
The 3D Printing Center for Health: Advancing Personalized
Healthcare Solutions Through Additive Manufacturing
Claudia Quaresma
1,2 a
, Ana Oliveira
2,3 b
, Carla Quintão
1,2 c
and Bruno Soares
2,3,4 d
1
LIBPhys, Laboratory for Instrumentation, Biomedical Engineering and Radiation Physics, Faculdade de Ciências e
Tecnologia, Universidade Nova de Lisboa, Caparica, Portugal
2
3D Printing Center for Health, Lisboa, Portugal
3
UNIDEMI, Department of Mechanical and Industrial Engineering, NOVA School of Science and Technology,
NOVA University of Lisbon, 2829-516, Caparica, Portugal
4
Laboratório Associado de Sistemas Inteligentes, 4800-058, Guimarães, Portugal
Keywords: 3D Printing, Customised, Healthcare, Technology, Co-Creation.
Abstract: The 3D Printing Center for Health is a non-profit association dedicated to advancing personalized healthcare
through the innovative application of 3D printing technology. By using a patient-centered, co-creation
methodology, the Center collaborates with patients, healthcare professionals, and engineers throughout the
design and development process. This approach enables the production of cost-effective, highly customized
medical devices, including prosthetics, orthotics, assistive devices, and anatomical models tailored to meet
the unique anatomical and functional needs of each patient. While partnering with hospitals and rehabilitation
centers, the Center addresses accessibility and affordability gaps often encountered in traditional healthcare,
making advanced solutions more widely available. Clinical studies have shown substantial improvements in
patient mobility and satisfaction, as well as a significant reduction in production costs due to the efficiency of
additive manufacturing. This paper provides an overview of the Center’s mission, methods, and main
achievements, highlighting its contributions to healthcare innovation and improvements in patient-specific
care through advanced 3D printing technologies. The Center’s commitment to social responsibility,
innovation, and patient-specific design is setting new standards in rehabilitative care and establishing a
foundation for future advancements in accessible, high-quality healthcare solutions.
1 INTRODUCTION
Additive Manufacturing, also known as Three-
dimensional (3D) printing technology, has been
emerging as a transformative tool in healthcare, as it
enables the production of highly customized, patient-
specific medical devices that traditional
manufacturing methods often cannot achieve (Pereira
et al., 2022; Pathak et al., 2023; Nizam et al., 2024).
3D printing applications in healthcare range from
creating prosthetic limbs and orthotic devices to
producing detailed anatomical models for surgical
planning and education. By building objects layer by
layer from digital models, 3D printing allows for
a
https://orcid.org/0000-0001-9978-261X
b
https://orcid.org/0009-0005-3145-6830
c
https://orcid.org/0000-0003-1015-4655
d
https://orcid.org/0000-0003-2737-1154
unparalleled precision and customization,
significantly enhancing the quality and accessibility
of medical solutions (Tian et al., 2021).
Despite these advancements, significant gaps
remain in the healthcare sector, especially concerning
common prosthetic and orthotic solutions. Usually,
these devices are costly and often lack
personalization, leading to discomfort and limited
functionality for patients, which results in high
rejection rates (Kumar Banga et a. 2021).
Furthermore, access to affordable, high-quality
rehabilitation tools is limited, particularly for
pediatric patients who require frequent adjustments
due to children’s development (Pathak et al., 2023).
Quaresma, C., Oliveira, A., Quintão, C. and Soares, B.
The 3D Printing Center for Health: Advancing Personalized Healthcare Solutions Through Additive Manufacturing.
DOI: 10.5220/0013262000003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 197-204
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
197

To address these challenges, the 3D Printing Center
for Health association was established with the
mission of developing accessible, cost-effective, and
highly customized healthcare solutions using
advanced 3D printing techniques. This non-profit
association brings together a multidisciplinary team
of engineers, healthcare professionals, and
researchers who collaborate on the design and
implementation of tailored medical devices. By
working closely with hospitals and rehabilitation
facilities, the 3D Printing Center for Health ensures
that these innovations are implemented in real-world
sceneries, thereby directly addressing the needs of
patients who, otherwise, would have limited access to
specialized care.
