
Improving Classification in Skin Lesion Analysis Through Segmentation
Mirco Gallazzi
a
, Anwar Ur Rehman
b
, Silvia Corchs
c
and Ignazio Gallo
d
Department of Theoretical and Applied Science, University of Insubria, 21100 Varese, Italy
Keywords:
YOLO, Swin Transformer, Object Detection and Segmentation, Medical Imaging, Dermatology, Skin Cancer.
Abstract:
Deep Learning plays a vital role in medical imaging, especially in classification and segmentation tasks es-
sential for diagnosing diseases from images. However, current methods often struggle to differentiate visually
similar classes and accurately delineate lesion boundaries. This study builds on prior findings of classification
limitations, investigating whether segmentation can improve classification performance for skin lesion analy-
sis with Transformer-based models. We benchmarked the segmentation capabilities of the Swin Transformer,
YOLOv8, and DeepLabV3 architectures on the HAM dataset, which contains 10,015 images across seven skin
lesion classes. Swin outperformed others in segmentation, achieving an intersection over union of 82.75%,
while YOLOv8 achieved 77.0%. However, classification experiments using classification datasets after seg-
menting and cropping the lesion of interest did not produce the expected improvements, with classification
accuracy showing slight drops in the segmented data. For example, on the original HAM dataset, the model
achieved a Test Accuracy (TA) of 84.64%, while Swin trained on segmented data showed a slight decline to
a TA of 84.13%. These findings suggest that segmentation alone may not effectively support classification.
Based on this, we propose future research into a sequential transfer learning approach, where segmentation
knowledge could be progressively transferred to improve classification.
1 INTRODUCTION
In medical imaging, classification and segmentation
support in-depth disease analysis. Classification cat-
egorizes images by pathology, while segmentation
highlights specific structures like lesions, adding spa-
tial information that may improve class differentiation
in dermatology.
Advances in Deep Learning (DL), such as those
demonstrated by Esteva et al. (Esteva et al., 2017),
have underscored both the potential and limitations
of classification in skin lesion analysis, particularly
in managing nuanced visual distinctions essential for
accurate diagnosis. It has been shown in the litera-
ture that Transformer-based models, specifically the
Swin Transformer (Swin) (Liu et al., 2021), achieved
high classification accuracy across multiple skin le-
sion types, affirming the model’s promise in skin can-
cer analysis (Gallazzi et al., 2024). However, persis-
tent challenges remain, particularly in differentiating
between classes with subtle visual similarities, such
a
https://orcid.org/0009-0000-3850-8086
b
https://orcid.org/0000-0002-9384-8988
c
https://orcid.org/0000-0002-1739-8110
d
https://orcid.org/0000-0002-7076-8328
as Nevus (NV) and Melanoma (MEL), suggesting that
classification alone may lack the precision required
for reliable distinction.
Given these limitations, we hypothesize that seg-
mentation, when applied as a supportive task, could
enhance classification performance by embedding
spatial context around lesion boundaries and struc-
tures, providing a refined feature set for complex le-
sion types. This study begins by investigating seg-
mentation as an isolated task, examining its effective-
ness on the HAM dataset (Tschandl and Rosendahl,
2018), which comprises over 10,000 images across
seven skin lesion classes. We benchmark the seg-
mentation capabilities of Swin, YOLOv8 (Ultralytics,
2024), and a ResNet(DeepLabV3) (He et al., 2016),
assessing whether segmentation independently con-
tributes meaningful improvements. Following this
evaluation, we use cropped images of the segmented
regions from each model as input for classification
tasks, enabling a direct comparison of classification
accuracy with and without segmented data.
Our findings reveal that using cropped images
from segmented regions alone does not significantly
enhance classification performance and may even in-
troduce minor declines, suggesting that segmentation
696
Gallazzi, M., Rehman, A. U., Corchs, S. and Gallo, I.
Improving Classification in Skin Lesion Analysis Through Segmentation.
DOI: 10.5220/0013247900003905
In Proceedings of the 14th International Conference on Pattern Recognition Applications and Methods (ICPRAM 2025), pages 696-703
ISBN: 978-989-758-730-6; ISSN: 2184-4313
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)

and classification independently may not sufficiently
enhance model accuracy. These observations lead us
to consider a Sequential Transfer Learning (STL) ap-
proach, where the knowledge from segmentation of
cropped regions is sequentially transferred to classifi-
cation. This framework could allow segmentation to
embed boundary and structural details that classifica-
tion can then refine, addressing the interpretative chal-
lenges presented by visually similar lesion types. This
approach raises two primary questions: ”How can
segmentation knowledge be effectively transferred to
improve classification performance?” and “In what
ways does a sequential integration of segmentation
and classification impact interpretative accuracy and
robustness in skin lesion analysis?”
