
Short-Term Effects of Mindful Uni-Nostril Breathing on
Cardio-Autonomic Functions: A Randomized Controlled Trial
Satyam Tiwari
a
and Arnav Bhavsar
b
Indian Knowledge System and Mental Health Applications Centre, IIT Mandi, India
Keywords: Uni-Nostril Breathing, Breath-Based Mindfulness, Autonomic Regulation, Cardiovascular Health, Svara
Yoga.
Abstract: Uni-nostril mindful breathing, an ancient yogic practice, has been suggested to influence autonomic nervous
system function differentially, yet systematic evidence remains limited. This randomized controlled trial
investigated the effects of nostril-specific breathing techniques on autonomic nervous system modulation in
healthy adults. Ninety participants were randomly assigned to one of three groups: left-nostril breathing, right-
nostril breathing, or a control group performing unstructured breathing for 10 minutes. HRV parameters and
systolic and diastolic blood pressures were collected pre-and post-intervention. Left nostril breathing
significantly decreased HRV parameters (SDNN: -27.0%, RMSSD: -25.1%) while increasing SI (+37.4%)
and SNS activity (+98.7%), therefore suggesting increased sympathetic activation. With little impact on other
autonomic indicators, right-nostril breathing showed significant decreases in both systolic (-5.5 mmHg) and
diastolic blood pressure (-3.3 mmHg). These results support nostril-specific breathing as a simple, non-
pharmacological technique for autonomic modulation, offering prospective applications in stress and
cardiovascular management, with varying effects dependent upon nostril selection.
1 INTRODUCTION
Breathing patterns, characterized by their rate, depth,
and rhythm, are essential for physiological control
and health preservation (Russo et al., 2017). These
patterns are not only mechanical activities; they act as
a bridge between voluntary and involuntary
physiological control systems, significantly
impacting autonomic nervous system function,
emotional states, and cognitive performance (Brown
& Gerbarg, 2009).
Recent research has further emphasized how
controlled breathing patterns can significantly
modulate autonomic responses, with particular
attention to the timing and awareness aspects of
breathing interventions (Gerritsen et al., 2023).
Various breathing patterns can induce unique
physiological responses; slow, deep breathing often
promotes parasympathetic activation and reduces
stress, while fast breathing can increase sympathetic
arousal (Pal et al., 2014). Research has demonstrated
that specific breathing patterns can modulate heart
a
https://orcid.org/0009-0005-8070-5979
b
https://orcid.org/0000-0003-2849-4375
rate variability, blood pressure, and stress hormone
levels (Jerath et al., 2006). Specific nasal breathing
rhythms have been shown to affect hemispheric brain
activity and corresponding autonomic responses
(Shannahoff-Khalsa, 2015). Studies indicate that
conscious modification of breathing patterns can
serve as a therapeutic tool for various physiological
and psychological conditions, highlighting the
importance of understanding the mechanisms
underlying different breathing techniques (Telles et
al., 2011).
1.1 Background and Rationale
Despite extensive research on breathing practices, a
significant limitation remains in understanding the
unique autonomic effects associated with unilateral
nostril patterns. Although previous studies, like
Zelano et al. (2016), demonstrated the effect of nasal
breathing on limbic oscillations, and Kahana-Zweig
et al. (2016) delineated fundamental nasal cycles,
784
Tiwari, S. and Bhavsar, A.
Short-Term Effects of Mindful Uni-Nostril Breathing on Cardio-Autonomic Functions: A Randomized Controlled Trial.
DOI: 10.5220/0013262500003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 784-792
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

they largely ignored to differentiate the distinct
autonomic effects of each nostril.
More recent systematic investigations have
demonstrated that slow breathing techniques can
enhance cardiac vagal activity through specific
respiratory brain coupling mechanisms (Laborde et
al., 2022). However, these studies have not explicitly
examined nostril-specific breathing patterns.
Conventional research, such as that conducted by
Pal et al. (2004) and Telles et al. (2011), mainly
investigated alternating nostril breathing or combined
breathing methods, therefore ignoring the distinct
impacts of right or left nostril breathing in isolation.
The recent study by Noble and Hochman (2019)
on pulmonary afferent patterns, along with the work
of Van Diest et al. (2014) on inhalation/exhalation
ratios, indicates that the particulars of breathing
patterns have a significant impact on autonomic
responses. However, these studies did not examine
the lateralized effects associated with nostril-specific
breathing. Zaccaro et al. (2018) conducted a thorough
review of the psychophysiological correlates of slow
breathing; however, their analysis pointed out a
significant gap in research regarding the unique
autonomic signatures associated with sustained
unilateral nostril breathing.
Recent studies have specifically highlighted the
role of mindfulness in breathing interventions. Unlike
mechanical breathing exercises, mindful breathing
incorporates attention regulation and present-moment
awareness, potentially enhancing autonomic
regulation through distinct neural pathways.
However, research examining mindful unilateral
nostril breathing remains limited.
Furthermore, prior studies have demonstrated
limitations in both technique and scope. Several
studies utilized brief intervention durations or did not
account for natural nasal cycles (Gerritsen & Band,
2018). Limited research on unilateral breathing has
focused mainly on immediate, short-term effects,
ignoring the long-term implications for autonomic
regulation. Moreover, Courtney (2009) emphasizes
that the connection between breathing patterns and
their therapeutic uses is not fully understood,
particularly in relation to nostril-specific techniques.