This paper aims to present an overview of the 3D
Printing Center for Health, highlighting its primary
projects and the methodologies that guide its research
and applications. It presents an overview of the
Center’s mission, methodologies, and key
achievements, underscoring its role in advancing
healthcare innovation and patient-specific care
through 3D printing. Through a series of case studies,
the paper illustrates the Center’s impact on patient
care, demonstrating how 3D printing can be
leveraged to create affordable and tailored healthcare
solutions that bridge critical gaps in traditional
healthcare delivery.
2 MATERIALS AND METHODS
The 3D Printing Center for Health employs a
structured, dynamic co-creation methodology that
keeps the patient at the heart of every stage of the
development process (Clanchy et al., 2024). This
patient-centered, adaptable approach is designed to
evolve with the patient's needs over time, ensuring
continuous alignment with both individual and
clinical requirements. Multidisciplinary collaboration
among engineers, healthcare professionals,
researchers, and patients enable the development of
devices that are uniquely tailored to the needs of each
patient (Silva et al., 2024). The Center’s mission is to
establish itself as a leader in promoting health and
functional independence through innovative 3D
printing technologies. By focusing on developing
customized, accessible solutions, the Center seeks to
empower individuals with motor disabilities and
other health needs, underscoring its commitment with
the advance of patient-centered healthcare.
The Center’s work is composed of three main
projects: 3D Anatomical Printing, e-NABLE 3D
Printing Center for Health and Motion Seeker.
a) 3D Anatomical Printing
This project focuses on the construction of anatomical
models based on medical imaging data, with a
particular reliance on Computed Tomography (CT)
scans and Magnetic resonance imaging (MRI). CT
imaging provides cross-sectional data that enables the
creation of precise, three-dimensional representations
of complex anatomical structures. These structures
can then be 3D printed, whether by Fused Deposition
Modeling (FDM), using mainly Polylactic Acid
(PLA) or through Vat Photopolymerization (SLA)
using Resins. These 3D-printed models are essential
for surgical planning, allowing clinicians to visualize
and assess patient-specific anatomy in a tangible way.
In addition to surgical planning, these models are
invaluable for educational purposes, providing
trainees and medical students with realistic, patient-
specific models for hands-on learning. Moreover,
these models can be used to explain to the patients
their health problems and how the medical team is
going to approach them, therefore comforting the
patients. Overall, the detailed visualization enabled
by CT-based 3D printed models enhances the
clinicians’ ability to anticipate challenges and devise
tailored surgical approaches, ultimately improving
patient outcomes and procedural success rates.
b) e-NABLE 3D Printing Center for Health
This project is dedicated to the development of
custom prosthetic devices, which can be precisely
adapted to address the specific motor dysfunctions
and needs of each patient. Through open-source
designs from the e-NABLE organization
(https://enablingthefuture.org), the Center’s team
leverages a foundation of shared knowledge to deliver
highly individualized solutions. These prosthetics are
printed with rigid and flexible materials, usually PLA
and Thermoplastic Polyurethane (TPU), respectively.
These materials are chosen for their unique
properties. PLA, a biodegradable thermoplastic
derived from renewable resources like corn starch,
offers rigidity and stability, making it ideal for
structural components of prosthetics that require
durability and shape retention. TPU, a flexible and
elastic thermoplastic, is used where adaptability and
comfort are paramount, such as in joint areas or grip-
enhancing sections of the prosthesis.
The design process involves exploring a range of
mechanical functionalities that maximize ease of use
and patient comfort.