The main contributions are:
1. Compare Swin’s segmentation performance rel-
ative to YOLOv8 and DeepLabV3, assessing its
utility in medical imaging.
2. Evaluating whether using cropped images de-
rived from segmentation enhances class separabil-
ity when used as a preprocessing step for classifi-
cation.
3. Discuss the potential of an STL-based integration,
discussing how findings from our study and rel-
evant literature support task sequence alignment
for performance gains in future work.
Our paper is structured as follows: Section 2 reviews
current approaches in segmentation tasks. Section 3
provides details on the HAM dataset and model ar-
chitectures, and Section 4 presents our experimental
results. Finally, Section 5 discusses our findings and
outlines future directions for a potential STL pipeline
in skin lesion detection.
2 RELATED WORKS
DL models have shown significant success in both
classification and segmentation tasks, enhancing per-
formance in various real-time applications such
as autonomous driving, agriculture (Gallo et al.,
2023), industrial automation (Rehman and Gallo,
2024)(Rehman et al., 2023), and especially medical
imaging. Dermatology, in particular, benefits from
these advancements, yet certain nuances in skin lesion
differentiation remain challenging, necessitating fur-
ther exploration of methods that could improve class
separability in complex cases(Gallazzi et al., 2024).
Convolutional Neural Networks (CNNs) (O’Shea,
2015) and Transformer-based architectures have
yielded strong results in skin lesion classification,
often using datasets like HAM. Esteva et al. (Es-
teva et al., 2017) developed a CNN for skin cancer
classification with performance comparable to der-
matologists, marking a significant milestone in the
field. ResNet, introduced in (He et al., 2016), is a
widely adopted CNN architecture in medical imag-
ing due to its residual connections, which allow for
deeper networks and improved detection of subtle
differences. Integrated into encoder-decoder frame-
works, such as U-Net (Ronneberger et al., 2015),
DeepLabv3’s deep layers enable robust feature ex-
traction and fine-grained segmentation.
U-Net’s encoder-decoder architecture with skip
connections has become foundational in medical seg-
mentation due to its precise spatial reconstruction ca-
pabilities. Adaptations of U-Net with attention mech-
anisms and multi-scale processing (Azad et al., 2024)
have addressed challenges specific to complex medi-
cal images, including dermatology.
The adaptation of Transformer-based models, par-
ticularly Swin, has advanced segmentation accu-
racy by capturing global dependencies within high-
resolution images. Liu et al. (Liu et al., 2021)
demonstrated Swin’s strengths in managing complex
lesion boundaries in dermatoscopic images. Swin-
Unet (Wang et al., 2022) combines Swin’s attention
features with U-Net’s localization capabilities, mak-
ing it highly suitable for anatomically complex re-
gions in challenging datasets.
YOLO-based models, originally developed for
object detection, have been adapted to skin lesion
segmentation to address scale and feature extrac-
tion challenges. With real-time processing capabil-
ities and the integration of attention mechanisms,
YOLO models demonstrate increased sensitivity to
lesion boundaries, facilitating effective segmentation
in high-resolution dermoscopic images. Multi-step
approaches, such as the combination of YOLO and
SegNet proposed in (Taghizadeh and Mohammadi,
2022), have been shown to optimize segmentation ac-
curacy.
Given these advancements, the potential of STL
has become an area of interest, especially for tasks
where segmentation might support or refine classifi-
cation. STL involves training a model on one task,
such as segmentation, to capture intricate structural
features before fine-tuning it for classification. This
approach could leverage the strengths of each task se-
quentially. According to (Mao, 2020), this framework
may allow models to better adapt knowledge from
segmentation to classification, addressing class sep-
arability challenges in visually complex datasets.
