
Predicting Adverse Events in Developmental Disabilities Population
James P. McGlothlin
1
, Micah Price-Offerman
1
, Robbie Beyer
1
, George Casey
1
and John P. Barile
2
1
RSM US LLP, Chicago, IL, U.S.A.
2
University of Hawai‘i at Mānoa, Havaii, U.S.A.
Keywords: Artificial Intelligence, Development Disabilities, Population Health, Analytics, Business Intelligence,
Predictive Analytics, Machine Learning, Supervised Learning.
Abstract: Individuals with development disabilities can experience a variety of adverse events. We have found that
these events are often unreported. In this project, we work with a large government program which assists
such individuals. The goal of the project is to use artificial intelligence (AI) and other modern technologies to
predict adverse events. This will allow case managers to better avoid adverse events, prepare for them and
help the program participants. Our initial results show very good accuracy and precision in identifying risk
and predicting participant adverse events.
1 INTRODUCTION
The Developmental Disabilities Division (DDD)
supports ~3,600 active participants. DDD is the
operating state agency for the Medicaid 1915(c)
Home and Community Based Services (HCBS)
Waiver for Individuals with Intellectual and
Developmental Disabilities. The department’s
mission is to “Foster partnerships and provide quality
person-centered and family-focused services and
supports that promote self-determination.”. The
department’s vision is “Individuals with intellectual
and developmental disabilities have healthy, safe,
meaningful and self-determined lives.”
The goal of this product is to determine which
participants are most at risk for an adverse event. Our
study looks at 10 types of adverse events:
1. Suspected abuse, neglect or financial
exploitation
2. Behavior Change
3. Change in Health Requiring Medical
Treatment
4. Any Use of Restraints
5. Injury from a Known/Unknown Cause
Requiring Medical Treatment
6. Medication Errors and/or Unexpected
Reaction to Medications or Treatment
7. Participant's Whereabouts Unknown
8. Use of Prohibited Intervention
9. Use of Seclusion
10. Death
Our research has three goals. The first goal is to
identify the majority of adverse events. DDD uses a
custom participant records system called Inspire.
Anecdotally, it is expected that less than half of
adverse events are correctly documented in Inspire.
Therefore, we leverage Medicaid claims data to
augment the participant records. As the vast majority
of the participants have coverage through the
government Medicaid program, this method should
allow us to identify any hospital visits, clinical visits
or medication dispenses for the participants.
The second goal is to predict adverse events
before they happen. This is the central exercise of this
paper and experiment.
For the purpose of our data and models, the grain
is one row per participant per month. So if the
participant has been in the program for a year they
will have 12 rows. For each participant and month,
we predict yes there will be an adverse event or no
there will not be. Therefore, while our system does
not attempt to predict exactly when an adverse event
will occur, it does predict it within the calendar
month.
The third and final goal is to utilize these
predictions to take prescriptive action and prevent
adverse events. This will require making suggestions
for interventions and tracking the input of these
interventions on participant outcomes.
844
McGlothlin, J. P., Price-Offerman, M., Beyer, R., Casey, G. and Barile, J. P.
Predicting Adverse Events in Developmental Disabilities Population.
DOI: 10.5220/0013318200003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 2: HEALTHINF, pages 844-849
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

2 ARTIFICIAL INTELLIGENCE
The goal of this product is to predict adverse events
before they occur. To do this, we leverage machine
learning and predictive analytics.
Machine learning (ML) has become a
transformative tool in various sectors, and public
health is no exception. At its core, machine learning
involves algorithms that can learn from data, identify
patterns, and make decisions or predictions without
explicit programming for each task. In public health,
ML is applied to analyze large volumes of data such
as electronic health records, genomic information,
and social determinants of health. This enables the
identification of trends and patterns that may not be
immediately obvious to human researchers. With the
power of ML, public health systems can improve
outcomes through early disease detection, predictive
modeling, and more efficient resource allocation
(Jordan, 2015) (Bi, 2019).
One significant application of machine learning in
public health is disease prediction and prevention.