Through this approach, the project not only
addresses the immediate functional needs of patients
but also paves the way for next-generation prosthetic
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
198

devices that are adaptable, comfortable, and
responsive to each individual’s unique requirements.
c) Motion Seeker
The Motion Seeker project is focused on designing
and developing customized assistive devices to
enhance functional independence for patients with
physical disabilities. Each assistive device is
meticulously tailored to support specific activities,
with the goal of empowering users to completely
engage in their daily tasks and social interactions. The
design process is conducted entirely by the Motion
Seeker team, consisting of engineers, healthcare
professionals, and designers who collaborate closely
with end-users and their families to ensure that each
device meets their unique functional needs. These
solutions are tailored to assist specific activities,
ensuring practical applicability and user comfort. As
with the developed prostheses within the scope of e-
NABLE 3D Printing Center for Health, the Motion
Seeker devices are predominantly printed with PLA
and TPU. However, more complex solutions may be
printed with higher performance materials, like
Acrylonitrile Styrene Acrylate (ASA), Acrylonitrile
Butadiene Styrene (ABS) or Nylon.
Systematic Co-Creation Process
The following steps are uniformly applied across all
projects to ensure consistency in device development:
1. Identification the Requirements: This initial
phase is crucial for establishing a clear understanding
of the specific needs and functional goals of each
assistive or prosthetic device. The requirements are
identified through a structured co-creation
methodology, which, as previously described,
actively involves patients, clinicians, and caregivers
in the decision-making process. By engaging all
stakeholders from the outset, this approach ensures
that the device aligns with the user’s unique
anatomical, functional, and lifestyle needs. During
this stage, detailed discussions and assessments are
conducted to gather insights into the patient’s daily
challenges, preferred usage scenarios, and any
existing limitations with traditional devices. The co-
creation process involves gathering both qualitative
and quantitative data. Qualitative insights are
collected through interviews and questionnaires with
patients and healthcare providers, while quantitative
measurements, such as anatomical measurements,
range of motion and strength assessments, are
performed to ensure that the functional requirements
of the device are fully understood. This collaborative
step is essential for ensuring that the project is
precisely tailored to each patient and provides a
foundation for the following design and development
phases. By defining these requirements
comprehensively, the team establishes a roadmap for
the next steps. This systematic approach ensures that
each device development is highly customized,
patient-centered, and responsive to the real-world
needs of users, setting the stage for a successful
design outcome.
2. Image Processing/3D Scanning: This phase
involves capturing patient-specific anatomical data
through advanced 3D scanning techniques to ensure
the precision required for a customized fit and
functionality of the device. Using technologies such
as CT, MRI, or high-resolution optical scanning,
detailed digital representations of the patient’s
anatomy are obtained. These imaging techniques
allow for precise measurements of bone structure,
soft tissue contours, or any unique anatomical
variations that may impact device design. Once
acquired, the scanned data undergoes initial image
processing to refine and optimize the anatomical
model. This includes segmentation, where specific
areas of interest (such as bones, muscles, or joints) are
isolated and refined, ensuring that only the most
relevant anatomical details are used in the design
process. This processing step is critical for removing
noise and highlighting key structural features, which
allows for greater accuracy in subsequent modelling
stages. In some cases, multiple imaging modalities
are combined to create a comprehensive 3D model
that captures both the internal and external anatomy.
For instance, CT scans may provide detailed skeletal
structure, while MRI data can add soft tissue
information, producing a more holistic anatomical
model. This level of detail is particularly valuable for
complex cases, enabling the design of devices that
closely conform to the patient's anatomy and offer
improved comfort and functionality. The resulting 3D
model is then imported into Computer-Aided Design
(CAD) software.
• 3. Computer-Aided Design (CAD) Modelling: In
this phase, the anatomical data captured through 3D
scanning is transformed into an accurate digital model
using Computer-Aided Design (CAD) software. The
3D Printing Center for Health typically relies on
Autodesk Fusion 360 (https://www.autodesk.com) for
creating patient-specific medical devices. Fusion 360s
advanced capabilities allow the design team to
replicate anatomical contours precisely, ensuring that
the digital model reflects the unique dimensions and
The 3D Printing Center for Health: Advancing Personalized Healthcare Solutions Through Additive Manufacturing
199

structural nuances of each patient’s body. The CAD
model functions as a flexible template for
customization, enabling the design team to adjust the
device’s shape, size, and functional elements to meet
the specific needs identified during the requirements-
gathering phase. This customization includes
designing features to support movement, applying
ergonomic principles to enhance comfort, and adding
adjustable components as necessary. Fusion 360
collaborative tools further allow for real-time design
adjustments based on continuous feedback from both
patients and clinicians, ensuring that the model remains
closely aligned with clinical requirements and user
preferences. Through an iterative design process, the
digital model is refined through multiple cycles.