Improving Classification in Skin Lesion Analysis Through Segmentation
697
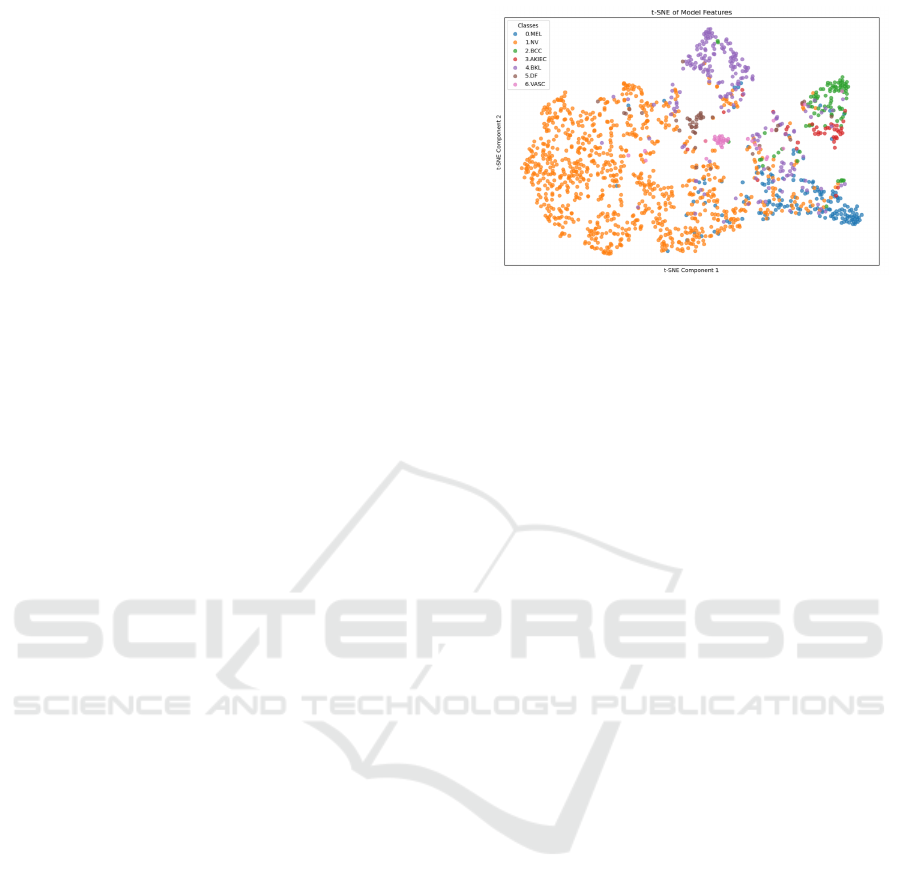
3 METHODOLOGY
Following the Introduction 1 and Related Works 2, we
now delve into the challenges of applying STL to skin
cancer classification, focusing on distinguishing be-
tween visually similar classes. Although transform-
ers like Swin have shown high accuracy across mul-
tiple medical imaging tasks, a detailed T-Distributed
Stochastic Neighbor Embedding (t-SNE) (Cai and
Ma, 2022) visualization of the feature space reveals
an inherent limitation in this context in Figure 1.
The plot shows that lesion classes with subtle visual
similarities—such as Nevus and Melanoma—tend to
cluster closely, leading to substantial overlap. This
clustering highlights the challenge of forming well-
defined boundaries between classes that share intri-
cate visual patterns, underscoring a critical gap in
model interpretability and reliability.
This limitation prompts an investigation into
whether segmentation can enhance class separability
by introducing spatial details and isolating key char-
acteristics that classification struggles to distinguish.
Segmentation, by improving boundary detection and
emphasizing lesion-specific features, could provide a
refined representation of each class, potentially en-
hancing classification outcomes.
In the following subsections, we examine the
dataset and model architectures used in this study to
address the challenges. Specifically, we explore the
effectiveness of YOLO, Swin, and DeepLabV3 mod-
els—each selected for its unique advantages in seg-
mentation and/or classification. YOLO’s strengths in
real-time object detection make it a valuable candi-
date for efficient segmentation, particularly in high-
resolution dermoscopic images, bringing great bene-
fits to creating a segmented dataset through its use.
With a slight modification to the original architecture,
Swin could effectively capture long-range depen-
dencies by handling complex boundary delineations
within high-resolution images. DeepLabV3, with its
residual connections, is widely adopted in medical
imaging and serves as a robust baseline for classifica-
tion and segmentation tasks due to its strong feature
extraction capabilities. Together, these models cover
a range of learning frameworks (hierarchical, resid-
ual, and attention-based), offering a comprehensive
approach for testing segmentation’s impact on clas-
sification. Section 2 has highlighted the adaptability
of these architectures across various medical imaging
tasks, and here, we aim to assess their performance
specifically on the HAM dataset, which provides both
classification labels and segmentation masks to sup-
port a comprehensive evaluation across both tasks.
Figure 1: T-SNE visualization of class embeddings, illus-
trating the significant overlap between visually similar skin
lesion classes, underscoring the challenge of achieving clear
class separability in classification tasks.
3.1 Human Against Machine (HAM)
The HAM dataset, which includes both classification
labels and segmentation masks across seven diverse
dermatological classes, serves as a benchmark in our
study, allowing a robust evaluation of model perfor-
mance in analyzing distinct yet visually similar lesion
types.
The training dataset encompasses 10,015 images
distributed across the following seven classes: MEL,
NV, Basal Cell Carcinoma (BCC), Basal Cell Car-
cinoma (BCC), Actinic Keratoses (AKIEC), Benign
Keratosis Like (BKL), Dermatofibroma (DF), Vascu-
lar (VASC) comprises 1,113, 6,705, 514, 327, 1,099,
115, and 142 images respectively. Each class repre-
sents a unique diagnostic category, contributing to the
dataset’s complexity and offering a suitable challenge
for classification and segmentation models. Figure 2
illustrates an example image from each class.
