different stimulation parameters, including
frequency, intensity, and modulation, and to identify
the most effective protocols for modulating neural
activity and improving motor and cognitive function.
To advance our research on this topic, this study
focused on examining the influence of AS on cerebral
rhythms in the unmodified mouse brain. The goal was
to determine whether an AS signal with defined
parameters (see methods) could optimally induce
persistent gamma entrainment and potentially
counteract EEG slowing, which could have
preclinical and clinical implications for the
application of AS paradigms in PD.
Modulating Cerebral Rhythms in Parkinson’s Disease: Insights on the Role of Auditory Stimulation
753

2 MATERIALS AND METHODS
2.1 Animals
Adult male mice CD1 (outbred strain; Charles River
Laboratories; RRID:MGI:5649524) and C57BL/6
Ola (inbred strain; Jackson Laboratory;
RRID:MGI:2162680), both aged 3 and 6 months
were used in these studies. All procedures were
conducted in compliance with national ethical and
legal standards and were authorized by the Ethical
Committee of the Universidad Politécnica de Madrid,
and the regional government of Madrid (authorization
code PROEX 108.0/20). The reporting of this
manuscript was conducted according to the Animal
Research Reporting In Vivo Experiments (ARRIVE)
guidelines.
To minimize potential damage to the head-
mounted electrode caps, the mice were housed
individually in polycarbonate cages (267 × 208 mm).
The animals were provided with ad libitum access to
food and water, with cellulose bedding and cotton
enrichment for environmental stimulation. The
animals were maintained and monitored by a
veterinarian and qualified personnel at the Centre for
Biomedical Technology (Pozuelo de Alarcón, Spain).
The housing conditions were strictly controlled, with
a temperature of 21 ± 2°C, a relative humidity of 40-
60%, and a 12-hour light/dark cycle. All mice were
deemed healthy based on serological and PCR
testing, adhering to Federation of Laboratory Animal
Science Associations (FELASA) recommendations.
Daily routines were conducted between 7 AM and
4 PM, with consistent and qualified personnel
assigned to each experimental procedure. To
characterize auditory capacity, a preliminary test was
conducted with four groups of four mice each based
on both mouse strains and two ages (3 and 6 months)
to select the strain with optimal hearing for
subsequent AS protocols. To investigate the effect of
two AS protocols in EEG, two groups of five mice
each for both protocols were selected from the best
hearing condition.
2.2 Electrodes Implantation
Mice underwent surgery for chronic implantation of
electrodes in parietal-temporal areas to cortical
auditory evoked potentials (CAEPs). We also
implanted intracranial electrodes for
electroencephalography (iEEG) recordings. In both
procedures, the animals were anesthetized with
ketamine (Imalgene, 80 mg/kg, i.p.) and xylazine
(Rompun, 10 mg/kg, i.p.), and afterward by
iodopovidone (Betadine, Avrio Health). Alcohol
wipes were applied to the skin. Anaesthetic depth was
monitored every 5 minutes by assessing pedal
withdrawal reflex. Prior to surgery, mice were treated
with an ophthalmic solution to prevent eye drying.
The rectal temperature was maintained during
surgical procedures by a heating pad (RTC-1 Thermo
Controller Surgery Table, Cibertec) at a body
temperature of 37 ± 0.5 °C.
Craniotomies were performed at the electrode
locations specified below using a dental drill. Four
stainless-steel electrodes (P1 Technologies) were
implanted for CAEPs recordings on the right (AP -2.5
mm; L -4.3 mm; DV 0.0 mm, anatomic locations
from bregma) and left auditory cortices (AP -2.5 mm;
L +4.3 mm; DV 0.0 mm). Two additional electrodes
were implanted as reference (AP 1.5 mm; L +2.0 mm;
DV 0.0 mm from bregma) and ground (AP 1.5 mm;
L -2.0 mm; DV 0.0 mm). For intracranial EEG
(iEEG) recordings, the electrodes were implanted in
the right hemisphere in frontal (AP +1.0 mm; L +10
mm; DV 0.0 mm) and parietal cortex areas (AP -3.5
mm; L +1.0 mm; DV 0.0 mm), following the same
anatomic locations reported in a previous study (Lee
et al., 2018). Reference (AP -5.3 mm; L 0.0 mm; DV
0.0 mm from bregma) and ground (AP -2.0 mm; L
+1.5 mm; DV 0.0 mm) electrodes were also
implanted (Figure 1A).
Figure 1: Experimental Setup. (A) Electrode placement for
frontal and parietal intracranial EEG (iEEG) recording. (B)
Experimental setup for simultaneous auditory stimulation
and iEEG recording.
After the electrode’s implantation, the skull was
dried, the coated portions of the wires were secured
with gel glue (Henkel, Loctite 454) and covered with
dental cement (DuraLay, Inlay Pattern Resin Powder
and Liquid). A header (P1 Technologies) was
connected to the wires and angled upwards.
For pain management, buprenorphine (Buprex,
0.05 mg/kg) was administered subcutaneously before
and 8 hr after surgery. The animals were allowed to
recover for 1 hr in a warm environment before
returning to the standard housing environment.
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
754
2.3 CAEPs Recordings
Mice hearing thresholds and auditory discrimination
were study by CAEPs recordings following Mei et al.
(2021) and Martorell et al. (2019) methodologies and
our previous knowledge on evoked potentials
recordings (Barios et al., 2016; Fernández-García et
al., 2016, 2018; Fernández-Serra et al., 2022)
Animals recovered for at least 1 week after electrodes
implantation. For evoked potentials recordings, mice
were first anaesthetized with ketamine and xylazine
and treated with an ophthalmic solution to prevent
eye drying. During auditory stimulation, anaesthetic
depth and body temperature were maintained. Two
speakers (Logitech Multimedia Speakers Z200) were
place facing each other and 25 cm away from left and
right mice ears. Stimulus consisted of a series of 50
ms tones of 0.5 and 1 kHz with a stimulating rate of 1
Hz, from a level of sound pressure level (SLP; SC310
sound level meter, CESVA) of 100 dB decreasing in
10 dB steps until no response was detected (hearing
threshold) or sound intensity level reached the
ambient noise level (~54.3 dB). The recording time
window was 500 ms, starting 50 ms before the
beginning of the stimulation, with 4,096 Hz sampling
rate. The signal was bandpass filtered at 0.2–2000 Hz
and averaged 300 times. Signals were recorded in an
isolated chamber by using a portable
electromyography (EMG)-evoked potentials (EP)
device (Micromed, Italy) and SystemPLUS Evolution
software (v.1.4; Micromed). Peak amplitudes of the
main potential wave (N1 component) were measured
in relation to the first 50 s as baseline.