Feedback from clinicians addresses functional aspects,
such as stability and support, while patient input
focuses on comfort, fitting, and aesthetic preferences.
This iterative cycle enables the team to make precise
adjustments that optimize the device’s usability and
effectiveness for real-world application. Once the
CAD model is finished, it undergoes through final
validation within the software to ensure structural
integrity and compatibility with 3D printing
specifications. The validated model is then prepared
for the next phase, material selection and 3D Printing.
This CAD modelling phase is essential to achieve a
highly customized, user-centered device, as it provides
the digital framework that guides fabrication.
4. Material Selection and 3D Printing: Suitable
materials are chosen based on the device's intended
function and patient needs. The majority of materials
used in device fabrication include PLA and/or TPU,
selected for their specific properties suited to patient
needs and the intended function of each device. PLA
provides rigidity and stability, ideal for structural
components, while TPU offers flexibility, making it
suitable for areas requiring greater adaptability and
comfort. Devices are fabricated using FDM, the most
common 3D printing technology, which enables rapid
prototyping and allows for iterative adjustments
throughout the design process. This ensures each
device meets high standards of functionality and
patient comfort.
5. Prototype Testing with Healthcare
Professionals and Patients: This phase is dynamic
and iterative, centered on testing the initial prototype
with both healthcare professionals and patients to
ensure it meets all functional, clinical, and
occupational needs. The first prototype is rigorously
evaluated for fitting, comfort, and usability in real-life
applications, with extensive feedback gathered from
all participants, both from interviews and from the use
of the System Usability Scale (SUS). (Bangor,
Kortum, and Miller 2008) This feedback is
invaluable, as it allows the team to make necessary
design adjustments that better align with the unique
requirements of each patient and the clinical
expectations. If modifications are needed, the design
is refined, reprinted, and subjected to further rounds
of testing. This cycle of adjustment, reprinting, and
retesting continues as needed, enabling the team to
optimize the device until it achieves the desired
outcomes. Throughout this process, the interests of
the patient, including both clinical and occupational
aspects, remain at the core of decision-making,
ensuring that the final device not only fulfills
technical specifications but also enhances the
patient's quality of life and independence. This
collaborative testing phase is essential for balancing
clinical functionality with patient comfort, as the
active involvement of patients and clinicians ensures
that the final device is as practical and effective as
possible. The comprehensive methodology enables
the Center to produce cost-effective, high-quality
devices tailored to address specific clinical needs
while maintaining rapid adaptability to feedback and
continuous improvement. The Center’s projects
leverage a structured, dynamic co-creation
methodology, ensuring each device meets patient-
specific anatomical and functional requirements.
Through a collaborative process involving patients,
clinicians, and engineers, device requirements are
iteratively refined, with adjustments made based on
ongoing feedback and clinical testing.
By applying this methodology consistently across all
projects, the 3D Printing Center for Health ensures
the production of cost-effective, high-quality devices
that meet specific clinical and patient needs. This
systematic approach enhances the Center’s ability to
adapt designs in response to feedback, enabling
continuous improvement and alignment with real-
world requirements.
3 RESULTS
The 3D Printing Center for Health has achieved
significant impact through its three main projects,
with measurable improvements in patient care and
functionality.
a) 3D Anatomical Printing
Anatomical models created through this project have
been used in various hospitals, providing clinicians
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
200

with detailed, patient-specific visual aids for surgical
planning and training. Different anatomical
structures, including a thoracic cage model printed in
PLA (Figure 1), have been produced to support
hands-on training exercises. Feedback from medical
professionals indicates that these models enhance
precision in complex surgical procedures by
improving anatomical visualization and enabling the
medical team to practice techniques in a realistic and
controlled environment.
