
Relationships Between Central Quality Assurance Criteria for the
Assessment of Statutory Health Insurance Patients for Further
Development Using Medical Informatics Methods
Mareike Burmester
1,5
, Paul-Ulrich Menz
2
, Vera Ries
3
, Klaus-Peter Thiele
3
, Bernhard van Treeck
4
and Reinhard Schuster
1,5
1
Medical Advisory Board of Statutory Health Insurance in Northern Germany (MD Nord), 23554 L
¨
ubeck, Germany
2
Medical Advisory Board of Statutory Health Insurance in Westphalia-Lippe (MD Westfalen-Lippe), 48153 M
¨
unster,
Germany
3
Medical Advisory Service Institution of the Statutory Health Insurance in North Rhine (MD Nordrhein), 40212 D
¨
usseldorf,
Germany
4
Federal Joint Committee (G-BA), 10587 Berlin, Germany
5
Institute of Mathematics, University of L
¨
ubeck, 23562 L
¨
ubeck, Germany
Keywords:
Medical Quality Assurance, Statutory Health Insurance in Germany, Linear Optimization, Correlations and
Partial Correlations.
Abstract:
In recent years, a comprehensive system has been developed both in terms of content and IT for quality
assurance in the assessment for the granting of benefits for persons with statutory health insurance by the
Medical Advisory Boards, which has also been enshrined in German legislation. In addition to not insignificant
formal criteria and criteria relating to specific assessment areas, four criteria relevant to the entire assessment
spectrum are evaluated in detail. One- and two-dimensional criteria provide an overview as an introduction to
the topic. Similar to the procedure in image processing, linear optimization methods are used to infer relevant
intervals of the detailed parameters from row and column totals. Using correlations and partial correlations, the
relationship between the central quality criteria is shown. Methods of spherical trigonometry are generalized.
For each of the three sides of the quadrilateral of the four central criteria, it is of central importance that the
partial correlations are greater or smaller than the correlations overall. This is determined by the modulus
value, which in the application under consideration produces the same results on all sides of the tetrahedron
under consideration.
1 INTRODUCTION
The Medical Advisory Board of Statutory Health In-
surance (SHI) in Germany is responsible for conduct-
ing assessments of SHI-insured individuals in order
to ascertain their medical and socio-medical require-
ments for benefit decisions by the statutory health
and long-term care insurance funds. Additionally, the
Medical Advisory Board provides advice to the afore-
mentioned insurance funds on a case-by-case basis.
The assessments of the Medical Advisory Board out-
side of long-term care insurance in the area of health
insurance are predominantly carried out by doctors.
They are impartial in their assessment and are only
bound by their conscience; however, they are required
to adhere to the legal framework and current medical
knowledge to a significant extent, see (Gemeinsamer
Bundesausschuss, 2022). The relationship between
law and medicine is a key topic in the field of so-
cial medicine. The abstract legal claims of insured
persons are concretised by multi-layered committees,
resulting in binding national regulations. Medical
findings, legislation and individual case decisions by
courts in various instances resulting from insufficient
concretisation set precedents for subsequent similar
decisions, which may be cited as such in future in-
stances. These developments and the further develop-
ment of regulations by the committees as a framework
for decision-making are naturally delayed to a lesser
or greater extent. This has direct consequences for
the ongoing necessary further training of all those in-
volved as experts in this interdisciplinary context, see
712
Burmester, M., Menz, P.-U., Ries, V., Thiele, K.-P., van Treeck, B. and Schuster, R.
Relationships Between Central Quality Assurance Criteria for the Assessment of Statutory Health Insurance Patients for Further Development Using Medical Informatics Methods.
DOI: 10.5220/0013255600003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 2: HEALTHINF, pages 712-719
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

(Altenstetter and Busse, 2005).
In 2016, Medical Advisory Board initiated a joint
evaluation of existing quality assurance initiatives and
the development of a comprehensive, standardised
quality assurance process for all areas of assessment.
This process was developed with the involvement of
all regional Medical Advisory Boards and specialist
competence units, which set the standards for assess-
ment in each specialist area. The Medical Advisory
Boards have trialled the new national quality assur-
ance plan in three test runs throughout Germany, pi-
loted it in the largest assessment area and then gradu-
ally implemented it in practice in all assessment areas.
Finally, the national quality assurance plan was even
taken up by the legislature, incorporated into the Ger-
man Social Code and, following a broad national dis-
cussion among all stakeholders and interested parties,
adopted in a guideline confirmed by the Federal Min-
istry of Health with sub-legal binding force. Quality
assurance follows the generally recognised principles
of the quality assurance cycle for continuous eval-
uation and further development, see (Gemeinsamer
Bundesausschuss, 2023).
The assessment is carried out by the Medical Ad-
visory Boards, which are essentially organised by fed-
eral state. Accordingly, an initial quality check is car-
ried out in the regionally responsible Medical Advi-
sory Board. For quality assurance purposes, the as-
sessments are randomly drawn on a nationwide basis.
The special feature of the newly implemented system
is that a partial sample is sent to another Medical Ad-
visory Boards for a second independent assessment.