HAM also provides two distinct external test sets
for classification and segmentation to enable a thor-
ough evaluation. The classification test set comprises
1,511 images, categorized as follows: 171 MEL, 908
NV, 93 BCC, 43 AKIEC, 217 BKL, 44 DF, and 35
VASC. This distribution reflects the variety and im-
balance in clinical datasets, testing model robustness
across less frequent classes and those with visual sim-
ilarities.
HAM provides a separate segmentation test set of
1,000 images, each with a corresponding segmenta-
tion mask. This segmentation set is critical for as-
sessing model capabilities in identifying and isolat-
ing lesion boundaries. We hypothesize that this task
may help address classification limitations by provid-
ing spatially precise lesion delineations.
By utilizing both test sets, we evaluate our models
on classification and segmentation performance, com-
paring how each approach handles the specific chal-
ICPRAM 2025 - 14th International Conference on Pattern Recognition Applications and Methods
698

lenges posed by the HAM dataset’s complex and di-
verse lesion types.
3.2 Deep Models: DeepLabV3 vs Swin
DeepLabV3 and Swin are adapted into encoder-
decoder structures for effective pixel-wise predictions
in segmentation tasks. DeepLabV3’s convolutional
backbone is the encoder, extracting increasingly ab-
stract features through residual blocks, while the de-
coder restores spatial resolution via transposed convo-
lutions or upsampling layers. Skip connections retain
low-level details, enhancing boundary accuracy. The
residual function in each encoder block processes the
input X as:
F(X) = σ(W X + b)+ X (1)
where F(X) is the output combining learned trans-
formations and original input, W and b are the weight
matrix and bias, and σ is the non-linear activation
function.
This formulation allows gradients to bypass the
transformation layer, ensuring stable training even in
deep networks, as the residual connections mitigate
vanishing gradient issues.
In contrast, the Swin adapts its hierarchical
transformer blocks into an encoder-decoder frame-
work specifically suited for segmentation by utiliz-
ing window-based multi-head self-attention within
the encoder. This segmentation approach applies self-
attention within small, shifted windows, capturing
spatial dependencies locally while maintaining com-
putational efficiency. The self-attention computation
for each window can be represented as:
Attention(Q, K, V ) = softmax
QK
T
√
d
V (2)
where Q, K, and V represent the query, key, and
value matrices, and d is the scaling factor. Swin’s
decoder restores spatial resolution with patch em-
beddings, blending local details and global context.
Its multi-scale architecture aligns with the encoder-
decoder paradigm, enhancing boundary delineation at
multiple resolutions.
For the decoder, Swin applies patch embedding
layers to restore the spatial resolution, using attention
mechanisms to integrate local detail with global con-
text. Furthermore, Swin’s multi-scale structure pro-
vides a natural fit for the encoder-decoder paradigm,
with feature maps at varying resolutions feeding into
corresponding levels of the decoder to produce seg-
mentation maps with accurate boundary delineations.
Given that self-attention in Swin’s encoder scales
with complexity:
O((H ×W ) ×d
2
) (3)
where H and W denote the height and width of the
input feature map, and d the window size.
This complexity highlights the balance Swin
achieves between global context capture and compu-
tational feasibility, using a manageable number of at-
tention computations within each window.
ResNet’s convolutional encoder is proficient at
identifying localized patterns, ideal for tasks with
clear boundaries. Swin, however, captures long-
range dependencies and complex spatial relationships
through attention mechanisms, excelling in images re-
quiring both local and global context. While ResNet-
based decoders often use convolutional upsampling,
Swin’s attention-based upsampling integrates multi-
scale global features, improving boundary and texture
precision in segmentation outputs.
3.3 You Only Look Once (YOLO)
YOLO is a family of models designed for real-time
object detection that treats the detection task as a
regression problem rather than a classification prob-
lem combined with region proposals (Redmon, 2016)
(Rehman and Gallo, 2024). Unlike previous object
detection methods, YOLO divides the image into an
S ×S grid, and each grid cell is responsible for de-
tecting objects whose centers fall within it. For each
grid cell, YOLO predicts B bounding boxes, a confi-
dence score, and class probabilities. The key benefits
of YOLO models are their speed and efficiency, al-
lowing real-time detection even on limited hardware.
This paper adopted the YOLOv8 model, which is the
latest version of the YOLO series of object detection
models, designed to enhance the accuracy and speed
of the previous versions. YOLOv8 improves over
YOLOv7 with more efficient feature extraction and
better loss function optimization (Ultralytics, 2024).
Unlike its predecessors, YOLOv8 can perform classi-
fication, object detection, and instance segmentation,
making it an all-in-one solution for computer vision
tasks.
YOLOv8 introduces several key improvements com-
pared to earlier versions, including an improved back-
bone network for feature extraction, an advanced
anchor-free detection head, enhanced loss functions
for more accurate training, and additional support for
instance segmentation tasks.