Steffen et al. (2023) have highlighted the
significant relationship between controlled breathing
practices and heart rate variability, emphasizing the
need for more targeted research on specific breathing
techniques. Their review suggests that while general
slow breathing patterns show apparent autonomic
effects, the specific mechanisms of unilateral nostril
breathing remain understudied.
The current study addresses these gaps by:
• Investigating the specific autonomic effects
of mindful unilateral nostril breathing
• Implementing rigorous controls while
accounting for mindfulness components
• Examining the interaction between
mindfulness and nostril-specific breathing
patterns in autonomic regulation
1.2 Objectives
This study aims to assess the impact of mindful left-
and right-nostril breathing techniques on autonomic
and cardiovascular health indicators, with particular
emphasis on heart rate variability (HRV) parameters
and blood pressure. The integration of mindfulness
with nostril-specific breathing provides a novel
approach to understanding autonomic modulation.
2 METHODS
The study used a randomized controlled trial design
to assess the impact of unilateral nostril breathing on
autonomic and cardiovascular parameters. The
Institutional Review Board of IIT Mandi approved
the study protocol, and all participants provided
written informed consent prior to participation.
2.1 Participants
Ninety healthy participants, aged 18 to 34 years, were
recruited and randomly assigned to three groups, each
consisting of 30 individuals: left-nostril breathing
(LNB), right-nostril breathing (RNB), and a control
group. Randomization was conducted utilizing a
computer-generated sequence. The demographics
and baseline characteristics of participants were
similar across groups (Table 1). Inclusion criteria
required participants to be in good physical health
(absence of diagnosed medical conditions and normal
vital signs at screening) with no history of
cardiovascular or respiratory disorders. Exclusion
criteria included chronic obstructive pulmonary
disease (COPD), heart disease, recent surgeries, or
recent exposure to stimulants (consumption of
caffeine, nicotine, or energy drinks within 12 hours
before the intervention).
To control for exercise as a potential confounding
factor, all participants were instructed to avoid
moderate to vigorous physical activity for 24 hours
prior to testing. Additionally, participants were asked
to maintain their normal daily activities but avoid any
form of exercise on the day of testing until the
completion of all measurements.
Short-Term Effects of Mindful Uni-Nostril Breathing on Cardio-Autonomic Functions: A Randomized Controlled Trial
785

Table 1 presents the demographic characteristics
and baseline measurements of participants across all
three groups. No significant differences were
observed in age (F (2,87) = 0.14, p = .87) or gender
distribution (χ2 = 0.42, p = .81) between groups.
Table 1: Participant Demographics and Baseline
Characteristics.
Characteristic
LNB Group
(n=30)
RNB Group
(n=30)
Control Group
(n=30)
p-value
Age (years) 21.1 ± 1.4 21.3 ± 1.6 21.2 ± 1.5 0.87
Female (%) 33 30 37 0.81
2.2 Intervention Protocol
Participants in the experimental groups performed
their respective breathing techniques for 10 minutes.
The LNB group practiced breathing exclusively
through the left nostril, while the RNB group used
only the right nostril. The control group maintained
normal breathing.
All participants maintained a standardized seated
posture with eyes closed and followed a regulated
breathing rhythm (6-second inhalation, 6-second
exhalation) guided by a digital timer for 10 minutes.
Participants were familiarized with the digital timer's
audio cues before the intervention. The timer
produced soft beeps (40dB), indicating inhalation and
exhalation phases, allowing participants to maintain
the breathing rhythm with their eyes closed. Room
temperature and environmental conditions were
controlled throughout the sessions.
2.3 Outcome Measures
Primary outcome measures included both time-
domain and frequency-domain heart rate variability
(HRV) parameters, along with blood pressure
measurements. The time-domain parameters
included:
RMSSD (Root Mean Square of Successive
Differences): Quantifying short-term beat-to-beat
variations
SDNN (Standard Deviation of Normal-to-Normal
intervals): Representing overall variability of heart
rhythms
Frequency-domain parameters included:
• LF (Low Frequency) power: 0.04-0.15 Hz band
• HF (High Frequency) power: 0.15-0.40 Hz
band
• LF/HF ratio: Indicating sympathovagal balance
Secondary outcomes comprised:
• Stress Index (SI): Calculated using Baevsky's
formula (SI = AMo/2Mo × MxDMn)
• Sympathetic Nervous System (SNS) activity:
Evaluated through Low-Frequency power
• Parasympathetic Nervous System (PNS)
activity: Assessed through High-Frequency
power
• Blood pressure parameters (systolic and
diastolic)
All measurements were recorded at baseline (pre-
intervention) and immediately after the practice
(post-intervention) using calibrated equipment. HRV
parameters were measured using the EM Wave Pro
device during 5-minute recording periods with a
sampling frequency of 370 hertz, and blood pressure
was assessed using a calibrated sphygmomanometer
following standard protocols.