Figure 1: Thoracic cage model printed in PLA.
b) e-NABLE 3D Printing Center for Health
The e-NABLE 3D Printing Center for Health has
successfully developed and delivered custom
prosthetic devices specifically designed for pediatric
upper-limb applications, providing vital support for
numerous patients with motor impairments. Using
open-source models from the e-NABLE organization
as a foundation, the Center customizes each
prosthesis to address the unique anatomical and
functional needs of each child. This approach allows
for the production of prostheses that enhance comfort,
functionality, and adaptability, promoting greater
independence and confidence in daily activities.
To meet the specific requirements of different
types of amputations, the Center has developed
various prosthetic models, including transcarpal
(Figure 2), transradial (Figure 3), and transhumeral
prostheses (Figure 4).
Each type is tailored according to the level of
amputation, ensuring an optimal fit and alignment
with the patient’s remaining limb structure. This
customization process enables children to perform
tasks more effectively and comfortably, with designs
that accommodate their growth and evolving needs.
Figure 2: Prosthesis designed for a child with a transcarpal
amputation.
Figure 3: Prosthesis designed for a child with a transradial
amputation.
Figure 4: Prosthesis designed for a child with a transumeral
amputation.
The 3D Printing Center for Health: Advancing Personalized Healthcare Solutions Through Additive Manufacturing
201
By integrating a patient-centered approach and
advanced 3D printing technology, the Center is able
to offer accessible, cost-effective prosthetic solutions
that significantly improve the quality of life for
pediatric patients with upper-limb deficiencies. The
SUS results were 95, 85 and 98 respectively which
show that this approach works. Furthermore, long
term monitoring showed that all patients still use the
prosthesis, contrary to the normal rejection rates after
6 months (Resnik L., Borgia M., Biester S., Clark
M.A., 2021), even when compared with the E-Nable
standard models.
With these prostheses, children are now able to
perform tasks and gestures they had previously been
unable to accomplish.
Currently, the Center is actively engaged in
research focused on the development of myoelectric
prosthetics, aiming to create devices that offer
enhanced control and responsiveness. Ongoing
research on myoelectric prosthetics suggests
promising future applications, with potential for more
intuitive, user-friendly designs that could further
improve patient autonomy.
c) Motion Seeker
One of the key projects under the 3D Printing Center
for Health, the Motion Seeker, exemplifies the
transformative potential of 3D-printed assistive
devices. This project focuses on developing devices
that enable patients to regain independence in
performing daily tasks. Devices developed within this
framework have notably improved the ability of
patients with motor handicaps to engage in previously
challenging activities, such as playing musical
instruments or using everyday objects (Figure 5).
Figure 5: Assistive device for daily tasks.
These devices are specifically designed from the
ground up to ensure maximum functionality and
comfort, from the initial conceptualization and design
phase up to the final 3D printing process.
The Center’s approach to designing devices based
on individual anatomical specifications ensures
optimal performance across a range of tasks, thus
improving the patient's overall independence.
Feedback from both patients and caregivers has
been overwhelmingly supportive, with reports
indicating a marked improvement in the ease of use
and comfort of these devices. Since January 2022
through October 2024, the Center has developed a
total of 82 devices, representing 43 unique designs,
which have helped 78 patients across 9 services from
various hospitals. Patients and caregivers have
expressed particular, appreciation for the adaptability
of these devices, noting how seamlessly they can be
used for different tasks, further emphasizing their
effectiveness in daily life. The combination of
tailored designs and advanced manufacturing
processes has led to assistive devices that not only
meet functional requirements but also provide long-
term comfort, contributing to enhanced patient
satisfaction and overall well-being, with an overall
SUS value of 91.
The 3D Printing Center for Health has made
significant advancements in medical device
development, demonstrating measurable clinical and
economic benefits through its innovative applications
of 3D printing technology in healthcare.