2.4 iEEG Recordings and Analysis
For recovery and habituation, the mice were moved
to the recording room, 1 week prior to the start of the
recording sessions. iEEG signals were acquired in
mice with freedom of movement in a 30 × 30 × 30 cm
cage during 3-hr sessions (Figure 1B), following
previous methodologies of our group (García-Peña et
al., 2023; Herrero et al., 2021)The header was
attached to a flexible cable (P1 Technologies) and
connected to single-channel AC amplifiers (Grass,
78D). The power line frequency was removed using
a 50 Hz notch filter. The cortex signals were
amplified by 8,000x, bandpass filtered (CyberAmp,
Axon Instruments, 380) at 0.3–100 Hz. Signals were
then digitally converted (National Instruments, BNC-
2090A) at a sampling frequency of 500 Hz and
recorded using the LabVIEW Biomedical Toolkit
software (v.2012; National Instruments;
RRID:SCR_014325).
For spectral analysis, the power in the range of 0–
100 Hz with a 1024-bin size was calculated using a
customized code from MATLAB software (version
2022b). EEG frequency bands were classified into
delta (δ; 0.5–4 Hz), theta (θ; 4–8 Hz), alpha (α; 8–12
Hz), beta (β; 14–30 Hz) and gamma (γ; 30–100 Hz).
Before (PRE) and after (POST) relative band power
change was calculated subtracting POST to PRE
relative power for each band power studied.
Spectrograms representing powers at frequencies
up to 100 Hz were obtained using a Hamming
window with a block size of 512 and 2048 for the 0.5-
4 Hz and the 30-100 Hz spectrograms, respectively,
calculated using Spike2 (v. 6.18, Cambridge
Electronic Design; RRID:SCR_000903).
2.5 Auditory Stimulation Experimental
Designs
All AS protocols were generated by custom-built
program under LabVIEW Virtual Instrument and
delivered via two opposite speakers (Logitech
Multimedia Speakers Z200) placed 20 cm above the
ground of the cage (Figure 1B).
2.5.1 AS-Protocol 1
The proposed protocol adapts Lee et al. (2018)
auditory stimulation protocol. Briefly, mice
underwent a 1-day duration auditory stimulation of 1-
hr session accompanied by 1-hr iEEG recordings
immediately before (PRE) and after (POST)
stimulation. One day after starting the protocol (basal;
B; D0) and in non-stimulation days iEEG recordings
were performed but no stimulation (Figure 2).
Figure 2: AS-protocol 1 adapted from Lee et al. (2018). 2-
h auditory stimulation (AS) of 500-s ON (40 Hz tones) and
700-s OFF periods was preceded and followed by 1 h of
iEEG recordings, PRE and POST respectively.
Mice were exposed to tones of 40 Hz. 20 pulses
of 10 ms delivered at a SPL of 90 dB were played.
Animals were presented with 1.2-s cycles alternating
500 ms of audio stimulation (ON period) interleaved
with 700 ms of no tones (OFF period). Stimuli were
presented in this manner for 2-hr sessions for 7200
ON-OFF cycles according to protocol (Table 1).
Modulating Cerebral Rhythms in Parkinson’s Disease: Insights on the Role of Auditory Stimulation
755

Table 1: Comparison of AS protocols.
As-protocol 1 AS-protocol 2
Tones 40 Hz 10 kHz
Modulation None 40 Hz
Cycles
500 ms ON
700 ms OFF
10 s ON
10 s OFF
SLP 90 dB 90 dB
Sessions 2 h 1 h
Adapted
from
(Lee et al.,
2018)
Martorell et al.
(2019)
and Lee et al.
(2018)
2.5.2 AS-Protocol 2
The proposed protocol adapts Martorell et al. (2019)
and Lee et al. (2018) auditory stimulation protocols.
Briefly, mice underwent a 5-day duration with
alternating days auditory stimulation of 1-hr session
(AS; D1, D3 and D5) interleaved with non-
stimulating days (resting period; RP; D2 and D4)
accompanied by 1-hr iEEG recordings immediately
before (PRE) and after (POST) stimulation. One day
after starting the protocol (basal; B; D0) and in non-
stimulation days iEEG recordings were performed
but no stimulation (Figure 3).
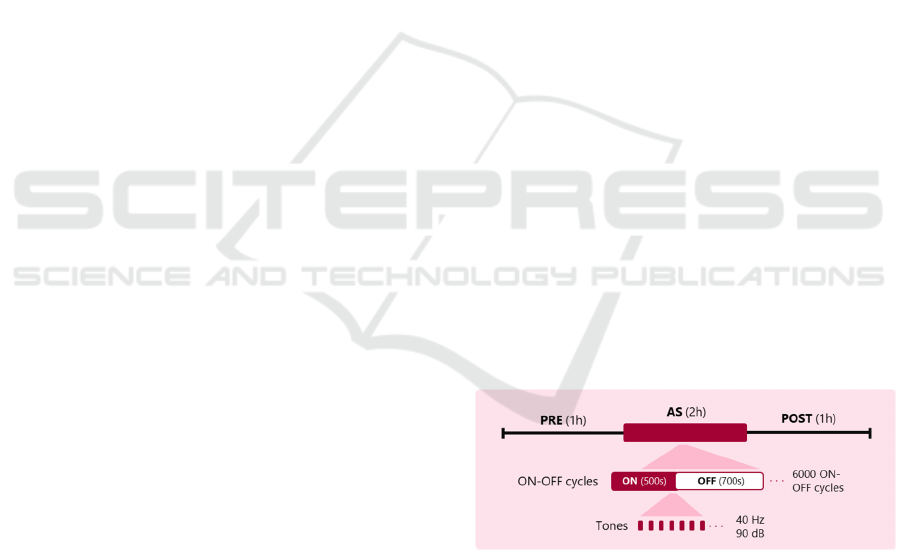
Figure 3: AS-protocol 2 adapted from Martorell et al.
(2019) and Lee et al. (2018). 1-h auditory stimulation (AS)
of 10-s ON (40 Hz pulses modulating 10 kHz tones) and
10-s OFF periods was preceded and followed by 1 h of
iEEG recordings, PRE and POST respectively, in
alternating days (D1, D3 and D5). Basal day (D0) and
resting days (D2 and D4), iEEG recording was performed
but no stimulation was not.
Mice were exposed to 1-ms pulses of 10 kHz
tones presented at 40 Hz. Pulses were delivered at 25
ms intervals (40 Hz with a 4% duty cycle) and at SPL
of 90 dB. Animals were subjected to 20-s cycles
alternating between 10-s periods of audio stimulation
(ON periods) interleaved with 10-s periods without
stimulation (OFF periods). These stimuli were
presented during 1-hr sessions, each consisting of 180
ON-OFF cycles, administered on AS days in
accordance with the protocol (Table 1).