2.4 Statistical Analysis
Statistical analyses were performed using SPSS
version 25.0. Paired t-tests compared pre-post
differences within groups, while between-group
differences were analyzed using one-way ANOVA
with post-hoc Tukey tests. Statistical significance was
set at p < .05. Effect sizes were calculated using
Cohen's d for significant findings.
3 RESULTS
3.1 Heart Rate Variability Parameters
3.1.1 Time-Domain Analysis
No significant differences were observed in baseline
HRV parameters between groups (SDNN: F (2,87)
=0.34, p=.71; RMSSD: F (2,87) =0.29, p=.75),
indicating comparable autonomic states at study
onset. Analysis of HRV parameters revealed
significant changes in the left-nostril breathing group,
while the right-nostril and control groups showed
minimal variations (Table 2). The left-nostril
breathing group demonstrated significant reductions
in both SDNN and RMSSD (p < .01).
Table 2: Changes in Heart Rate Variability Parameters.
Parameter Group Pre Post
Change
(%)
p-value
SDNN
(
ms
)
Left 86.20 ± 23.4 62.90 ± 18.7 -27.0 0.002
Ri
g
ht 83.45 ± 22.1 81.23 ± 20.9 -2.7 0.456
Control 84.12 ± 21.8 83.89 ± 21.2 -0.3 0.891
RMSSD
(
ms
)
Left 86.23 ± 24.1 64.57 ± 19.2 -25.1 0.002
Ri
g
ht 84.67 ± 22.8 82.34 ± 21.4 -2.7 0.478
Control 85.01 ± 23.2 84.56 ± 22.1 -0.5 0.867
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
786
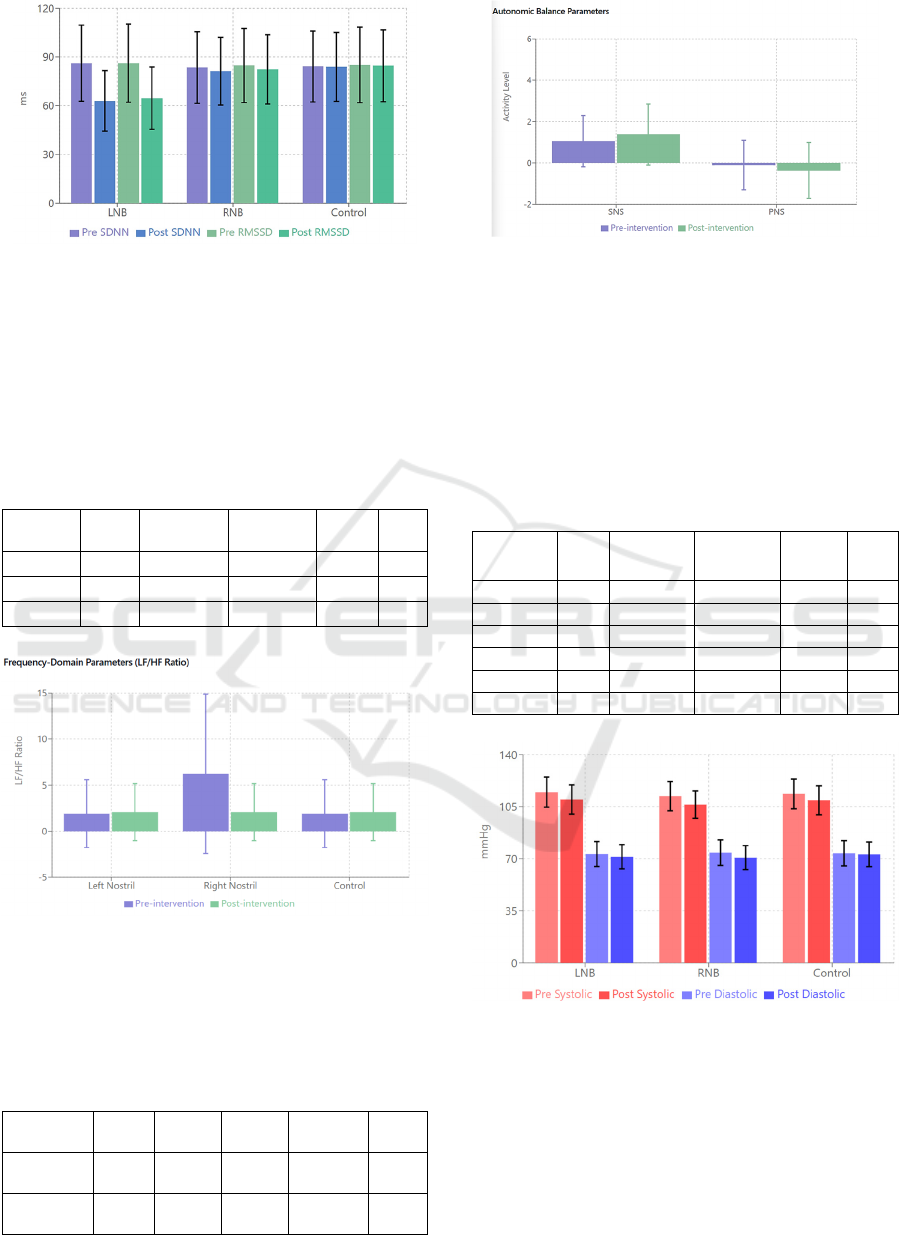
Figure 1: Heart Rate Variability Parameters – Pre-
intervention and post-intervention SDNN and RMSSD
values by group.