If there are noticeable differences between the self-
assessment and external assessment, a further qual-
ity check is carried out by a third Medical Advisory
Board.
The entire process of drawing up and providing
expert opinions, along with all the stages of the audit
described above, is conducted via a central server on
which all the expert opinions specified for the audit
are accessible in anonymised form, see (Medizinis-
cher Dienst Bund, 2023), (Ries et al., 2020) and (Ries
et al., 2023).
Should discrepancies remain following the third
stage of the examinations, a discussion and consen-
sus are reached on the specific case construction at
nationwide consensus conferences. After consensus
conferences were held for the first time in 2018 as
part of the feasibility study, they are now established
as a nationwide quality assurance instrument. They
promote mutual learning and take place twice a year
in each of the nine assessment areas, once in person
and once online via video conference. Representa-
tives of all regional Medical Advisory Boards take
part in each consensus conference, as well as at least
one representative of the assigned specialist compe-
tence unit. This makes it possible to promptly im-
plement the need for further development in the regu-
lations identified in the consensus conferences, as the
competence units set the standards for the assessment.
A total of 20 criteria have been established for the
evaluation of the assessment, which are standardised
across all assessment areas. Furthermore, additional
technical criteria are currently being harmonised with
the general criteria. In addition to the aforementioned
formal criteria, there are four criteria of central im-
portance for standardised assessment processes in the
regional Medical Advisory Boards, cf. (Gaertner and
van Essen, 2024), (Gaertner and Gnatzy, 2011).
Three of these central criteria are operationalised
as three test aspects for the accuracy of the expert
opinion, namely criteria 14, 15 and 16. Triggered by
limitations on the transfer of the case file due to data
protection regulations that vary in strictness from re-
gion to region, the national quality assurance plan has
created the new requirement for expert opinions with
the fourth central criterion 9 that they must be con-
vincing with a concise presentation of the character-
istics of the individual case that are relevant to the
assessment.
2 MATERIALS AND METHODS
The following analysis focuses on the medical fields
(cf. (Anja Dippmann, 2024)):
• 100: Incapacity of work
• 200: Hospital care
• 400: New and unconventional examination and
treatment methods / pharmacy
• 500: Prevention and rehabilitation
• 700: Medical supplies
In the year under review 2023, 16942 internal and
4953 external evaluations were carried out in these
medical fields. In addition, only the central criteria
• Criterion 9: The report contains the information
necessary to assess the facts of the case
• Criterion 14: The expert opinion takes into ac-
count current medical knowledge
• Criterion 15: The expert opinion takes into ac-
count the socio-medical requirements
• Criterion 16: The result of the expert opinion is
plausible and understandable in the context of the
facts presented
Relationships Between Central Quality Assurance Criteria for the Assessment of Statutory Health Insurance Patients for Further
Development Using Medical Informatics Methods
713

taken into account.
The results of the assessments of the expert opin-
ions are presented on a three-level scale (green, yel-
low, red). The colour red means that the criterion to be
assessed has not been met. Green means that the cri-
terion has been fully met, while yellow indicates that
there is room for improvement (cf. (Gerlach, 2001)).
The first step is to compare the internal and ex-
ternal ratings, differentiated by cause group. The fre-
quency of occurrence of each scale value is analysed.
This is followed by a specification where the distribu-
tion of criteria 14 and 16 is analysed separately.
The aim of consistent quality assurance across all
assessment regions is to achieve the same assessments
both within and between regions. The first step is
to compare the internal and overall ratings. This re-
sults in a total of nine different combinations of rat-
ings with the three possible scale values (green/green,
green/yellow, ... red/yellow, red/red). This can also
be visualised as a nine-box table, in the same way as
a four-box table. The marginal sums can be calculated
from the available values. In the present constellation,
a total of six proportional values can be identified,
with the sum of the vertical and horizontal marginal
totals having the same value. This situation is typi-
cal of medical image processing, where the object is
to be inferred from certain sums per viewing direction
(see (Zeng, 2010), (Jan, 2005)). Compared to medical
imaging, the dimension here is small, but it is suitable
for demonstrating basic aspects.
The extent to which the actual combination de-
viates from the possible extreme values is of inter-
est for standardised quality assurance. In the follow-
ing, we will determine the interval in which the val-
ues in the nine-field table lie if the boundary values
are specified. A linear optimisation problem is solved
for this purpose. It is assumed that the modelling is
carried out using linear optimisation and that mono-
tonicity is given. In addition, the proportions of the
joint assessment are assumed to be between 0% and
100%. The target value is defined as the interval in
which either the largest valuation differences or iden-
tical valuations occur. If there are no values of com-
mon valuations in the practical application, but only
the marginal totals, intervals for deviations can thus
be determined. A separate analysis is also carried out
for the individual criteria.
The comparison of internal and external assess-
ments is not the only application that enables this ap-
proach. It is also possible to analyse the interrelation-
ships between the criteria. In the following, medical
field 100 for incapacity for work is analysed, whereby
the assessments of criteria 14 (medical content) and
16 (comprehensibility of the results) are compared.
Furthermore, an analysis is carried out for medical
field 200 (hospital care).