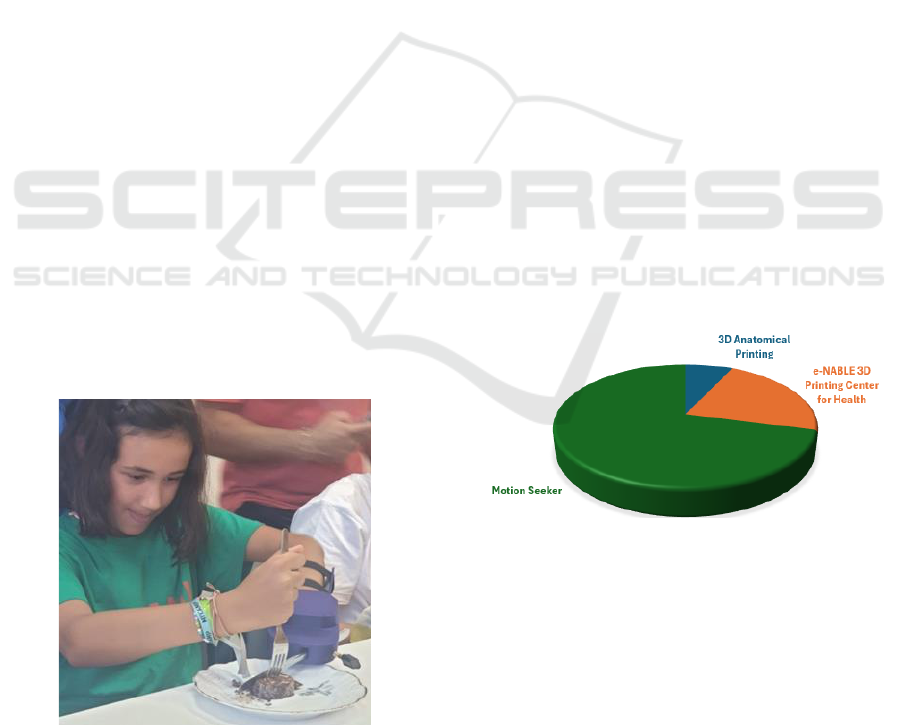
Figure 6 illustrates the distribution of project
activity within the 3D Printing Center for Health
across its three main initiatives.
Figure 6: Distribution of activity across each project.
The data indicate that most of the Center's efforts,
71%, are concentrated on the Motion Seeker project,
which focuses on developing customized assistive
devices aimed at improving functional independence
for patients with physical disabilities. The e-NABLE
3D Printing Center for Health project, dedicated to
creating personalized prosthetics primarily for
pediatric patients, represents 22% of the Center’s
work. Finally, 3D Anatomical Printing, which
involves creating detailed anatomical models for
surgical planning and educational purposes, accounts
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
202

for 7% of the Center’s activities. This distribution
reflects the Center’s strategic emphasis on assistive
devices for enhancing patient autonomy, followed by
efforts in prosthetics and anatomical modelling.
Among the Center’s most notable achievements,
clinical studies indicate a remarkable 90%
improvement in patient mobility among users of 3D-
printed rehabilitation devices. This improvement has
been attributed to the highly customized designs,
which align closely with individual anatomical and
functional needs compared to standard devices
available on the market. Additionally, the Center's
use of Additive Manufacturing has led to a 30%
reduction in production costs, an achievement that
greatly enhances the affordability and accessibility of
essential rehabilitation solutions.
The impact on patients has been equally profound,
with user testimonials reflecting a 95% satisfaction
rate. Patients consistently report enhanced comfort
and functionality, emphasizing how these devices are
helpful in their daily activities. For many users, these
assistive devices and prostheses have enabled them to
perform tasks and gestures that were previously
unachievable, underscoring the Center’s success in
addressing both physical and psychological needs.
The patient-centered approach, focusing on the
customization and refinement of 3D-printed devices,
has proven to be highly effective, reinforcing the 3D
Printing Center for Health’s role as a leader in
advancing patient care through innovative,
accessible, and responsive healthcare technologies.
4 CONCLUSIONS
The 3D Printing Center for Health has effectively
demonstrated the transformative potential of additive
manufacturing in healthcare through the creation of
customized, functional, and accessible assistive
devices. By employing a patient-centered design
approach and leveraging advanced 3D printing
technologies, the Center has elevated both the quality
and adaptability of medical devices, aligning them
closely with the specific clinical and occupational
needs of diverse users. Furthermore, the Center
addresses critical gaps in traditional healthcare,
particularly for underserved communities, by creating
high-quality, customized, and affordable devices that
enhance users' quality of life and promote social
inclusion. Future initiatives include expanding
research into myoelectric devices with
electromyographic control for real-time
responsiveness and exploring biocompatible smart
materials to enhance adaptability and durability.