3.1.2 Frequency-Domain Analysis
Frequency analysis demonstrated distinct
autonomic responses:
Table 3: Changes in Frequency-Domain Parameters.
Parameter Group
Pre-
Intervention
Post-
Intervention
Change
(%)
p-
value
LF/HF Left 1.90 ± 3.67 2.07 ± 3.09 +8.9 0.716
Right 6.24 ± 8.66 2.07 ± 3.09 -66.8 0.125
Control 1.90 ± 3.67 2.07 ± 3.09 +8.9 0.678
Figure 2: Frequency-Domain Parameters – LF/HF ratio
(mean ± SD) showing autonomic balance changes pre- and
post-intervention across groups.
3.1.3 Autonomic Balance Indicators
Table 4: Changes in Autonomic Parameters.
Parameter Group Pre Post
Change
(%)
p-
value
SNS
Activity
Left
1.06 ±
1.24
1.38 ±
1.48
+30.2 0.013
PNS
Activity
Left
-0.10 ±
1.20
-0.36 ±
1.36
-260.0 0.087
Figure 3: Autonomic Activity – SNS and PNS activity
levels (mean ± SD) pre- and post-intervention,
demonstrating relative changes in autonomic regulation.
3.2 Blood Pressure Changes
Both experimental groups showed significant
reductions in systolic blood pressure, with the right-
nostril breathing group demonstrating additional
significant decreases in diastolic pressure (Table 5).
Table 5: Changes in Blood Pressure.
Parameter Group Pre-
Intervention
(
mmH
g)
Post-
Intervention
(
mmH
g)
Change
(mmHg)
p-
value
Systolic BP Left 114.9 ± 10.2 109.9 ± 9.8 -5.0 0.010
Right 112.1 ± 9.8 106.6 ± 9.2 -5.5 0.012
Control 113.7 ± 10.1 109.4 ± 9.7 -4.3 0.038
Diastolic BP Left 73.3 ± 8.4 71.5 ± 8.1 -1.8 0.064
Right 74.2 ± 8.6 70.9 ± 8.0 -3.3 0.048
Control 73.8 ± 8.5 73.1 ± 8.3 -0.7 0.452
Figure 4: Blood Pressure Changes – Systolic and diastolic
pressure pre-post intervention by group.
3.2.1 Stress Index and SNS Activity
The left-nostril breathing group showed significant
increases in both the Stress Index and SNS activity
(Table 6). The right-nostril group demonstrated
moderate increases in SI, while the control group
maintained stable levels.
Short-Term Effects of Mindful Uni-Nostril Breathing on Cardio-Autonomic Functions: A Randomized Controlled Trial
787
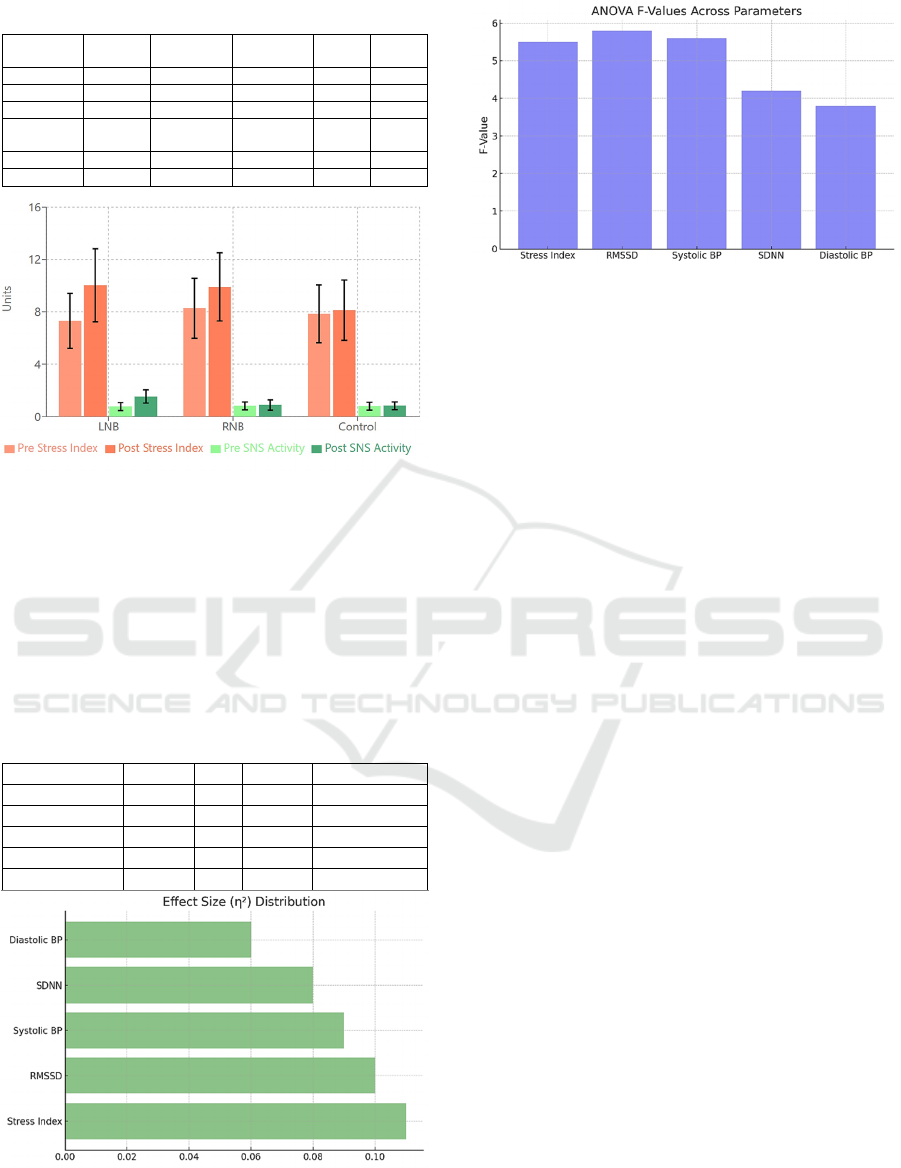
Table 6: Changes in Stress Parameters.