Finally, the correlations, the partial correlations
and the modulus value are analysed. For this purpose,
the random variables X
1
, X
2
, X
3
, X
4
are defined as the
four central criteria of quality assurance. The correla-
tion ρ
i j
= ρ
ji
describes the relationship between two
variables X
i
and X
j
, where i, j ∈ {1, 2, 3, 4}, i ̸= j ap-
plies. The partial correlations are calculated accord-
ing to the following formula:
ρ
i j,k
=
ρ
i j
− ρ
ik
ρ
jk
q
1 − ρ
2
ik
q
1 − ρ
2
jk
,
where i, j, k ∈ {1, 2, 3, 4} are pairwise different. The
modulus value
m =
1 − ρ
2
i j,k
1 − ρ
2
i j
is always identical for all three combinations of partial
correlation and the corresponding correlation. If the
modulus value m > 1, the following applies
m > 1
⇔
1−ρ
2
i j,k
1−ρ
2
i j
> 1
⇔ 1 − ρ
2
i j,k
> 1 − ρ
2
i j
⇔ ρ
2
i j
> ρ
2
i j,k
⇔ |ρ
i j
| > |ρ
i j,k
|.
The calculations described above are performed sepa-
rately for the internal and overarching assessment. In
addition, the internal valuation is analysed separately
between the medical fields considered.
Furthermore, calculations in linear optimization
with Mathematica from Wolfram Research are used.
3 RESULTS
Table 1 shows the differences between the internal
and the overarching assessment of the expert opinions
depending on the reason group for the four central cri-
teria 9, 14, 15 and 16. Only those expert opinions that
were subject to both an internal and an overarching
assessment were taken into account.
Both the red and amber assessments show a more
critical tendency in the overarching assessments. This
may be due to the fact that the approach to the ap-
praisal varies from region to region and that the struc-
ture of the appraisals is different and therefore less
well known. Both are reasons for standardisation.
When assessing the medical requirements in ac-
cordance with criterion 14, the best results are shown
HEALTHINF 2025 - 18th International Conference on Health Informatics
714
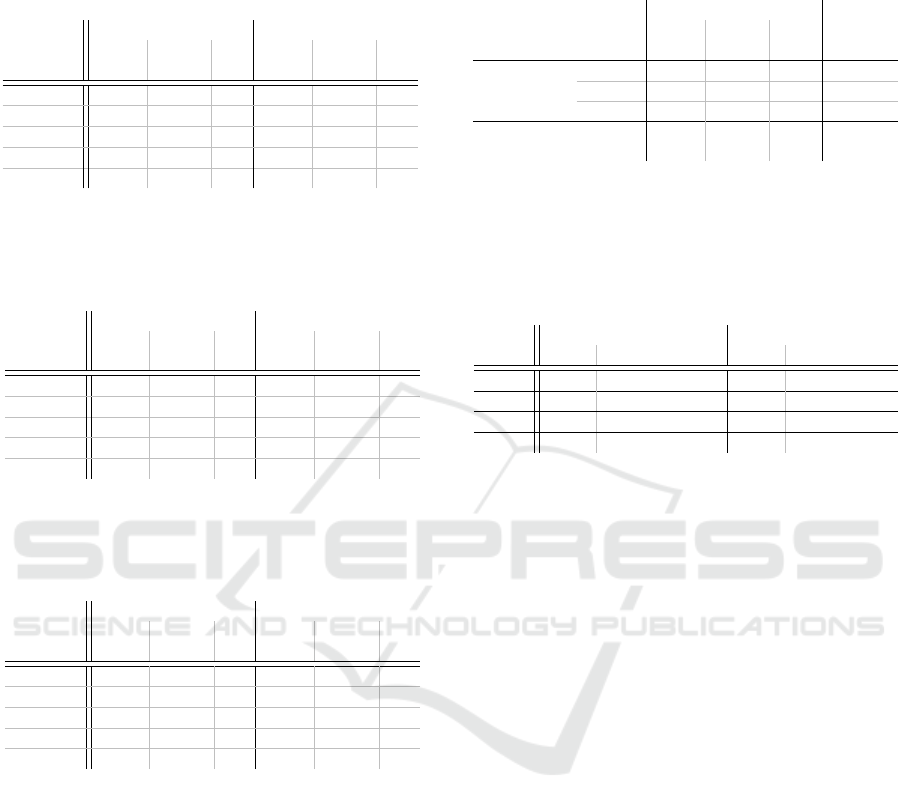
Table 1: The results of the internal and external assessment
of all Medical Advisory Boards in relation to criteria 9 and
14-16 for the year 2023 are presented herewith. The values
are expressed as a percentage (%).
Internal External
Medical
green yellow red green yellow red
Field
100 84.5 11.5 4.0 77.9 16.8 5.3
200 95.3 3.7 1.0 93.5 4.4 2.2
400 89.8 8.3 1.9 85.3 10.0 4.7
500 82.9 12.3 4.8 82.6 12.6 4.8
700 93.7 4.6 1.7 88.6 6.8 4.6
Table 2: The results of the internal and external assessment
of all Medical Advisory Boards in relation to criteria 14 for
the year 2023. The values are expressed as a percentage
(%).