Through this commitment to innovation and patient-
centered design, the 3D Printing Center for Health is
setting a new standard in rehabilitative care,
advancing healthcare technology, and promoting
equity across patient populations. Future initiatives
include developing myoelectric devices and
exploring biocompatible materials to enhance
adaptability and durability. Through this commitment
to innovation and accessibility, the Center sets a new
standard in rehabilitative care.
Additionally, the 3D Printing Center for Health
ensures the performance and safety of all devices by
adhering to local and international regulatory
requirements for medical devices. Each device
undergoes rigorous testing for structural integrity,
functionality, and safety before being approved for
clinical use. The Center collaborates with healthcare
institutions to guarantee compliance with applicable
legal frameworks. Additionally, a thorough risk
management process is implemented to identify and
address potential hazards throughout the
development cycle. This approach ensures that all
devices meet high standards of safety, reliability, and
efficacy, reinforcing the Center’s commitment to
advancing patient-centered healthcare solutions.
ACKNOWLEDGEMENTS
The authors acknowledge funding from Fundacão
para a Ciência e Tecnologia (FCT-MCTES) for its
financial support through the UNIDEMI projects
UIDB/00667/2020 and UIDP/00667/2020. dditional
support was provided by national funds from I.P.
through the DOI: 10.54499/UIDB/04559/2020
(LIBPhys-UNL).
REFERENCES
Bangor, Aaron Kortum, Philip T. Miller, James T., (2008)
An empirical evaluation of the system usability scale,
International Journal of Human-Computer Interaction,
6, 574-594
Clanchy K, Mitchell J, Mulholland K, Jurd E, Kendall E,
Lloyd DG, Palipana D, Pizzolato C and Shirota C
(2024) Towards co-design of rehabilitation
technologies: a collaborative approach to prioritize
usability issues. Front. Rehabil. Sci. 5:1302179. doi:
10.3389/fresc.2024.1302179
Kumar Banga, H., Kalra, P., M. Belokar, R., & Kumar, R.
(2021). Design and Fabrication of Prosthetic and
Orthotic Product by 3D Printing. IntechOpen. doi:
10.5772/intechopen.94846
The 3D Printing Center for Health: Advancing Personalized Healthcare Solutions Through Additive Manufacturing
203

Nizam, M., Purohit, R., & Taufik, M. (2024). 3D printing
in healthcare: A review on drug printing, challenges,
and future perspectives. Additive Manufacturing, 40,
110199.
Pathak K, Saikia R, Das A, Das D, Islam MA, Pramanik P,
Parasar A, Borthakur P, Sarmh P, Saikia M, Borthakur
B. (2023). 3D printing in biomedicine: advancing
personalized care through additive manufacturing.
Explor Med, 4:1135–67.
Pereira HR, Barzegar M, Hamadelseed O, Esteve AV,
Munuera J. (2022). 3D surgical planning of pediatric
tumors: a review. Int J Comput Assist Radiol Surg.
2022;17(4):805–16.
Resnik L., Borgia M., Biester S., Clark M. A., A
Longitudinal study of prosthesis use in veterans with
upper limb amputation (2021) Prosthetics and Orthotics
International, 45 (1), pp. 26 - 35
Silva J, Silva M, Soares B, Quintão C, Londral AR,
Quaresma C. Multi-activity 3D printed assistive
technology in children: a case study. Assist Technol.
2024;00(00):1–6.
Tian, Y., Chen, C. X., Xu, X., Wang, J., Hou, X., Li, K.,
Lu, X., Shi, H., Lee, E. S., & Jiang, H. B. (2021). A
review of 3D printing in dentistry: technologies,
affecting factors, and applications. Scanning, 2021,
Article ID 9950131.
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
204