Parameter Group Pre Post Change
(
%
)
p-value
Stress Index Left 7.30 ± 2.1 10.03 ± 2.8 +37.4 <0.001
Ri
g
ht 8.27 ± 2.3 9.90 ± 2.6 +19.7 0.037
Control 7.85 ± 2.2 8.12 ± 2.3 +3.4 0.456
SNS
Activit
y
Left 0.77 ± 0.3 1.53 ± 0.5 +98.7 0.008
Ri
g
ht 0.82 ± 0.3 0.89 ± 0.4 +8.5 0.324
Control 0.80 ± 0.3 0.83 ± 0.3 +3.8 0.678
Figure 5: Stress Parameters – Pre- post intervention Stress
Index and SNS Activity by group.
3.2.2 Between-Group Analysis
One-way ANOVA revealed significant differences
between groups across all primary parameters (Table
7). Post-hoc Tukey tests indicated that the left-nostril
breathing group showed the most pronounced
changes in autonomic parameters.
Table 7: Between-Group ANOVA Results.
Parameter F-value df p-value Effect Size (η²)
Stress Index 5.31 2,87 <0.01 0.109
SDNN 4.12 2,87 <0.05 0.087
RMSSD 5.25 2,87 <0.05 0.108
Systolic BP 4.75 2,87 <0.01 0.098
Diastolic BP 3.42 2,87 <0.05 0.073
Figure 6: Effect Size Distribution – η² effect sizes across
various metrics.
Figure 7: ANOVA F-Values – F-values for different
metrics indicating group variance.
4 DISCUSSIONS
The present study examined the effects of three
different breathing interventions—left Inhale-Exhale
(LNB Group), Right Inhale-Exhale (RNB Group),
and normal breathing (Control Group)—on key
autonomic and cardiovascular parameters. The
findings reveal distinct physiological impacts based
on the specific nostril employed, which aligns with
and extends previous research on breathing
techniques and their influence on autonomic balance.
4.1 Interpretation of Primary Findings
The LNB Group, engaging in left-nostril breathing,
demonstrated significant increases in Stress Index
(SI) and Sympathetic Nervous System (SNS) activity,
coupled with notable reductions in heart rate
variability (HRV) as measured by SDNN and
RMSSD. These changes are indicative of heightened
sympathetic activity.
The finding contrasting interpretations from
previous studies (Russo et al., 2017) may suggest that
increased sympathetic activity might indicate left-
nostril breathing, under certain controlled durations
and contexts, can enhance alertness, responsiveness,
etc, via stress arousal rather than inducing a strictly
calming effect. The RNB Group, using right-nostril
breathing, demonstrated significant reductions in
systolic and diastolic blood pressure, indicating
potential cardiovascular advantages without causing
considerable sympathetic arousal. The Control
Group, showing normal breathing, demonstrated
slight variance across these parameters, consequently
affirming that the effects observed in the
experimental groups are directly linked to the specific
breathing interventions.
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
788

4.2 Detailed Discussion of Autonomic
Modulation via HRV Parameters
The significant changes in HRV parameters observed
in this study require further investigation, especially
considering the differences from traditional
understandings of nostril-specific breathing effects.
The left-nostril breathing group exhibited significant
reductions in SDNN (-27.0%) and RMSSD (-25.1%),
surpassing the typical magnitudes observed in
breathing intervention studies. According to the Task
Force of the European Society of Cardiology and the
North American Society of Pacing and
Electrophysiology (1996), such significant alterations
in HRV parameters indicate meaningful shifts in
autonomic balance, particularly when SDNN
reductions exceed 20% from baseline values.
The simultaneous increase in Stress Index (SI:
+37.4%, p<0.001) and SNS activity (+98.7%,
p=0.008) in the left-nostril breathing group presents
an intriguing autonomic profile that challenges
traditional perspectives. These results particularly
contradict traditional assumptions in yogic and
autonomic literature, according to which left nostril
breathing usually corresponds with parasympathetic
activity (Pal et al., 2004).