2.6 Statistical Analysis
All statistical analyses were performed using
SigmaPlot v.12.0 (Systat, Germany). Unless
otherwise indicated, all statistical data are presented
as mean ± standard error of the mean (SEM). Outliers
were detected and removed using the z-score method
with a threshold of 2.0. Removal of outliers had no
significant impact on the results of the analysis.
Shapiro-Wilk normality test and the test for equality
of variance were performed. For normally distributed
data, an analysis of variance (one-way ANOVA)
followed by Bonferroni multiple comparison post-
hoc test were applied to determine significant
differences in the POST–PRE relative band power.
For not-normally distributed data, Kruskal-Wallis H
test followed by Dunn multiple comparison post-hoc
test were applied. Statistical significance was defined
as a p-value less than .05 (p < .05). Statistical values
were expressed as: *, if .05 > p > .005; **, if .005 > p
> .001; and ***, if p > .001.
3 RESULTS
To establish optimal experimental conditions for
investigating auditory-evoked neuronal oscillations,
we first conducted a comparative analysis of the
auditory capacity in CD1 and C57BL/6 mice, to select
the more suitable mouse strain where to test the effect
of auditory stimulation on spontaneous EEG. The
choice of CD1 and C57BL/6 mice strains was based
on their distinct genetic profiles, allowing for a
comprehensive evaluation of AS efficacy across
diverse genetic backgrounds, thereby increasing the
generalizability of the findings to a broader
population.
CAEPs recordings revealed a pronounced age-
dependent decline in hearing sensitivity in CD1 mice,
with complete auditory loss observed at 3 and 6
months of age at 0.5 and 5 kHz (0 of 8 CD1 mice of
both ages periods responded at the maximum of 100
dB; data not shown). In contrast, C57BL/6 mice aged
3 months maintained auditory function for 5 kHz
(41.57 ± 10.005 mV of N1 peak at 90 dB in the right
hemisphere) and 0.5 kHz (33.98 ± 9.067 mV) (Figure
4). Also, C57BL/6 exhibited a notable reduction in
hearing sensitivity at 6 months with no detectable
evoked responses in 2 of 4 mice studied at ~60 dB for
5 kHz and at ~70 dB for 0.5 kHz stimulation in the
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
756
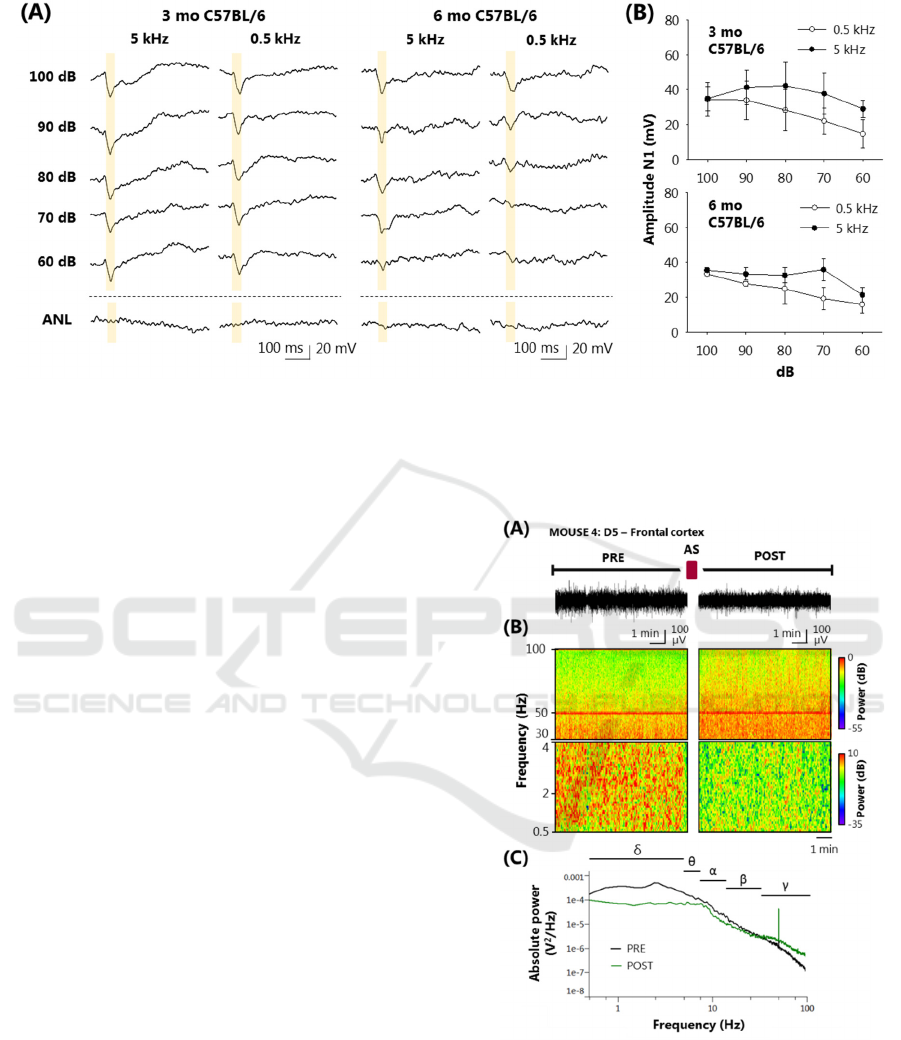
Figure 4: C57BL/6 mice auditory capacity evaluation by CAEPs. (A) CAEPs recordings of 3 (3 mo) and 6 (6 mo) months old
C57BL/6 mice at 5 and 0.5 kHz stimuli from 100 to 60 dB SPL dB. ANL means ambient noise level. (B) N1-peak amplitude
from first 50-ms baseline of 3 (upper graph) and 6 months old (lower graph) C57BL/6 mice at 5 and 0.5 kHz. Recordings
without an identifiable N1-peak were removed from the mean. Data is shown as means ± SEM analysed from four recordings
from each condition. No statistical analysis was performed.
right hemisphere. Among 6-months C57BL/6
mice with a detected N1-wave, a reduction auditory
function at 5 kHz (33.52 ± 3.55 mV of N1 peak at 90
dB in the right hemisphere) and 0.5 kHz (27.70 ± 1.21
mV) was observed compared with 3-months
C57BL/6 (Figure 4). N1 amplitude showed no
significant differences between the left and right
hemispheres in CD-1 and C57BL/6 mice with all the
parameters studied. These findings indicate how
crucial is to select an adequate mouse strain in
auditory research and suggest that C57BL/6 mice at 3
months might represent an appropriate model for
studying auditory-driven neural activity.