The YOLOv8 architecture is built upon three main
components: the backbone, the neck, and the head.
The backbone extracts features from the input im-
age. In YOLOv8, CSP (Cross Stage Partial) networks
(Wang et al., 2020) is used as the backbone, which
reduces computational complexity while maintaining
feature quality. The input image of size W ×H ×3 is
Improving Classification in Skin Lesion Analysis Through Segmentation
699

Figure 2: Examples of each of the seven classes of skin disease images from the HAM dataset.
transformed into a set of feature maps through convo-
lutional layers and down-sampling operations.
The neck aggregates features from different backbone
levels to improve detection accuracy for objects of
different sizes. YOLOv8 employs a PANet (Path Ag-
gregation Network) (Liu et al., 2018) that ensures ef-
ficient information flow from high-resolution to low-
resolution layers and vice versa.
The detection head predicts bounding boxes, object-
ness scores, class labels, and segmentation masks.
Unlike anchor-based approaches in earlier versions,
YOLOv8 uses an anchor-free mechanism that directly
predicts the object’s center, eliminating the complex-
ity associated with anchor boxes.
Loss Function: YOLOv8’s training relies on several
loss functions to optimize object detection and seg-
mentation accuracy. However, the most commonly
used loss function is GIoU loss.
It aims to provide a better measure for non-
overlapping boxes by considering the smallest enclos-
ing box C:
L
GIoU
= 1 −IoU +
|C −(B
p
∪B
g
)|
|C|
. (4)
4 EXPERIMENTS AND RESULTS
Our primary goal in implementing Swin,
DeepLabV3, and YOLO architectures on the
HAM dataset was to explore whether using seg-
mentation to derive cropped images could enhance
classification outcomes, especially in dermatolog-
ical settings where visually similar lesion classes
are challenging to differentiate. By studying the
segmentation and classification performance using
the segmented images that these models generate,
this investigation seeks to confirm the generalization
capabilities and robustness of each model under the
unique constraints and characteristics of HAM data.
To assess segmentation and classification perfor-
mance on the HAM dataset, we employed several key
metrics: Intersection over Union (IoU) for segmenta-
tion accuracy and accuracy, precision, recall, and F1-
score for classification. Each metric offers specific
insights, with IoU indicating the extent of overlap be-
tween predicted and ground-truth regions in segmen-
tation and the other metrics assessing the accuracy
and robustness of classification across classes with
varying visual similarities.
The metrics used are defined as follows:
IoU =
T P
T P + FP + FN
(5)
Accuracy =
T P + T N
Total Samples
(6)
Precision =
T P
T P + FP
(7)
Recall =
T P
T P + FN
(8)
F1-score = 2 ×
Precision ×Recall
Precision + Recall
(9)
Where ”TP” indicates the ”True Positives”, ”TN”
the ”True Negatives”, ”FP” ”FN” indicates the ”False
Positives” and the ”False Negatives”, respectively.
All computations were performed on a container-
ized environment utilizing 1 Nvidia A100 80GB
GPU, 16 cores of an AMD Epyc 7742 64-core CPU,
and 64GB of DDR4-3200 RAM, all connected to a
76TB RAID6 storage server via a 25Gbps low-latency
network. The environment used in our experiments
uses PyTorch version 2.1.0 with CUDA version 11.8
and Python version 3.11.6.
4.1 Yolo vs ResNet/Swin in
Segmentation
Table 1: Segmentation Results on HAM Test Set.
Experiment Type Model IoU (%)
Seg(Challenge) MaskRcnn2 80.2
Seg DeepLabV3 81.66
Seg Swin 82.75
Seg YOLO 77.0
In our first experiment, we evaluate the segmentation
capabilities of three models—YOLOv8, DeepLabV3
architecture (Chen et al., 2018), and Swin—on the
HAM dataset. The dataset was split into 80% for
training and 20% for validation. Each model was
trained to learn to segment skin lesions by directly
comparing predictions against the true segmentation
masks provided in HAM. After each training epoch,
model performance was assessed on the external
HAM segmentation test set, identical to the test set
used in the HAM segmentation challenge (ISIC Chal-
lenge, 2024). This alignment enables a direct bench-
mark comparison, allowing us to assess our results in
the context of the 2018 competition standards.
ICPRAM 2025 - 14th International Conference on Pattern Recognition Applications and Methods
700

Table 2: Classification results on the HAM test set. TA, TP, TR, and TF1 indicate Test Accuracy, Test Precision, Test Recall,
and Test F1 Score (%). The ”Dataset” column shows the HAM dataset segmented by different models.