The observed increase in the Stress Index
following mindful breathing, particularly in the LNB
group, may be attributed to enhanced autonomic
engagement during focused attention. This
heightened autonomic response could reflect the
active nature of mindful breathing practice rather than
passive relaxation, suggesting a complex interaction
between attention regulation and autonomic control
mechanisms. This finding aligns with the recent
understanding that mindfulness practices can initially
increase alertness and sympathetic activation as part
of the attention-regulation process.
These changes were accompanied by significant
alterations in frequency-domain measures,
particularly the LF/HF ratio, suggesting shifts in
autonomic balance. The frequency-domain analysis
provided additional insights:
• Left-nostril breathing: Increased LF/HF ratio
(+8.9%), suggesting sympathetic predominance
• Right-nostril breathing: Notable decrease in
LF/HF ratio (-66.8%), indicating
parasympathetic activation
• Control group: Minimal changes in autonomic
balance
The simultaneous analysis of SNS (+30.2%) and
PNS (-260.0%) activity in the left-nostril group
supports a complex pattern of autonomic modulation
rather than simple sympathetic activation. These
findings align with Malliani et al.'s (1991) framework
of cardiovascular neural regulation, suggesting that
sustained changes in autonomic parameters often
reflect intricate regulatory mechanisms.
The observed changes align with the
cardiovascular neural regulation framework proposed
by Malliani et al. (1991), who established that
sustained changes in autonomic parameters often
reflect complex regulatory mechanisms rather than
simple linear responses. The magnitude of these
changes indicates the activation of neuronal
respiratory components that, as suggested by Jerath et
al. (2006), might induce different autonomic
reactions via cardiorespiratory coupling processes,
although further research is needed.
While previous research by Pal et al. (2004)
suggested predominantly parasympathetic effects of
left nostril breathing in short-term practices, our
results indicate a more complex autonomic response
pattern. This complexity aligns with Telles et al.'s
(2011) findings on high-frequency yoga breathing,
which demonstrated that specific breathing patterns
can elicit varied autonomic responses based on
practice parameters.
These findings contribute to the broader
understanding of breath-based interventions outlined
by Brown and Gerbarg (2005), who emphasized the
importance of technique-specific effects in
autonomic modulation. The observed effect sizes for
HRV parameters (η²=0.087 for SDNN, η²=0.108 for
RMSSD) suggest potentially meaningful clinical
applications, particularly when considered alongside
Russo et al.'s (2017) analysis of physiological effects
in slow breathing practices. However, as
demonstrated by our results and supported by Telles
and Naveen (2008), such pronounced autonomic
shifts may require further assessments for application
based on baseline autonomic status and therapeutic
goals.
4.3 Cardiovascular Impacts
The RNB Group exhibited significant reductions in
both systolic and diastolic blood pressure post-
intervention, which aligns with literature suggesting
right-nostril breathing may modulate cardiovascular
responses favorably (Telles et al., 2011). Studies
indicate that right-nostril breathing can stimulate
autonomic adjustments that reduce blood pressure
without necessarily activating the sympathetic system
in the same way as left-nostril breathing. Lehrer et al.
(2000) demonstrated similar blood pressure benefits
through controlled breathing exercises, highlighting
Short-Term Effects of Mindful Uni-Nostril Breathing on Cardio-Autonomic Functions: A Randomized Controlled Trial
789

that right-nostril breathing may lower blood pressure
while maintaining autonomic stability. The minimal
changes in SNS activity and HRV metrics in the RNB
Group support this distinction, suggesting right-
nostril breathing as a potential cardiovascular
intervention with limited sympathetic activation.
4.4 Comparison with Existing
Literature
This study’s findings partially align with, yet diverge
from, past research on nostril-specific breathing
techniques. Pal et al. (2004) and Shannahoff-Khalsa
(2007) posited that left-nostril breathing enhances
parasympathetic responses, a view commonly
supported by traditional yogic practices. However,
our findings suggest that left-nostril breathing, under
specific conditions, may stimulate sympathetic
responses, contrasting with the relaxation effects
typically associated with it (Brown & Gerbarg, 2005).
The consistency of our findings with Lehrer et al.
(2000) and Telles et al. (2011) in terms of right-nostril
breathing’s effect on blood pressure, however,
underscores its potential as a low-intensity
intervention for cardiovascular regulation. The
observed sympathetic elevation in the LNB Group
further reflects the unique duality in nostril-specific
breathing, suggesting autonomic responses that vary
based on intensity, duration, and nostril dominance.
4.5 Possible Mechanisms of Action
The differential effects observed can be understood
through the concept of autonomic lateralization,
where each hemisphere of the brain exerts contrasting
influences on autonomic output. Specifically, right-
hemisphere stimulation (through left-nostril
breathing) has been associated with heightened
sympathetic arousal. In contrast, left-hemisphere
stimulation (through right-nostril breathing) can
foster a parasympathetic response or a balanced
autonomic tone (Craig, 2005). The increased SI and
SNS activity seen in the LNB Group suggests right-
hemispheric activation, which results in sympathetic
engagement. At the same time, the substantial BP
decreases in the RNB Group might correspond with
left-hemispheric dominance, signifying a more
balanced cardiovascular response. This lateralization
approach corresponds with previous research
highlighting the complex autonomic changes induced
by nostril-specific breathing (Shannahoff-Khalsa,
2007).