Internal External
Medical
green yellow red green yellow red
Field
100 97.4 1.8 0.8 95.6 3.8 0.6
200 99.4 0.5 0.1 99.1 0.7 0.2
400 89.6 9.0 1.4 82.8 13.4 3.8
500 95.8 3.1 1.0 93.9 4.0 2.1
700 97.5 1.5 1.0 95.1 2.5 2.4
Table 3: The results of the internal and external assessment
of all Medical Advisory Boards in relation to criteria 16 for
the year 2023. The values are expressed as a percentage
(%).
Internal External
Medical
green yellow red green yellow red
Field
100 75.0 19.5 5.4 68.3 26.8 5.0
200 91.6 6.8 1.6 88.6 8.4 3.0
400 92.3 5.4 2.4 85.7 9.1 5.2
500 78.1 17.3 4.6 78.7 16.4 4.9
700 88.7 9.4 1.9 82.3 11.5 6.2
even more clearly in Table 2 in medical field 200
‘Hospital care’.
The plausibility of the results in criterion 16 in Ta-
ble 3 is assessed more critically than in the previously
analysed criterion 14. Here too, the results for hospi-
tal care show the best results.
Equal internal and overarching assessments are
desirable for standardised quality assurance across all
regions of the assessment. In this context, the ques-
tion arises as to what extent the actual combination of
criteria deviates from possible extreme values.
Linear optimisation as a processing method first
requires the meaningful formulation of inequalities
and target values, which are then compared with re-
gard to their results. It seems sensible to maximise the
number of equal evaluations. Alternatively, it would
be possible to minimise the greatest differences. In a
Table 4: The percentage of identical or dissenting votes
for the identical appraisals in the internal and external ap-
praisals.
Internal evaluation
green yellow red
Sum
external
External
evaluation
green 80.0 5.0 1.5 86.5
yellow 7.2 1.6 0.5 9.3
red 3.0 0.7 0.5 4.2
Sum
90.2 7.3 2.5 100
internal
Table 5: Results of the optimisation problem separately for
the four main criteria. In the left-hand column are the actual
values for the red/green deviation case, with the correspond-
ing calculated intervals in which the value could lie with
the specified limits. On the right, these values for the case
where green/green, yellow/yellow and red/red were chosen.
Crit.
Maximum Difference Consensus
Real Interval Real Interval
9 5.9 [2.1 , 10.1] 77.2 [69.1 , 86.6]
14 2.2 [0.5 , 2.4] 91.9 [90.8 , 96.5]
15 4.8 [1.4 , 6.4] 82.2 [78.3 , 91.4]
16 4.8 [1.5 , 7.5] 77.0 [68.4 , 86.4]
combination of both considerations, it would first be
necessary to determine which weights would lead to
a sensible solution.
Another option would be to analyse the equally
problematic ratings. The assumption that a com-
bined analysis would not result in equally poorly as-
sessed appraisals is not based on empirical experi-
ence. Therefore, a minimum level can be assumed
for other optimisation criteria. Alternatively, the real
value with the minimum and maximum possible val-
ues without further target values can be considered for
empirical determination.
With regard to the differentiation between internal
and overarching audits with regard to the four defined
criteria, the following can be stated:
With the exception of a small deviation, the values
in each row and column are monotonically decreas-
ing. For the linear optimisation problem with the fur-
ther restriction that there are expert opinions that are
rated red (criterion not fulfilled) both internally and
across the board, an example of 0.4% with 0.5% in
reality is assumed. This means that in reality there
is complete agreement (green/green, yellow/yellow,
red/red) for 82.1% of the expert opinions. Assum-
ing that the minimum of common criteria assessed in
red is not restricted in the expert opinion, the interval
ranges from 76.6% to 90.2%. With the restriction, the
minimum increases slightly to 77.8%.