Experiment Type Dataset Model TA (%) TP (%) TR (%) TF1 (%)
Classification HAM Swin 84.64 84.77 84.64 84.57
Classification HAM segmented YOLO Swin 84.01 84.09 84.12 83.53
Classification HAM segmented DeepLabV3 Swin 84.12 84.38 84.12 83.75
Classification HAM segmented Swin Swin 84.13 84.41 84.12 83.58
Segmentation performance was evaluated using
the IoU (5) metric, consistent with the evaluation cri-
teria used in the HAM challenge. In the challenge, the
winning score reached an IoU of 80.2%. Our results
showed in Table 1 indicate improved performance,
with DeepLabV3 achieving an IoU of 81.66% and
Swin reaching 82.75% while YOLO implementation
didn’t surpass the bench value, achieving a 77.0%.
These results highlight residual and transformer-
based architectures’ effectiveness in medical image
segmentation, especially in distinguishing complex
lesion boundaries.
The segmentation experiment underscores the ca-
pacity of Swin and DeepLabv3-based architectures to
generalize effectively in medical segmentation tasks,
establishing a strong foundation for further integra-
tion of segmentation into a comprehensive classifica-
tion task.
4.2 Yolo vs ResNet/Swin in
Classification
Following the results of the segmentation experi-
ment in 4.1, we created three separate versions of
cropped HAM images, each derived from the segmen-
tation masks generated by the three models: YOLO,
DeepLabV3, and Swin. Each model produced a sep-
arate segmented dataset, leveraging its own segmen-
tation capabilities to generate unique annotations for
the HAM images. This approach allowed us to ex-
plore how different segmentation models might influ-
ence classification performance when used as prepro-
cessed inputs.
Each of these newly created datasets of cropped
images from the segmented regions was subsequently
fed into the Swin model to train it on classification,
aiming to assess any potential improvements in dis-
tinguishing between dermatological multi-classes. In
this experiment, we evaluated classification perfor-
mance using accuracy (6), precision (7), recall (8),
and f1-score (9) to gauge whether segmentation could
enhance the Swin model’s ability to differentiate be-
tween classes with subtle visual similarities.
The results presented in Table 2 show that using
cropped images derived from segmented regions of
the HAM dataset did not yield a meaningful improve-
ment in classification performance. The Swin model
achieved a Test Accuracy (TA) of 84.64%, Test Pre-
cision (TP) of 84.77%, Test Recall (TR) of 84.64%,
and Test F1 Score (TF1) of 84.57% when trained on
the original HAM dataset, serving as a benchmark.
In contrast, when trained on cropped images
segmented by each of the three models—YOLO,
DeepLabV3, and Swin itself—performance declined
slightly across all metrics. The YOLO-segmented
dataset yielded a TA of 84.01%, TP of 84.09%, TR
of 84.12%, and TF1 of 83.53%. DeepLabV3’s seg-
mented dataset resulted in a TA of 84.12%, TP of
84.38%, TR of 84.12%, and TF1 of 83.75%. Simi-
larly, Swin’s own segmented dataset achieved a TA of
84.13%, TP of 84.41%, TR of 84.12%, and TF1 of
83.58%.
These findings indicate that using segmented data
for classification may introduce noise or obscure crit-
ical details, particularly in classes with similar visual
features. This leads to slightly reduced accuracy and
other metrics. This outcome underscores the limita-
tions of using segmented images alone for classifica-
tion tasks. It reinforces the need for an approach that
can sequentially optimize segmentation and classifi-
cation within a unified framework. Moreover, while
segmentation provides valuable spatial details, its role
as a preprocessing step for classification did not yield
the expected improvements. This study thus moti-
vates a shift toward STL as a potential solution for
sequentially combining these tasks to enhance overall
model performance in future work.
4.3 Integrating Segmentation in
Classification: Discussion
The experiments in Section 4.1 confirmed that the se-
lected models, particularly Swin, perform effectively
in skin lesion segmentation, demonstrating robustness
in capturing nuanced boundaries and structural de-
tails important for medical imaging. This supports
Swin’s utility in segmentation tasks, where precise
delineation is essential for reliable lesion analysis.
The strong performance in segmentation highlights
the potential of Swin to capture spatial details that
could be valuable for other tasks, such as classifica-
tion.
Improving Classification in Skin Lesion Analysis Through Segmentation
701

However, as shown in Section 4.2, our subse-
quent classification experiments—using datasets seg-
mented by each model—did not yield the anticipated
improvements. This indicates that the segmented
datasets may have removed critical information or in-
troduced noise, which is especially problematic when
distinguishing between visually similar lesion classes.
These results highlight a potential limitation in using
segmented images alone for classification, particu-
larly when segmentation is treated as a preprocessing
step rather than being integrated into a unified frame-
work.
Given these findings, an intriguing question arises:
If segmentation alone does not improve classifica-
tion outcomes, could sequentially transferring knowl-
edge from segmentation to classification enhance per-
formance? Recent research on Sequential Transfer
Learning (STL) supports this hypothesis. Studies
such as Paulsen and Casey (Paulsen and Casey, 2023)
have shown the benefits of STL, where segmenta-
tion pre-training improves the classification of com-
plex visual classes by leveraging spatial information
gained in the initial phase. Similarly, Chan et al.