To investigate the impact of 40 Hz auditory
stimulation on iEEG activity, studies in 3-month-old
C57BL/6 mice were conducted following AS-
protocol 1 and 2 detailed in 2.4 subsection of
Materials and Methods, that include frequencies
higher than 0.5 kHz and SPL above 60 dB.
AS-protocol 1 adapted from Lee et al. (2018) with
40 Hz auditory pulses without high-frequency carrier
tone, showed no effects of auditory stimulation in
EEG power (data not shown). No longitudinal study
was performed.
AS-protocol 2 adapted from Martorell et al.
(2019) and Lee et al. (2018) with 10 kHz carrier tone
modulated by 40 Hz auditory pulses, showed EEG
changes produced by AS (Figure 5). iEEG recordings
were stable during 1-hr recording and total power
showed no statistical differences between days (data
not shown). Baseline-day (B) recordings revealed no
significant differences in POST–PRE relative
Figure 5: Spectral analysis of pre- (PRE) and post- (POST)
stimulation periods at day 5 from AS-protocol 2. (A)
Representative 10-min intracranial electroencephalography
(iEEG) recordings of D5 PRE and POST periods. (B)
Spectrograms (0.5–4 Hz, delta, upper panel; 30–100 Hz,
gamma, lower panel) obtained from the two same PRE and
POST regions showed in panel A. (C) Absolute power
iEEG of same PRE and POST regions showed in panel A
showing delta (δ) and theta (θ), and alpha (α), beta (β), and
gamma (γ) bands.
Modulating Cerebral Rhythms in Parkinson’s Disease: Insights on the Role of Auditory Stimulation
757
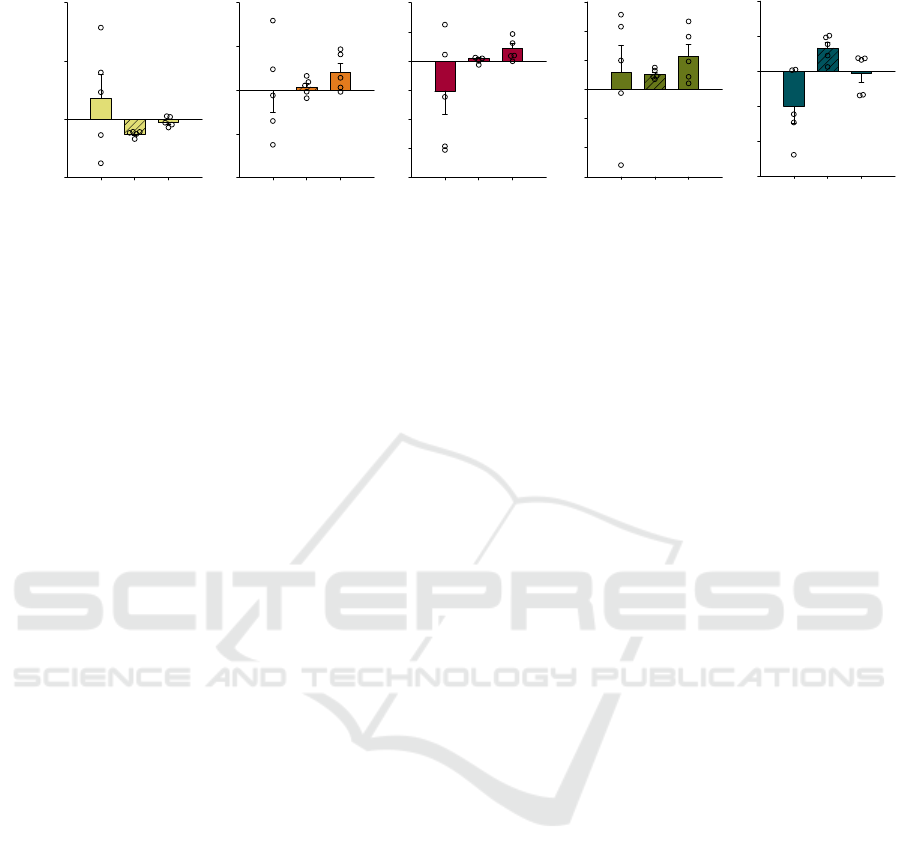
Figure 6: Relative power bands difference between pre- (PRE) or post-stimulation (POST) during basal day (B), auditory
stimulation days (D1, D3 and D5) and resting period days (D2 and D4) obtained from iEEG recordings of five mice at AS-
protocol 2. Data is shown as means ± SEM analyzed from five mice. No statistical test were performed.
potentials and in all frequency bands studied (Figure
6). However, during AS, frontal cortex showed a
consistent increase in gamma POST–PRE relative
power (+1.31 ± 0.38 %) accompanied by a marked
reduction in delta POST–PRE relative power (-2.54 ±
0.25 %), compared to their basal. The parietal cortex
showed the same observations in delta and gamma
POST–PRE relative powers (data not shown).
A longitudinal analysis of the data revealed that
delta relative power decreased during the post-
stimulation period compared to the pre-stimulation
period (Figure 7). However, subsequent resting days
(D2 and D4) showed a recovery of delta power to
baseline levels. Gamma relative power, on the other
hand, was increased during the post-stimulation
period and this effect persisted for at least one day
post-stimulation, as indicated in resting days (D2 and
D4). However, the effect of gamma power
enhancement appeared to dissipate by the second
resting day, as the PRE period of the subsequent
stimulation day (D3-PRE and D5-PRE) returned to
baseline levels. There were not statistically
significant differences in one-way ANOVA analysis.
4 DISCUSSION
The present study aims to establish a preclinical
model of AS to investigate its potential to modulate
aberrant neural oscillations in PD. Despite promising
findings in human studies demonstrating the capacity
of AS to normalize EEG power and enhance
functional connectivity (Gálvez et al., 2018;
González et al., 2023) and promising mice studies in
AD (Lee et al., 2018; Martorell et al., 2019; Murdock
et al., 2024), the optimal parameters for AS in PD
remain elusive. A standardized preclinical model is
crucial to systematically explore the effects of various
stimulation parameters to ensure accurate,
reproducible, and translatable results into effective
clinical interventions. Even though PD mice models
do not fully replicate all features of human pathology,
PD mice models are useful for studying specific
aspects of the disease, performing moderate invasive
procedures or chronic recordings and stimulations
that are unfeasible in humans (Blandini & Armentero,
2012; Chesselet & Richter, 2011; Meredith &
Rademacher, 2011).
In this study, we compared two distinct
bibliography-based AS protocols into the best hearing
mouse strain. The choice of CD1 and C57BL/6 mice
was strategic, as these strains exhibit contrasting
genetic profiles. CD1 mice is a nonconsanguineous
mouse that aimed to mimic the genetic diversity of
the human population.