For the largest valuation differences, which are
present in 4.5% of cases in real terms, the interval
Relationships Between Central Quality Assurance Criteria for the Assessment of Statutory Health Insurance Patients for Further
Development Using Medical Informatics Methods
715
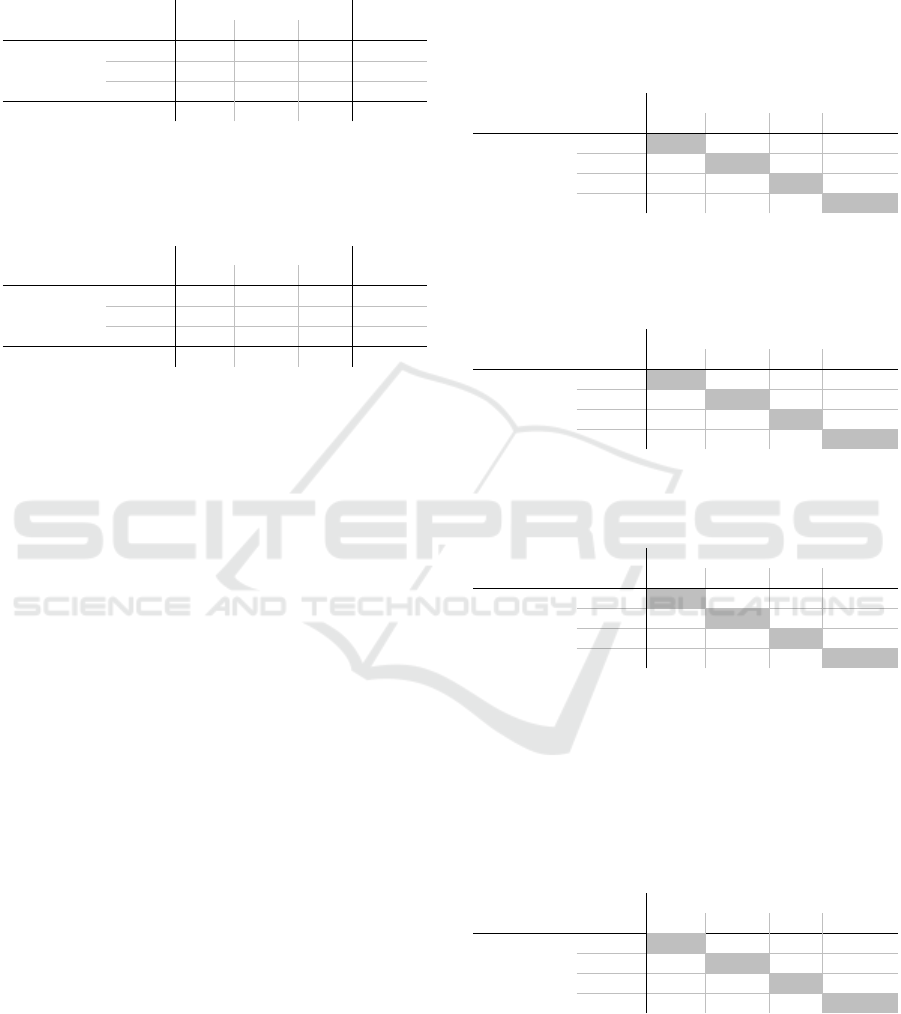
Table 6: Describes the percentages of how often green, yel-
low and red were selected in criteria 14 and 16 in medical
group 100, and how often which combination of values oc-
curred.
Criteria 16
green yellow red Sum
Criteria
14
green 73.6 19.5 4.3 97.4
yellow 0.4 1.2 0.4 2.0
red 0.1 0.1 0.4 0.6
Sum 74.1 20.8 5.1 100
Table 7: Describes the percentages of how often green, yel-
low and red were selected in criteria 14 and 16 in medical
group 200, and how often which combination of values oc-
curred.
Criteria 16
green yellow red Sum
Criteria
14
green 90.6 6.8 1.8 99.2
yellow 0.1 0.4 0.1 0.6
red 0.0 0.0 0.2 0.2
Sum 90.7 7.2 2.1 100
ranges from 1.4% to 6.6% without the additional re-
striction. With the additional restriction, the upper
limit is reduced to 5.4%.
With regard to the individual criteria, the follow-
ing result is obtained for the variant without additional
restrictions (Table 5):
The comparison of the ratings between criteria 14
and 16 in the medical field 100 results in the following
nine-field table (Table 6).
When looking at criteria 14 and 16, it is initially
noticeable that there is a greater asymmetry in the
evaluation matrix. In addition, the monotony in the
rows and columns is more limited in the medical field
200 than in the medical field 100. This is related to the
dependency between the criteria, which is considered
next with the correlations.
In the medical field 100, the interval from 4.4% to
5.7% (i.e. outside the real value) lies at 4.3% max-
imum valuation differences with monotonicity as-
sumption and without monotonicity assumption wider
between 2.5% to 5.7%. The complete match is 75.3%
in real terms with monotonicity assumption we un-
derestimate the interval from 71.5% to 74.1%. With-
out the monotonicity assumption, on the other hand,
a wider interval of 71.5% to 76.7% is achieved.
In the medical field 200, higher levels of agree-
ment can again be observed (Table 7).
The largest valuation differences in real terms
are 1.8%, with the intervals [2.4%, 6.9%] exhibiting
monotonicity and [1.4%, 2.4%] lacking a monotonic-
ity assumption. In real terms, there is complete agree-
ment at 91.1% with the intervals [89.8%, 90.6%] with
monotonicity and [89.8%, 91.4%] without a mono-
tonicity assumption.
Finally, the measures of correlation, partial corre-
lation and modular value of the four central criteria
are analysed in more detail.
Table 8: Describes the correlation between two of the four
central criteria. All evaluated reports were analysed inter-
nally for this purpose. The matrix is symmetrical due to the
symmetry property of correlation.
Second Criteria
9 14 15 16
First
Criteria
9 0.295 0.393 0.601
14 0.295 0.371 0.348
15 0.393 0.371 0.496
16 0.601 0.348 0.496
Table 9: The correlations between criteria 9, 14, 15, 16
for all externally assessed reports, regardless of the medi-
cal fields.