(Chan et al., 2023) demonstrated that STL approaches
involving pre-training on large datasets before fine-
tuning on target tasks can significantly enhance model
accuracy. Tirinzoni et al. (Tirinzoni et al., 2020) fur-
ther highlighted that spatial insights gained in the first
stage of STL can be effectively transferred to improve
performance in downstream tasks.
In addition to these findings, Wang et al. (Wang
et al., 2023) proposed a Collaborative Learning Deep
Convolutional Neural Networks model, which em-
phasizes the interdependence between segmentation
and classification tasks. Their work demonstrates that
segmentation can improve classification by provid-
ing lesion contour information, while classification
can enhance segmentation through target localization
maps. This highlights the potential of collaborative
learning to exploit the correlation between tasks, par-
ticularly when sample data are limited. The findings
from Wang et al. further motivate exploring methods
like STL that sequentially or collaboratively integrate
segmentation and classification.
Building on these insights, we propose an STL
framework that first trains the Swin model on seg-
mentation to capture essential boundary and struc-
tural characteristics, followed by fine-tuning for clas-
sification. Unlike traditional approaches that treat
these tasks in isolation, STL allows the model to pro-
gressively refine its feature representations, leverag-
ing segmentation-derived spatial insights to enhance
lesion differentiation in classification.
Future work will focus on evaluating the impact of
this STL approach, aiming to establish a more accu-
rate and robust pipeline for skin lesion analysis. The
core idea is that STL can capitalize on the strengths of
each task in sequence, with the hypothesis that Swin’s
performance can be enhanced by incrementally refin-
ing features relevant to segmentation and classifica-
tion, thereby capturing finer details that may be over-
looked in a traditional isolated setup.
5 CONCLUSIONS
This study aimed to evaluate the Swin model’s effec-
tiveness in skin lesion classification using segmented
images, to benchmark Swin’s segmentation capa-
bilities alongside YOLO and DeepLabV3 architec-
tures, and to explore the potential for a segmentation-
classification pipeline based on a sequential approach
in dermatological analysis. The experiments provided
valuable insights into each of these objectives.
Firstly, we assessed the standalone effectiveness
of Swin, YOLO, and DeepLabV3 for skin lesion
segmentation, comparing their performances on the
HAM dataset. Our results suggest that Swin performs
competitively with the other models, effectively man-
aging complex boundary details and demonstrating its
potential as a reliable tool for medical segmentation
tasks.
Secondly, we investigated whether segmentation
could improve class separability in classification tasks
by using cropped images of the segmented regions as
inputs for classification. The findings indicated that,
while segmentation captures detailed structural infor-
mation, using segmented data alone did not improve
classification accuracy. This outcome suggests that
segmentation, although beneficial for boundary delin-
eation, may inadvertently remove essential contextual
information needed for distinguishing between visu-
ally similar lesion classes.
This study introduces the potential of STL for se-
quentially aligning segmentation and classification.
Using segmentation to first capture boundary details
and then refine classification may improve model ac-
curacy by incrementally enhancing task-specific fea-
tures. This suggests a promising path for robust, uni-
fied models in skin cancer detection.
However, adopting an STL approach also intro-
duces challenges. Sequentially transferring segmen-
tation features to classification may amplify noise or
propagate inaccuracies from the segmentation phase,
especially if segmentation boundaries are imprecise.
Furthermore, balancing the computational cost of se-
quential training and the risk of overfitting in each
task stage are essential considerations that require
ICPRAM 2025 - 14th International Conference on Pattern Recognition Applications and Methods
702

careful assessment. Addressing these challenges will
be crucial to validate the effectiveness of STL and en-
sure that it generalizes well across various dermato-
logical datasets.
Looking forward, we aim to investigate whether
this STL approach can be generalized beyond medical
imaging or whether its effectiveness is uniquely suited
to clinical applications. Understanding its adaptabil-
ity to broader contexts could reveal new possibilities
for versatile models capable of handling complex vi-
sual recognition tasks across various domains.
REFERENCES
Azad, R., Aghdam, E. K., Rauland, A., and Bozorgpour, A.
(2024). Medical image segmentation review: The suc-
cess of u-net. IEEE Transactions on Pattern Analysis
and Machine Intelligence.
Cai, T. T. and Ma, R. (2022). Theoretical foundations of
t-sne for visualizing high-dimensional clustered data.
Journal of Machine Learning Research, 23(301):1–
54.
Chan, J. Y.-L., Bea, K. T., Leow, S. M. H., Phoong, S. W.,
and Cheng, W. K. (2023). State of the art: a review of
sentiment analysis based on sequential transfer learn-
ing. Artificial Intelligence Review, 56(1):749–780.