Despite of that, these distinct genetic
characteristics can influence auditory sensitivity as
CD1 mice exhibited an age-related decline in auditory
sensitivity that has also observed in some studies
(Shone et al., 1991; Wu & Marcus, 2003). On the
other hand, C57BL/6, which provides a more
homogenous genetic background, maintained their
auditory function until 6 months of age. These results
should be treated with caution as other studies have
found C57BL/6 to develops progressive age-related
sensorineural hearing loss (Walton et al., 1995;
Willott, 1986). Perhaps the normal hearing of
C57BL/6 reported in our hands is related to the
specific substrain used. The “Ola” C57BL/6 substrain
was studied in our work whereas, unfortunately, most
studies do not report the specific examined substrains,
which as we have demonstrated here, can influence
hearing outcomes (Mekada et al., 2009).
Our findings also underscore the critical
importance of considering age and strain-specific
differences in auditory processing when designing
preclinical studies. A comprehensive characterization
of mice using objective auditory tests, such as
CAEPs, prior to initiating any auditory stimulation
protocol is essential.
DELTA
BASRP
POST-PRE relative
power difference (%)
-10
0
10
20
THETA
BASRP
-8
-4
0
4
8
ALPHA
BASRP
-16
-12
-8
-4
0
4
8
BETA
BASRP
-3
-2
-1
0
1
2
3
GAMMA
BASRP
-6
-4
-2
0
2
4
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
758
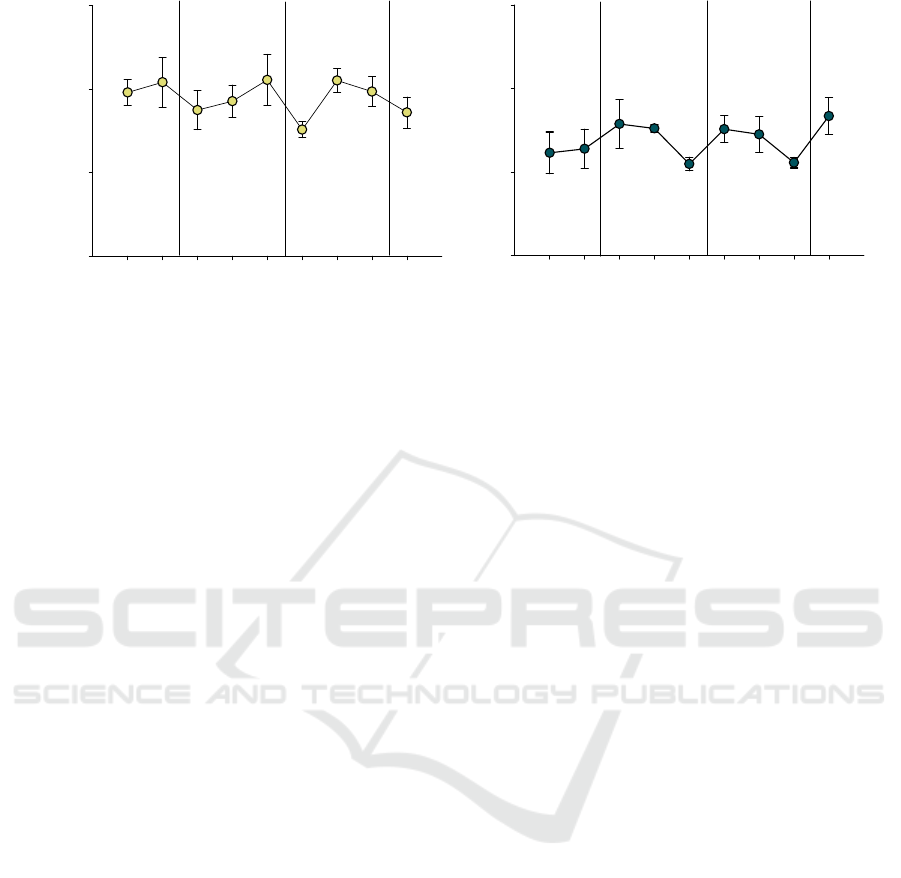
Figure 7: Relative delta (left panel) and gamma (right panel) power at baseline day (D0), pre- (PRE) and post-stimulation
(POST) periods days (D1, D3 and D5) and resting days (D2 and D4) obtained from iEEG recordings of five mice at AS-
protocol 2. Vertical black lines represent auditory stimulation (AS) periods. Data is shown as means ± SEM analyzed from
five mice. One-way analysis of variance (ANOVA) was performed.
Previous studies have demonstrated the efficacy
of prolonged auditory stimulation protocols, lasting
up to two weeks, in inducing long-lasting changes in
brain oscillations (Lee et al., 2018). However, our
study provides a novel perspective by evaluating the
effects of a shorter, more focused stimulation
protocol. By focusing on a shorter stimulation period,
we have been able to determine whether a more
concise intervention is enough to induce the desired
neuronal entrainment. This strategy has several
significant advantages as it allows for a reduction in
the time of exposure to stimulation, minimizing the
risk of adverse effects and facilitating patient
adherence to long-term treatment.
Surprisingly, when faithfully replicating the AS
protocol described Lee et al. (2018) in a mouse model
of AD, we did not observe the same effects in
reducing gamma power in the short-term. In
particular, the claim that 40 Hz pulses were used to
stimulate the auditory system of mice raises
questions. Considering the auditory range of rodents,
stimuli of this frequency are likely not optimal for
inducing significant changes in neuronal activity
(Heffner & Heffner, 2007; Naff et al., 2007;
Ohlemiller et al., 2016). Previous studies have shown
that the use of tones within the auditory range of mice,
combined with frequency modulations, is a more
effective strategy for modulating neuronal activity
(Kilgard et al., 2001; Martorell et al., 2019).
Consequently, we modified AS-protocol 1 exploring
the use of audible tones following with the aim of
optimizing the efficacy of stimulation and replicating
the results reported in peer-reviewed publications.
In contrast, the AS-protocol 2, incorporating a 10
kHz tone presented in 40 Hz auditory pulses, induced
notable changes in EEG power. Specifically, we
observed a significant increase in gamma band power
and a decrease in delta band power that is consistent
with previous studies demonstrating the potential of
AS to enhance cortical excitability and promote
neural synchrony (Gálvez et al., 2018; González et
al., 2023).
The observed increase in gamma power is
particularly intriguing, as gamma oscillations are
implicated in cognitive processes, including
attention, memory, and sensory perception. The
enhancement of gamma power may counteract the
slowing of neural oscillations, a hallmark of PD
(Soikkeli et al., 1991) Furthermore, the persistence of
gamma power enhancement for at least one day post-
stimulation suggests a potential, although reversible,
long-lasting impact of AS on neural networks.
However, further longitudinal studies with
continuous AS are needed to fully elucidate the time
course of these effects and to determine the optimal
stimulation parameters for maximizing therapeutic
benefits.