Second Criteria
9 14 15 16
First
Criteria
9 0.290 0.444 0.623
14 0.290 0.458 0.433
15 0.444 0.458 0.458
16 0.623 0.433 0.458
Table 10: Describes the difference between internal and ex-
ternal ratings and the correlation between the four central
criteria.
External
9 14 15 16
Internal
9 0.252 0.345 0.529
14 0.252 0.392 0.370
15 0.345 0.392 0.490
16 0.529 0.370 0.490
In order to obtain an indication of how strongly
the four central criteria correlate in the internal assess-
ment of the expert opinions in the individual cause
groups, the cause groups are analysed separately once
again.
Table 11: Correlation of the criteria based on internal re-
ports of the medical field 100. In this case, n = 1576 expert
opinions were analysed.
Second Criteria
9 14 15 16
First
Criteria
9 0.211 0.499 0.567
14 0.211 0.203 0.261
15 0.499 0.203 0.548
16 0.567 0.261 0.548
The highest correlation between the four criteria
analysed can be seen in the expert opinions on medic-
inal products and on new and unconventional treat-
ment methods (medical field 400), see Table 13, cf.
(Schuster, 2022). The highest number of expert opin-
HEALTHINF 2025 - 18th International Conference on Health Informatics
716
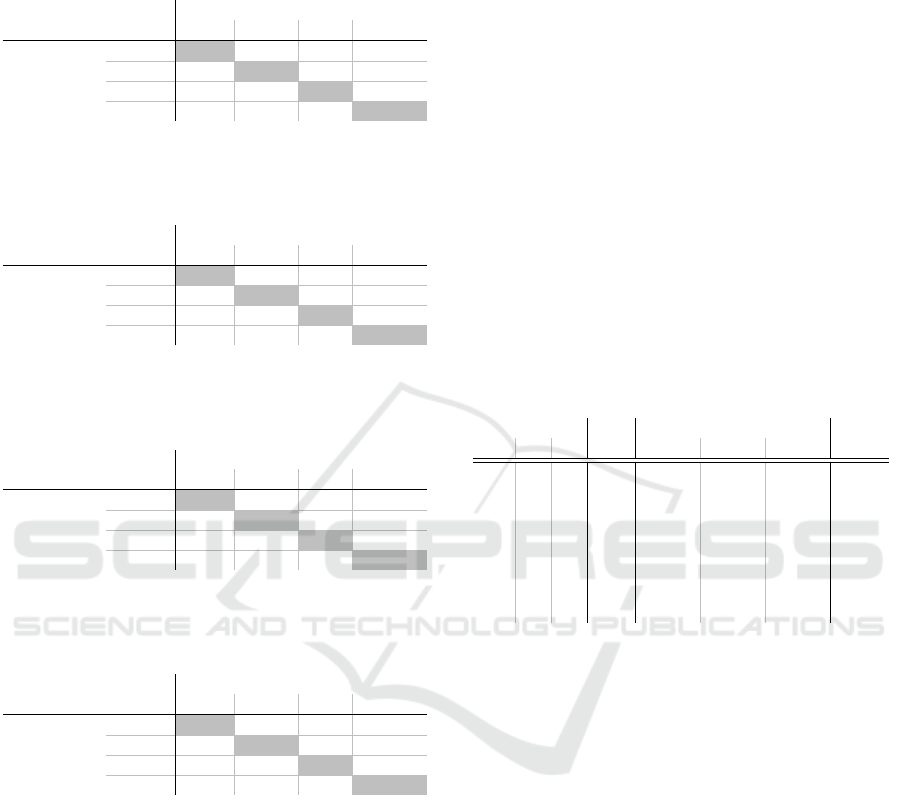
Table 12: Correlation of the criteria based on internal re-
ports of the medical field 200. Here n = 8040 expert opin-
ions were analysed.
Second Criteria
9 14 15 16
First
Criteria
9 0.227 0.137 0.600
14 0.227 0.238 0.292
15 0.137 0.238 0.354
16 0.600 0.292 0.354
Table 13: Correlation of the criteria based on internal re-
ports of the medical field 400. Here, n = 1404 expert opin-
ions were the subject of analysis.
Second Criteria
9 14 15 16
First
Criteria
9 0.513 0.525 0.568
14 0.513 0.507 0.512
15 0.525 0.507 0.542
16 0.568 0.512 0.542
Table 14: Correlation of the criteria based on internal re-
ports of the medical field 500. Here, n = 1375 reports were
analysed.
Second Criteria
9 14 15 16
First
Criteria
9 0.366 0.487 0.575
14 0.366 0.399 0.386
15 0.487 0.399 0.595
16 0.575 0.386 0.595
Table 15: Correlation of the criteria based on internal re-
ports of the medical field 700. In case of medical field 700,
n = 1380 expert opinions were analysed.
Second Criteria
9 14 15 16
First
Criteria
9 0.334 0.377 0.639
14 0.334 0.490 0.405
15 0.377 0.490 0.458
16 0.639 0.405 0.458
ions on hospital care is found in medical field 200
in Table 12 and thus also in the total number of ex-
pert opinions (Table 8). It is also worth noting that
the differences between internal (Table 8) and over-
all assessment (Table 9 and, moreover, the differences
between these assessments vary only moderately. If
internal and overall assessment were essentially the
same, the difference in table 10 would not differ in
this way. The smallest differences between the med-
ical assessments (criterion 14) and the socio-medical
assessments in the individual medical field analysed
are for medicines (400) (Table 13) and medical aids
(700) (Table 15).