4.6 Control of Confounding Factors
While our study demonstrated significant effects of
nostril-specific breathing on autonomic parameters,
we carefully controlled for potential confounding
factors, particularly exercise. The 24-hour restriction
on moderate to vigorous physical activity prior to
testing helped minimize exercise-induced variations
in autonomic function. This control was essential as
exercise can acutely alter HRV parameters, blood
pressure, and sympathetic activity (Shaffer &
Ginsberg, 2017). However, we acknowledge that
variations in participants' regular physical fitness
levels might still influence their autonomic baseline
measures. Future studies could benefit from
stratifying participants based on their regular physical
activity levels or including fitness assessment as a
covariate in the analysis.
4.7 Practical Applications and
Implications for Clinical Practice
The distinct effects of nostril-specific breathing have
practical implications for non-pharmacological
therapies in the control of autonomic and
cardiovascular health. The significant decreases in
blood pressure observed in the RNB Group suggest
that right-nostril breathing might function as a viable
method for persons with hypertension or for those
aiming to reduce blood pressure without medication
(Brown & Gerbarg, 2005).
Conversely, the increased sympathetic tone
associated with left-nostril breathing may have
applications for tasks requiring heightened alertness
and could be useful as a short-term energizing
practice for individuals needing increased focus
(Shaffer & Ginsberg, 2017). These findings extend
the therapeutic use of controlled breathing by
illustrating how nostril-specific techniques can be
tailored to specific autonomic goals, whether for
relaxation, alertness, or blood pressure management.
4.8 Scope and Future Directions
While this study offers insights into nostril-specific
breathing, it is limited by its short-term intervention
duration and the homogeneity of the young, healthy
participant group. Future research should explore
long-term effects of nostril-specific breathing across
diverse populations, and objective neuroimaging
techniques could further clarify the neural basis of
observed autonomic responses.
Additionally, longitudinal studies examining
sustained breathing practices may reveal the
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
790

cumulative effects on HRV, blood pressure, and
overall autonomic balance. Exploring combinations
of nostril-specific breathing with other autonomic
modulation techniques, such as mindfulness or
biofeedback, may provide a more robust framework
for autonomic health interventions.
5 CONCLUSIONS
The study evaluated the impacts of three breathing
interventions—Left Inhale-Left Exhale (LNB), Right
Inhale-Exhale (RNB), and normal breathing—on
cardiovascular and autonomic parameters, with
significant findings mainly cantered around the Stress
Index (SI), Sympathetic Nervous System (SNS)
activity, heart rate variability (HRV) metrics (SDNN
and RMSSD), and systolic and diastolic blood
pressure (BP). These findings highlight the unique
physiological impacts of targeted nostril breathing
techniques, with left nostril breathing linked to
elevated SNS activity and right nostril breathing
showing cardiovascular benefits.
REFERENCES
Brown, R. P., & Gerbarg, P. L. (2005). Sudarshan Kriya
yogic breathing in the treatment of stress, anxiety, and
depression: Part I—neurophysiologic model. Journal of
Alternative and Complementary Medicine, 11(1), 189-
201. https://doi.org/10.1089/acm.2005.11.189
Craig, A. D. (2005). Forebrain emotional asymmetry: A
neuroanatomical basis? Trends in Cognitive Sciences,
9(12), 566-571. https://doi.org/10.1016/j.tics.2005.10.0
05
Critchley, H. D., Rotshtein, P., Nagai, Y., O'Doherty, J., &
Mathias, C. J. (2011). Neural systems supporting
interoceptive awareness. Nature Reviews
Neuroscience, 12(2), 58-67. https://doi.org/10.1038/nr
n2811
Gerritsen, R. J. S., & Band, G. P. H. (2018). Breath of life:
The respiratory vagal stimulation model of
contemplative activity. Frontiers in Human
Neuroscience, 12, 397.
Gerritsen, R. J. S., Lafave, H. L., Bidelman, G. M., & Band,
G. P. H. (2023). Slow breathing modulates cardiac
autonomic responses and behavioral performance via
respiratory-brain coupling mechanisms. Neuroscience
& Biobehavioral Reviews, 146, 105013.
Jerath, R., Edry, J. W., Barnes, V. A., & Jerath, V. (2006).
Physiology of long pranayamic breathing: Neural
respiratory elements may provide a mechanism that
explains how slow deep breathing shifts the autonomic
nervous system. Medical Hypotheses, 67(3), 566-571.
https://doi.org/10.1016/j.mehy.2006.02.042
Kahana-Zweig, R., Geva-Sagiv, M., Weissbrod, A.,
Secundo, L., Soroker, N., & Sobel, N. (2016).
Measuring and characterizing the human nasal cycle.
PLoS One, 11(10), e0162918.
Koenig, J., & Thayer, J. F. (2016). Sex differences in
healthy human heart rate variability: A meta-analysis.