The reduction in delta power because of AS and
its subsequent recovery to baseline levels is also
noteworthy. Increased delta band power has been
identified as a potential early PD biomarker (Caviness
et al., 2015; Chu et al., 2021) that also associates with
dementia in PD (Bosboom et al., 2006). A study from
Stanley et al. (2019) in rhesus macaque monkey
examined the influence of gamma (40 Hz) click trains
on delta transient response followed finding an
entrainment of gamma and suppression of delta. Also,
D0
D1-PRE
D1-POST
D2
D3-PRE
D3-POST
D4
D5-PRE
D5-POST
Relative delta power (%)
20
30
40
50
D0
D1-PRE
D1-POST
D2
D3-PRE
D3-POST
D4
D5-PRE
D5-POST
Relative gamma power (%)
0
5
10
15
Modulating Cerebral Rhythms in Parkinson’s Disease: Insights on the Role of Auditory Stimulation
759

a construction of a computational model determined
that long duration gamma-rhythmic input stimuli
induce a steady-state containing entrainment at
gamma, and suppression of delta oscillations. This
suppression is achieved in the model by an action
driven by the thalamus.
Several limitations in this study warrant careful
attention. First, SPL used in AS protocols may be
high. Consequently, assessing auditory thresholds
before starting AS will improve animal welfare.
Second, given the potential for distortion in
conventional audio equipment, future studies should
rigorously control frequency behavior. Future
research will also refine AS-protocol 2 and
investigate the neural mechanisms modulated by AS
during the ON period. Further investigation should
focus on the therapeutic potential of the AS protocol
in PD models, particularly targeting early
pathological EEG biomarkers for timely intervention.
In conclusion, while our findings offer compelling
evidence supporting the capacity of AS to modulate
neural oscillatory activity in a preclinical PD model,
additional studies are imperative to delineate the
specific neural dynamics induced by AS and to
evaluate its long-term therapeutic efficacy.
5 CONCLUSIONS
In conclusion, this study represents a significant step
in elucidating and detailing an effective AS protocol
for PD. Our findings demonstrate that AS with 10
kHz tones presented with 40 Hz pulses can induce a
reduction in delta power and an increase in gamma
power, in the unmodified brain. AS-based
technologies could theoretically counteract EEG
changes linked with injury and neurodegeneration.
Our results reinforce the pathway toward the
identification of specific, increasingly optimized AS
paradigms for the treatment of PD.
ACKNOWLEDGMENTS
We thank Soledad Martinez for the excellent
technical assistance. This study was partially funded
by the Ministerio de Ciencia e Innovación (PID2023-
152058OB-I00) funded by MCIN/AEI/10.13039/
501100011033), Comunidad de Madrid (MINA-CM-
S2022/BMD-7236 and PEJ-2023-AI/SAL-GL-
26815) and the European Union's EIC-Pathfinder
Program, under the project THOR (Grant Agreement
number 101099719).
REFERENCES
Adaikkan, C., & Tsai, L. (2020). Gamma entrainment:
Impact on neurocircuits, glia, and therapeutic
opportunities. Trends in Neurosciences, 43(1), 24–41.
10.1016/j.tins.2019.11.001
Ahveninen, J., Kähkönen, S., Tiitinen, H., Pekkonen, E.,
Huttunen, J., Kaakkola, S., Ilmoniemi, R. J., &
Jääskeläinen, I. P. (2000). Suppression of transient 40-
hz auditory response by haloperidol suggests
modulation of human selective attention by dopamine
D2 receptors. Neuroscience Letters, 292(1), 29–32.
10.1016/S0304-3940(00)01429-4
Albouy, P., Martinez-Moreno, Z. E., Hoyer, R. S., Zatorre,
R. J., & Baillet, S. (2022). Supramodality of neural
entrainment: Rhythmic visual stimulation causally
enhances auditory working memory performance.
Science Advances, 8(8), eabj9782. 10.1126/sciadv.
abj9782
Barios, J. A., Pisarchyk, L., Fernandez-Garcia, L., Barrio,
L. C., Ramos, M., Martinez-Murillo, R., & Gonzalez-
Nieto, D. (2016). Long-term dynamics of
somatosensory activity in a stroke model of distal
middle cerebral artery oclussion. Journal of Cerebral
Blood Flow and Metabolism: Official Journal of the
International Society of Cerebral Blood Flow and
Metabolism, 36(3), 606–620. 10.1177/0271678X1560
6139
Benninger, D. H., Lomarev, M., Lopez, G., Wassermann,
E. M., Li, X., Considine, E., & Hallett, M. (2010).
Transcranial direct current stimulation for the treatment
of parkinson's disease. Journal of Neurology,
Neurosurgery & Psychiatry, 81(10), 1105–1111.
10.1136/jnnp.2009.202556
Benoit, C., Dalla Bella, S., Farrugia, N., Obrig, H., Mainka,
S., & Kotz, S. A. (2014). Musically cued gait-training
improves both perceptual and motor timing in
parkinson's disease. Frontiers in Human Neuroscience,
8, 494. 10.3389/fnhum.2014.00494
Blandini, F., & Armentero, M. (2012). Animal models of
parkinson's disease. The FEBS Journal, 279(7), 1156–
1166. 10.1111/j.1742-4658.2012.08491.x
Bosboom, J. L. W., Stoffers, D., Stam, C. J., van Dijk, B.
W., Verbunt, J., Berendse, H. W., & Wolters, E. C.
(2006). Resting state oscillatory brain dynamics in
parkinson's disease: An MEG study. Clinical
Neurophysiology: Official Journal of the International
Federation of Clinical Neurophysiology, 117(11),
2521–2531. 10.1016/j.clinph.2006.06.720
Bukowska, A. A., Krężałek, P., Mirek, E., Bujas, P., &
Marchewka, A. (2015). Neurologic music therapy
training for mobility and stability rehabilitation with
parkinson's disease - A pilot study. Frontiers in Human
Neuroscience, 9, 710. 10.3389/fnhum.2015.00710
Caviness, J. N., Hentz, J. G., Belden, C. M., Shill, H. A.,
Driver-Dunckley, E. D., Sabbagh, M. N., Powell, J. J.,
& Adler, C. H. (2015). Longitudinal EEG changes
correlate with cognitive measure deterioration in
parkinson's disease. Journal of Parkinson's Disease,
5(1), 117–124. 10.3233/JPD-140480
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
760

Chan, D., Suk, H., Jackson, B. L., Milman, N. P., Stark, D.,
Klerman, E. B., Kitchener, E., Fernandez Avalos, V. S.,
de Weck, G., Banerjee, A., Beach, S. D., Blanchard, J.,
Stearns, C., Boes, A. D., Uitermarkt, B., Gander, P.,
Howard, M., Sternberg, E. J., Nieto-Castanon, A., . . .