Chen, L.-C., Zhu, Y., Papandreou, G., Schroff, F., and
Adam, H. (2018). Encoder-decoder with atrous sepa-
rable convolution for semantic image segmentation. In
Proceedings of the European conference on computer
vision (ECCV), pages 801–818.
Esteva, A., Kuprel, B., Novoa, R. A., Ko, J., Swetter, S. M.,
Blau, H. M., and Thrun, S. (2017). Dermatologist-
level classification of skin cancer with deep neural net-
works. nature, 542(7639):115–118.
Gallazzi, M., Biavaschi, S., Bulgheroni, A., Gatti, T.,
Corchs, S., and Gallo, I. (2024). A large dataset to
enhance skin cancer classification with transformer-
based deep neural networks. IEEE Access.
Gallo, I., Rehman, A. U., Dehkordi, R. H., Landro, and
Nicola (2023). Deep object detection of crop weeds:
Performance of yolov7 on a real case dataset from uav
images. Remote Sensing, 15(2):539.
He, K., Zhang, X., Ren, S., and Sun, J. (2016). Deep resid-
ual learning for image recognition. In Proceedings of
the IEEE conference on computer vision and pattern
recognition, pages 770–778.
ISIC Challenge (Last accessed on 30-10-2024). Isic chal-
lenge webpage. https://challenge.isic-archive.com.
Liu, S., Qi, L., Qin, H., Shi, J., and Jia, J. (2018). Path ag-
gregation network for instance segmentation. In Pro-
ceedings of the IEEE conference on computer vision
and pattern recognition, pages 8759–8768.
Liu, Z., Lin, Y., Cao, Y., and Hu (2021). Swin trans-
former: Hierarchical vision transformer using shifted
windows. In Proceedings of the IEEE/CVF interna-
tional conference on computer vision, pages 10012–
10022.
Mao, H. H. (2020). A survey on self-supervised pre-training
for sequential transfer learning in neural networks.
arXiv preprint arXiv:2007.00800.
O’Shea, K. (2015). An introduction to convolutional neural
networks. arXiv preprint arXiv:1511.08458.
Paulsen, S. and Casey, M. (2023). Sequential transfer learn-
ing to decode heard and imagined timbre from fmri
data. arXiv preprint arXiv:2305.13226.
Redmon, J. (2016). You only look once: Unified, real-time
object detection. In Proceedings of the IEEE confer-
ence on computer vision and pattern recognition.
Rehman, A. U. and Gallo, I. (2024). Cross-pollination of
knowledge for object detection in domain adaptation
for industrial automation. International Journal of In-
telligent Robotics and Applications, pages 1–19.
Rehman, A. U., Gallo, I., and Lorenzo, P. (2023). A
food package recognition framework for enhancing
efficiency leveraging the object detection model. In
2023 28th International Conference on Automation
and Computing (ICAC), pages 1–6. IEEE.
Ronneberger, O., Fischer, P., and Brox, T. (2015). U-
net: Convolutional networks for biomedical image
segmentation. In Medical image computing and
computer-assisted intervention–MICCAI 2015: 18th
international conference, Germany, 2015, proceed-
ings, part III 18, pages 234–241. Springer.
Taghizadeh, M. and Mohammadi, K. (2022). The fast and
accurate approach to detection and segmentation of
melanoma skin cancer using fine-tuned yolov3 and
segnet based on deep transfer learning. arXiv preprint
arXiv:2210.05167.
Tirinzoni, A., Poiani, R., and Restelli, M. (2020). Sequen-
tial transfer in reinforcement learning with a genera-
tive model. In International Conference on Machine
Learning, pages 9481–9492. PMLR.
Tschandl, P. and Rosendahl (2018). The ham10000 dataset,
a large collection of multi-source dermatoscopic im-
ages of skin lesions. Scientific data, 5(1):1–9.
Ultralytics (2024). Yolov8 release notes. https://github.
com/ultralytics/yolov8. Available: https://github.com/
ultralytics/yolov8.
Wang, C.-Y., Liao, H.-Y. M., and Wu, Y.-H. (2020). Csp-
net: A new backbone that can enhance learning capa-
bility of cnn. In Proceedings of the IEEE/CVF con-
ference on computer vision and pattern recognition
workshops, pages 390–391.
Wang, H., Xie, S., Lin, L., and Iwamoto (2022). Mixed
transformer u-net for medical image segmentation. In
ICASSP 2022-2022 IEEE international conference on
acoustics, speech and signal processing (ICASSP),
pages 2390–2394. IEEE.
Wang, Y., Su, J., Xu, Q., and Zhong, Y. (2023). A collabo-
rative learning model for skin lesion segmentation and
classification. Diagnostics, 13(5):912.
Improving Classification in Skin Lesion Analysis Through Segmentation
703