The partial correlations between three of the four
variables considered in quality assurance are analysed
below. This can be viewed as three sides of a polyhe-
dron. The modulus value described in the methods
section indicates whether the correlations are larger
or smaller than the partial correlations. As described
in the methods section, this then applies to all permu-
tations of the three variables. For the interpretation
of the tetrahedron, it can be deduced that the modu-
lus values are greater than 1 on all three sides, which
means that the partial correlations are smaller than the
correlations in terms of amount.
In a comparison between the internal and overar-
ching tests, there are only moderate differences in the
correlations, partial correlations and the module value
for all combinations of criteria under consideration:
Table 16: The analysis of the correlation between the first
and second of the four criteria shows that if the correlation
via the third criterion is excluded, a value of the partial cor-
relation close to zero can be assumed, which means that
the two criteria considered can be regarded as independent.
Since all values of the modulus value are greater than 1, all
correlations are greater than the associated partial correla-
tions.
Criteria int./ Partial Correlation Modul
1 2 3 ext. ρ
12,3
ρ
13,2
ρ
23,1
value
9 14 15 int 0.175 0.290 0.319 1.062
9 14 15 ext 0.135 0.336 0.276 1.048
9 14 16 int 0.115 0.223 0.556 1.081
9 14 16 ext 0.071 0.288 0.485 1.062
9 15 16 int 0.136 0.354 0.509 1.160
9 15 16 ext 0.115 0.386 0.440 1.120
14 15 16 int 0.244 0.421 0.203 1.091
14 15 16 ext 0.261 0.403 0.222 1.102
A comparison of the criteria considered in the in-
ternal evaluation results in the following comparison
for the individual cause groups:
According to the explanations in the methods sec-
tion, all partial correlations are smaller in amount than
the correlations. This says nothing about the sign, i.e.
the change from positive to negative correlation or the
reverse. In the present analysis, a change of sign oc-
curs only once, namely in the medical field 200 (hos-
pital care) in the correlation without the criterion 14
(medical content).
4 CONCLUSIONS
The four quality criteria under examination interact
intensively, as evidenced by the fact that the partial
correlations are consistently smaller than the correla-
tions. It is frequently posited that the high correlation
observed between two variables is a consequence of
their dependence on a third variable. In this instance,
all three variables are of significant content relevance
when the triangular configuration of statistical corre-
Relationships Between Central Quality Assurance Criteria for the Assessment of Statutory Health Insurance Patients for Further
Development Using Medical Informatics Methods
717

Table 17: Analysis of the partial correlations considered in
table 16 and the module value separately for the individual
medical fields.
Criteria Med Partial Correlation Modul
1 2 3 field ρ
12,3
ρ
13,2
ρ
23,1
value
9 14 15 100 0.129 0.115 0.477 1.029
9 14 15 200 0.202 0.214 0.087 1.011
9 14 15 400 0.336 0.326 0.358 1.204
9 14 15 500 0.215 0.272 0.400 1.102
9 14 15 700 0.185 0.417 0.260 1.087
9 14 16 100 0.079 0.175 0.542 1.040
9 14 16 200 0.068 0.200 0.573 1.050
9 14 16 400 0.314 0.313 0.415 1.223
9 14 16 500 0.191 0.230 0.505 1.113
9 14 16 700 0.107 0.265 0.584 1.113
9 15 16 100 0.274 0.371 0.405 1.232
9 15 16 200 -0.101 0.343 0.596 1.009
9 15 16 400 0.314 0.348 0.397 1.224
9 15 16 500 0.221 0.441 0.406 1.247
9 15 16 700 0.123 0.305 0.566 1.148
14 15 16 100 0.074 0.523 0.183 1.037
14 15 16 200 0.150 0.306 0.229 1.036
14 15 16 400 0.318 0.381 0.328 1.210
14 15 16 500 0.229 0.521 0.201 1.127
14 15 16 700 0.374 0.326 0.233 1.131
lations is taken into account. This remains the case
when three edges of each of the four variables are
considered as tetrahedrons. It is important to note
that correlations and partial correlations are statistical
variables that may not be able to explain a causal rela-
tionship. In the context under consideration, there are
numerous substantive discussions between physicians
with the participation of computer scientists in the
consensus conferences described in the introduction.
The extensive details of the discussion provided a ba-
sis for statistical examination. The result was that the
substantive arguments put forward by doctors in the
review of expert opinions were largely confirmed sta-
tistically. This alternating suggestion between practi-
tioners and statisticians will contribute to the further
development of quality assurance in the Medical Ad-
visory Boards with high performance, cf. (N
¨
uchtern
et al., 2015), (Newhouse, 2002).
A further point of discussion in the further devel-
opment of quality assurance in medical assessments
is the relationship between cost and benefit. A re-
duction in the number of criteria to be assessed will
result in a reduction in the effort required. On the one
hand, the expert groups are engaged in a discussion
regarding the identification of criteria that are simi-
lar in content and, therefore, amenable to a summary.