Tsai, L. (2022). Gamma frequency sensory stimulation
in mild probable alzheimer's dementia patients: Results
of feasibility and pilot studies. PloS One, 17(12),
e0278412. 10.1371/journal.pone.0278412
Chesselet, M., & Richter, F. (2011). Modelling of
parkinson's disease in mice. The Lancet. Neurology,
10(12), 1108–1118. 10.1016/S1474-4422(11)70227-7
Chu, C., Zhang, Z., Wang, J., Liu, S., Wang, F., Sun, Y.,
Han, X., Li, Z., Zhu, X., & Liu, C. (2021). Deep
learning reveals personalized spatial spectral
abnormalities of high delta and low alpha bands in EEG
of patients with early parkinson’s disease. Journal of
Neural Engineering, 18(6), 066036. 10.1088/1741-
2552/ac40a0
Fernández-García, L., Marí-Buyé, N., Barios, J. A.,
Madurga, R., Elices, M., Pérez-Rigueiro, J., Ramos, M.,
Guinea, G. V., & González-Nieto, D. (2016). Safety
and tolerability of silk fibroin hydrogels implanted into
the mouse brain. Acta Biomaterialia, 45, 262–275.
10.1016/j.actbio.2016.09.003
Fernández-García, L., Pérez-Rigueiro, J., Martinez-
Murillo, R., Panetsos, F., Ramos, M., Guinea, G. V., &
González-Nieto, D. (2018). Cortical reshaping and
functional recovery induced by silk fibroin hydrogels-
encapsulated stem cells implanted in stroke animals.
Frontiers in Cellular Neuroscience, 12, 296.
10.3389/fncel.2018.00296
Fernández-Serra, R., Martínez-Alonso, E., Alcázar, A.,
Chioua, M., Marco-Contelles, J., Martínez-Murillo, R.,
Ramos, M., Guinea, G. V., & González-Nieto, D.
(2022). Postischemic neuroprotection of
aminoethoxydiphenyl borate associates shortening of
peri-infarct depolarizations. International Journal of
Molecular Sciences, 23(13), 7449.
10.3390/ijms23137449
Fujiki, M., Yee, K. M., & Steward, O. (2020). Non-invasive
high frequency repetitive transcranial magnetic
stimulation (hfrTMS) robustly activates molecular
pathways implicated in neuronal growth and synaptic
plasticity in select populations of neurons. Frontiers in
Neuroscience, 14, 558. 10.3389/fnins.2020.00558
Gálvez, G., Recuero, M., Canuet, L., & Del-Pozo, F.
(2018). Short-term effects of binaural beats on EEG
power, functional connectivity, cognition, gait and
anxiety in parkinson's disease. International Journal of
Neural Systems, 28(5), 1750055. 10.1142/S01290657
17500551
García-Peña, P., Ramos, M., López, J. M., Martinez-
Murillo, R., de Arcas, G., & Gonzalez-Nieto, D. (2023).
Preclinical examination of early-onset thalamic-cortical
seizures after hemispheric stroke. Epilepsia, 64(9),
2499–2514. 10.1111/epi.17675
González, D., Bruña, R., Martínez-Castrillo, J. C., López, J.
M., & de Arcas, G. (2023). First longitudinal study
using binaural beats on parkinson disease. International
Journal of Neural Systems, 33(6), 2350027.
10.1142/S0129065723500272
Goodwill, A. M., Lum, J. A. G., Hendy, A. M., Muthalib,
M., Johnson, L., Albein-Urios, N., & Teo, W. (2017).
Using non-invasive transcranial stimulation to improve
motor and cognitive function in parkinson's disease: A
systematic review and meta-analysis. Scientific
Reports, 7(1), 14840. 10.1038/s41598-017-13260-z
Heffner, H. E., & Heffner, R. S. (2007). Hearing ranges of
laboratory animals. Journal of the American
Association for Laboratory Animal Science: JAALAS,
46(1), 20–22.
Henao, D., Navarrete, M., Valderrama, M., & Le Van
Quyen, M. (2020). Entrainment and synchronization of
brain oscillations to auditory stimulations.
Neuroscience Research, 156, 271–278. 10.1016/j.neur
es.2020.03.004
Herrero, M. A., Gallego, R., Ramos, M., Lopez, J. M., de
Arcas, G., & Gonzalez-Nieto, D. (2021). Sleep-wake
cycle and EEG-based biomarkers during late neonate to
adult transition. Brain Sciences, 11(3), 298.
10.3390/brainsci11030298
Iaccarino, H. F., Singer, A. C., Martorell, A. J., Rudenko,
A., Gao, F., Gillingham, T. Z., Mathys, H., Seo, J.,
Kritskiy, O., Abdurrob, F., Adaikkan, C., Canter, R. G.,
Rueda, R., Brown, E. N., Boyden, E. S., & Tsai, L.
(2016). Gamma frequency entrainment attenuates
amyloid load and modifies microglia. Nature,
540(7632), 230–235. 10.1038/nature20587
Ingendoh, R. M., Posny, E. S., & Heine, A. (2023). Binaural
beats to entrain the brain? A systematic review of the
effects of binaural beat stimulation on brain oscillatory
activity, and the implications for psychological research
and intervention. Plos One, 18(5), e0286023.
10.1371/journal.pone.0286023
Kilgard, M. P., Pandya, P. K., Vazquez, J., Gehi, A.,
Schreiner, C. E., & Merzenich, M. M. (2001). Sensory
input directs spatial and temporal plasticity in primary
auditory cortex. Journal of Neurophysiology, 86(1),
326–338. 10.1152/jn.2001.86.1.326
Lee, J., Ryu, S., Kim, H., Jung, J., Lee, B., & Kim, T.
(2018). 40 hz acoustic stimulation decreases amyloid
beta and modulates brain rhythms in a mouse model of
alzheimer’s disease.10.1101/390302
Li, S., Ma, L., Wang, Y., Wang, X., Li, Y., & Qin, L.
(2018). Auditory steady-state responses in primary and
non-primary regions of the auditory cortex in neonatal
ventral hippocampal lesion rats. PloS One, 13(2),
e0192103. 10.1371/journal.pone.0192103
Liu, S., Lei, Q., Liu, Y., Zhang, X., & Li, Z. (2022).
Acoustic stimulation improves memory and reverses
the contribution of chronic sleep deprivation to
pathology in 3xTgAD mice. Brain Sciences, 12(11),
1509. 10.3390/brainsci12111509
Martorell, A. J., Paulson, A. L., Suk, H., Abdurrob, F.,
Drummond, G. T., Guan, W., Young, J. Z., Kim, D. N.,
Kritskiy, O., Barker, S. J., Mangena, V., Prince, S. M.,
Brown, E. N., Chung, K., Boyden, E. S., Singer, A. C.,
& Tsai, L. (2019). Multi-sensory gamma stimulation
ameliorates alzheimer's-associated pathology and
Modulating Cerebral Rhythms in Parkinson’s Disease: Insights on the Role of Auditory Stimulation
761

improves cognition. Cell, 177(2), 256–271.e22.