Neuroscience & Biobehavioral Reviews, 64, 288-310.
https://doi.org/10.1016/j.neubiorev.2016.03.007
Laborde, S., Allen, M. S., Borges, U., Hosang, T. J., Furley,
P., Mosley, E., & Dosseville, F. (2022). The influence
of slow-paced breathing on cardiac vagal activity:
Practical implications and protocol variations. Applied
Psychophysiology and Biofeedback, 47(1), 1-11.
Lehrer, P. M., Vaschillo, E., & Vaschillo, B. (2000).
Resonant frequency biofeedback training to increase
cardiac variability: Rationale and manual for training.
Applied Psychophysiology and Biofeedback, 25(3),
177-191. https://doi.org/10.1023/A:1009554825745
Malliani, A., Pagani, M., Lombardi, F., & Cerutti, S.
(1991). Cardiovascular neural regulation explored in
the frequency domain. Circulation, 84(2), 482-492.
https://doi.org/10.1161/01.CIR.84.2.482
Noble, D. J., & Hochman, S. (2019). Hypothesis:
Pulmonary afferent activity patterns during slow, deep
breathing contribute to the neural induction of
physiological relaxation. Frontiers in Physiology, 10,
1176.
Pal, G. K., Velkumary, S., & Madanmohan, M. (2004).
Effect of short-term practice of breathing exercises on
autonomic functions in normal human volunteers.
Indian Journal of Medical Research, 120(2), 115-121.
Russo, M. A., Santarelli, D. M., & O'Rourke, D. (2017).
The physiological effects of slow breathing in the
healthy human. Breathe, 13(4), 298-309.
https://doi.org/10.1183/20734735.009817
Sackett, D. L. (2000). Bias in analytic research. Journal of
Chronic Diseases, 32(1-2), 51-63. https://doi.org/
10.1016/0021-9681(79)90012-2
Schulz, K. F., & Grimes, D. A. (2002). Allocation
concealment in randomized trials: Defending against
deciphering. The Lancet, 359(9306), 614-618.
https://doi.org/10.1016/S0140-6736(02)07750-3
Shaffer, F., & Ginsberg, J. P. (2017). An overview of heart
rate variability metrics and norms. Frontiers in Public
Health, 5, 258. https://doi.org/10.3389/fpubh.2017.0
0258
Shannahoff-Khalsa, D. S. (2007). An introduction to
Kundalini yoga meditation techniques that are specific
for the treatment of psychiatric disorders. The Journal
of Alternative and Complementary Medicine, 13(7),
819-827. https://doi.org/10.1089/acm.2007.6750
Steffen, P. R., Austin, T., DeBarros, A., & Brown, T.
(2023). The Impact of Slow Breathing on Heart Rate
Variability and Self-Regulation: A Review of Recent
Research. Frontiers in Psychology, 14, 1130249.
Task Force of the European Society of Cardiology and the
North American Society of Pacing and
Electrophysiology. (1996). Heart rate variability:
Standards of measurement, physiological
Short-Term Effects of Mindful Uni-Nostril Breathing on Cardio-Autonomic Functions: A Randomized Controlled Trial
791

interpretation, and clinical use. Circulation, 93(5),
1043-1065. https://doi.org/10.1161/01.CIR.93.5.1043
Telles, S., & Desiraju, T. (1993). Autonomic changes in
Brahmakumaris Rajayoga meditation. International
Journal of Psychophysiology, 15(2), 147-152.
https://doi.org/10.1016/0167-8760(93)90040-8
Telles, S., & Naveen, K. V. (2008). Voluntary breath
regulation in yoga: Its relevance and physiological
effects. Biofeedback, 36(3), 70-73.
https://doi.org/10.5298/1081-5937-36.3.70
Telles, S., Singh, N., & Balkrishna, A. (2011). Heart rate
variability changes during high-frequency yoga
breathing and breath awareness. BioPsychoSocial
Medicine, 5(1), 4. https://doi.org/10.1186/1751-0759-
5-4
Thayer, J. F., Åhs, F., Fredrikson, M., Sollers, J. J., &
Wager, T. D. (2012). A meta-analysis of heart rate
variability and neuroimaging studies: Implications for
heart rate variability as a marker of stress and health.
Neuroscience & Biobehavioral Reviews, 36(2), 747-
756. https://doi.org/10.1016/j.neubiorev.2011.11.009
Van Diest, I., Verstappen, K., Aubert, A. E., Widjaja, D.,
Vansteenwegen, D., & Vlemincx, E. (2014).
Inhalation/Exhalation ratio modulates the effect of slow
breathing on heart rate variability and relaxation.
Applied Psychophysiology and Biofeedback, 39(3-4),
171-180.
Zaccaro, A., Piarulli, A., Laurino, M., Garbella, E.,
Menicucci, D., Neri, B., & Gemignani, A. (2018). How
breath-control can change your life: A systematic
review on psycho-physiological correlates of slow
breathing. Frontiers in Human Neuroscience, 12, 353.
Zelano, C., Jiang, H., Zhou, G., Arora, N., Schuele, S.,
Rosenow, J., & Gottfried, J. A. (2016). Nasal
respiration entrains human limbic oscillations and
modulates cognitive function. Journal of Neuroscience,
36(49), 12448-12467.
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
792