This is particularly pertinent to criteria that are em-
ployed solely within individual event groups. The ra-
tionale for these criteria was based on the assumption
that they could not be evaluated using the general cri-
teria. However, the testing experience from the dis-
cussions in the consensus conferences has shown that
this is certainly possible. This has resulted in a mod-
ification of the test criteria. This paper does not show
to what extent the audit has led to a significant im-
provement in the assessment. While a modification
and summarisation of the review criteria may appear
to result in a deterioration, it has, in fact, led to an
overall improvement.
In addition to the discussion of the proximity of
test criteria in terms of content, the question also
arises as to which criteria are viewed in the same way
by the physicians in each case without content-related
proximity. This concerns an association of judgement
errors. This can also motivate a reduction in the num-
ber of endpoints.
This paper focussed on the fact that there are crite-
ria whose further reduction would not make sense and
would be clearly counterproductive for quality assur-
ance overall.
REFERENCES
Altenstetter, C. and Busse, R. (2005). Health care reform in
germany: patchwork change within established gov-
ernance structures. Journal of health politics, policy
and law, 30(1-2):121–142.
Anja Dippmann, et al.. (2024). Qualtit
¨
atssicherung der Be-
gutachtung f
¨
ur die Gesetztliche Krankenversicherung
QSKV - Jahresbericht 2024 - Datenjahr 2023. Home-
page MD Bund.
Gaertner, T. and Gnatzy, W. (2011). Zum
sachverst
¨
andigenstatus im medizinischen dienst
der krankenversicherung am beispiel des mdk hessen.
GuP, 5:166–173.
Gaertner, T. and van Essen, J. (2024). Der Medi-
zinische Dienst - eine sozialmedizinische Institution
der Qualit
¨
atssicherung im Gesundheitssystem. Das
Gesundheitswesen.
Gemeinsamer Bundesausschuss (2022). Beschluss
des Gemeinsamen Bundesausschusses
¨
uber die
Ver
¨
offentlichung des Berichts des Medizinischen
Dienstes Bund gem
¨
aß §16 Teil A MD-QK-RL:
Bericht
¨
uber die im Jahr 2021 durchgef
¨
uhrten
Qualit
¨
atskontrollen. Homepage Gemeinsamer
Bundessausschuss.
Gemeinsamer Bundesausschuss (2023). Richtlinie des
Gemeinsamen Bundesausschusses nach §137 Absatz
3 SGB V zu Kontrolle des Medizinischen Dienstes
nach §275a SGB V. Homepage Gemeinsamer Bun-
dessausschuss.
Gerlach, F. M. (2001). Qualit
¨
atsf
¨
orderung in Praxis und
Klinik: eine Chance f
¨
ur die Medizin; 24 Tabellen.
Thieme.
Jan, J. (2005). Medical image processing, reconstruction
and restoration: concepts and methods. Crc press.
Medizinischer Dienst Bund (2023). Regelm
¨
aßige Berichter-
stattung der Medizinischen Dienste
¨
uber ihre T
¨
atigkeit
HEALTHINF 2025 - 18th International Conference on Health Informatics
718

und Personalausstattung. Richtlinie des Medizinischen
Dienstes Bund nach §283 Absatz 2 Satz 1 Nr. 8SGB V.
Newhouse, J. P. (2002). Why is there a quality chasm?
Health affairs, 21(4):13–25.
N
¨
uchtern, E., Bahemann, A., Egdmann, W., van Essen, J.,
Gostomzyk, J., Hemmrich, K., Manegold, B., M
¨
uller,
B., Robra, B., R
¨
oder, M., et al. (2015). Soziale
Sicherheit braucht Sozialmedizin Selbstverst
¨
andnis
von
¨
arztinnen und
¨
arzten in der sozialmedizinischen
Begutachtung und Beratung. Das Gesundheitswesen,
77(08/09):580–585.
Ries, V., Thiele, K.-P., Schuster, M., and Schuster, R.
(2020). It-structures and algorithms for quality assur-
ance in the health insurance medical advisory service
institutions in germany. In HEALTHINF, pages 353–
360.
Ries, V., Thiele, K.-P., van Treeck, B., Schroeer, S., Witt,
C., and Schuster, R. (2023). It-structures and algo-
rithms for quality assurance in the medical advisory
service institutions in germany. step 2: To err is hu-
man. consensus-conferences. In HEALTHINF, pages
271–278.
Schuster, R. (2022). Strukturelle Beratung der
Krankenkassen durch den Medizinischen Dienst
in der Pharmakotherapie. Die Verwendung epidemi-
ologischer und gesundheits
¨
okonomischer Analysen
im Zeitalter von Digitalisierung und Bid Data. Agor,
K and Knieps, F and Hartweg, HR.
Zeng, G. L. (2010). Medical image reconstruction, volume
530. Springer.
Relationships Between Central Quality Assurance Criteria for the Assessment of Statutory Health Insurance Patients for Further
Development Using Medical Informatics Methods
719