10.1016/j.cell.2019.02.014
Mei, L., Liu, L., Chen, K., & Zhao, H. (2021). Early
functional and cognitive declines measured by
auditory-evoked cortical potentials in mice with
alzheimer's disease. Frontiers in Aging Neuroscience,
13, 710317. 10.3389/fnagi.2021.710317
Mekada, K., Abe, K., Murakami, A., Nakamura, S., Nakata,
H., Moriwaki, K., Obata, Y., & Yoshiki, A. (2009).
Genetic differences among C57BL/6 substrains.
Experimental Animals, 58(2), 141–149. 10.1538/expani
m.58.141
Meredith, G. E., & Rademacher, D. J. (2011). MPTP mouse
models of parkinson's disease: An update. Journal of
Parkinson's Disease, 1(1), 19–33. 10.3233/JPD-2011-
11023
Miladinović, A., Ajčević, M., Busan, P., Jarmolowska, J.,
Deodato, M., Mezzarobba, S., Battaglini, P. P., &
Accardo, A. (2021). EEG changes and motor deficits in
parkinson’s disease patients: Correlation of motor
scales and EEG power bands. Procedia Computer
Science, 192, 2616–2623. 10.1016/j.procs.2021.09.031
Murdock, M. H., Yang, C., Sun, N., Pao, P., Blanco-Duque,
C., Kahn, M. C., Kim, T., Lavoie, N. S., Victor, M. B.,
Islam, M. R., Galiana, F., Leary, N., Wang, S., Bubnys,
A., Ma, E., Akay, L. A., Sneve, M., Qian, Y., Lai, C., .
. . Tsai, L. (2024). Multisensory gamma stimulation
promotes glymphatic clearance of amyloid. Nature,
627(8002), 149–156. 10.1038/s41586-024-07132-6
Naff, K. A., Riva, C. M., Craig, S. L., & Gray, K. N. (2007).
Noise produced by vacuuming exceeds the hearing
thresholds of C57Bl/6 and CD1 mice. Journal of the
American Association for Laboratory Animal Science:
JAALAS, 46(1), 52–57.
Nakao, K., & Nakazawa, K. (2014). Brain state-dependent
abnormal LFP activity in the auditory cortex of a
schizophrenia mouse model. Frontiers in
Neuroscience, 8, 168. 10.3389/fnins.2014.00168
Ohlemiller, K. K., Jones, S. M., & Johnson, K. R. (2016).
Application of mouse models to research in hearing and
balance. JARO: Journal of the Association for Research
in Otolaryngology, 17(6), 493. 10.1007/s10162-016-
0589-1
Ross, B., & Lopez, M. D. (2020). 40-hz binaural beats
enhance training to mitigate the attentional blink.
Scientific Reports, 10(1), 7002. 10.1038/s41598-020-
63980-y
Shone, G., Raphael, Y., & Miller, J. M. (1991). Hereditary
deafness occurring in cd/1 mice. Hearing Research,
57(1), 153–156. 10.1016/0378-5955(91)90084-m
Soikkeli, R., Partanen, J., Soininen, H., Pääkkönen, A., &
Riekkinen, P. (1991). Slowing of EEG in parkinson's
disease. Electroencephalography and Clinical
Neurophysiology, 79(3), 159–165. 10.1016/0013-
4694(91)90134-P
Stanley, D. A., Falchier, A. Y., Pittman-Polletta, B. R.,
Lakatos, P., Whittington, M. A., Schroeder, C. E., &
Kopell, N. J. (2019, October 22). Flexible reset and
entrainment of delta oscillations in primate primary
auditory cortex: Modeling and experiment., 812024.
Steinmetz, J. D., Seeher, K. M., Schiess, N., Nichols, E.,
Cao, B., Servili, C., Cavallera, V., Cousin, E., Hagins,
H., Moberg, M. E., Mehlman, M. L., Abate, Y. H.,
Abbas, J., Abbasi, M. A., Abbasian, M., Abbastabar,
H., Abdelmasseh, M., Abdollahi, M., Abdollahi, M., . .
. Dua, T. (2024). Global, regional, and national burden
of disorders affecting the nervous system, 1990–2021:
A systematic analysis for the global burden of disease
study 2021. The Lancet Neurology, 23(4), 344–381.
10.1016/S1474-4422(24)00038-3
Walton, J. P., Frisina, R. D., & Meierhans, L. R. (1995).
Sensorineural hearing loss alters recovery from short-
term adaptation in the C57BL/6 mouse. Hearing
Research, 88(1-2), 19–26. 10.1016/0378-5955(95)00
093-j
Will, U., & Berg, E. (2007). Brain wave synchronization
and entrainment to periodic acoustic stimuli.
Neuroscience Letters, 424(1), 55–60.
10.1016/j.neulet.2007.07.036
Willott, J. F. (1986). Effects of aging, hearing loss, and
anatomical location on thresholds of inferior colliculus
neurons in C57BL/6 and CBA mice. Journal of
Neurophysiology, 56(2), 391–408.
10.1152/jn.1986.56.2.391
Wu, T., & Marcus, D. C. (2003). Age-related changes in
cochlear endolymphatic potassium and potential in CD-
1 and CBA/CaJ mice. Journal of the Association for
Research in Otolaryngology: JARO, 4(3), 353–362.
10.1007/s10162-002-3026-6
Ye, X., Li, L., He, R., Jia, Y., & Poon, W. (2022). Rhythmic
auditory stimulation promotes gait recovery in
parkinson's patients: A systematic review and meta-
analysis. Frontiers in Neurology, 13, 940419.
10.3389/fneur.2022.940419
Zhang, J., Wang, T., Zhang, C., Zeljic, K., Zhan, S., Sun,
B., & Li, D. (2017). The safety issues and hardware-
related complications of deep brain stimulation therapy:
A single-center retrospective analysis of 478 patients
with parkinson’s disease. Clinical Interventions in
Aging, 12, 923. 10.2147/CIA.S130882
Zheng, L., Yu, M., Lin, R., Wang, Y., Zhuo, Z., Cheng, N.,
Wang, M., Tang, Y., Wang, L., & Hou, S. (2020).
Rhythmic light flicker rescues hippocampal low
gamma and protects ischemic neurons by enhancing
presynaptic plasticity. Nature Communications, 11(1),
3012. 10.1038/s41467-020-16826-0
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
762