ML algorithms are capable of processing complex
datasets to predict the likelihood of diseases based on
various risk factors. For example, ML models have
been used to predict the onset of chronic diseases like
diabetes and cardiovascular conditions (Siontis,
2012) (Collins, 2012). By analyzing factors such as
age, lifestyle, genetics, and environmental influences,
ML can forecast the potential for disease in
individuals or populations, allowing for early
interventions. This predictive power is particularly
valuable in resource-limited settings where
preventive measures can save lives and reduce
healthcare costs. Machine learning is making strides
is in epidemiology, especially in tracking and
controlling infectious diseases (Ghosh, 2024)
(Adegoke, 2024). ML algorithms are being used to
analyze patterns in disease spread and to create
models for forecasting outbreaks. During the
COVID-19 pandemic, ML models were widely
employed to predict the spread of the virus, assess
healthcare system burdens, and identify effective
intervention strategies (Van der Schaar, 2021)
(Malik, 2021) (Heidari, 2022). These models relied
on a combination of epidemiological data, mobility
data, and demographic information. In addition, ML
has been applied to track the emergence of antibiotic-
resistant bacteria, thereby enhancing surveillance
efforts and informing public health responses
(Brenda, 2024) .
In addition to modeling, ML is enhancing
personalized medicine and treatment in public health
(Srinivasaiah, 2024). By analyzing vast datasets,
machine learning can tailor healthcare interventions
to individuals based on their unique characteristics.
This is particularly important in managing chronic
diseases, where treatment regimens can vary
significantly from one person to another. For
instance, ML algorithms can help determine the most
effective treatment plans for cancer patients by
analyzing genetic data and patient responses to
previous treatments (Rafique, 2021) (Quazi, 2022)..
This precision medicine approach not only improves
individual outcomes but also reduces the
inefficiencies of one-size-fits-all healthcare
strategies.
Despite its potential, machine learning in public
health comes with challenges. These include data
privacy concerns, ethical issues regarding algorithmic
biases, and the need for sufficient training of
healthcare professionals in data science. Moreover,
the success of ML models in public health is heavily
dependent on the quality of the data used for training
these models. Inaccurate, incomplete, or biased data
can lead to misleading predictions and decisions. As
such, there is an ongoing need for collaboration
between data scientists, healthcare professionals, and
policymakers to ensure that ML applications are
designed, tested, and implemented responsibly.
Supervised learning is a type of machine learning
where the model is trained on labeled data, meaning
each input is paired with the correct output. The goal
is to learn a mapping from inputs to outputs so that,
when presented with new, unseen data, the model can
predict the correct result (Osisanwo, 2017). The
process involves using a dataset with known labels to
train the algorithm, which then fine-tunes itself by
adjusting its internal parameters to minimize errors
between predicted and actual outcomes. This form of
learning is widely used in tasks such as classification
and regression, where the model learns to categorize
data or predict continuous values based on historical
examples (Kotsiantis, 2006).
In healthcare, supervised learning has shown
significant potential in improving diagnostic
accuracy, personalized treatment plans, and
predicting patient outcomes. For instance, machine
learning models can be trained on medical images
like MRIs or X-rays, where the labels correspond to
specific diagnoses, enabling the algorithm to assist
radiologists in detecting diseases such as cancer or
tuberculosis with high accuracy (Sharma, 2025).
Supervised learning is also used in predicting patient
risk factors, such as the likelihood of developing
chronic diseases like diabetes or heart disease, based
on historical health data, lifestyle choices, and genetic
factors (Islam, 2024). This application helps
Predicting Adverse Events in Developmental Disabilities Population
845

healthcare professionals provide more tailored
treatments and preventative measures, thereby
improving patient care and reducing overall
healthcare costs (Razzak, 2018).
Predictive analytics models use statistical
algorithms and machine learning techniques to
analyze historical data and predict future outcomes.
These models are built upon data mining and pattern
recognition principles, helping organizations forecast
trends, behaviors, and events. For example,
regression analysis is often used in predictive
analytics to identify relationships between variables,
while classification algorithms like decision trees or
random forests help categorize data points into
predefined classes. These predictive models are
widely used in various sectors, including finance,
healthcare, marketing, and supply chain management,
offering insights that guide decision-making and
improve operational efficiency (Berrar, 2019).
One of the key advantages of predictive analytics
is its ability to enhance decision-making by offering
actionable insights based on historical data. Machine
learning models, particularly deep learning and neural
networks, allow for complex, nonlinear relationships
within data to be understood, improving the accuracy
of predictions over traditional statistical methods. For
instance, in the healthcare sector, predictive models
can help identify patients at risk of developing
chronic conditions, thereby enabling early
intervention and personalized care plans (Chung et
al., 2018). These capabilities empower businesses to
proactively address issues, reduce costs, and increase
customer satisfaction by anticipating needs and
actions.
However, despite their power, predictive analytics
models also come with challenges. The effectiveness
of these models is highly dependent on the quality and
volume of the data being analyzed. Inaccurate,
incomplete, or biased data can lead to incorrect
predictions, which could be detrimental in areas like
finance or healthcare. Moreover, predictive models
can be resource-intensive, requiring significant
computational power and expertise to develop and
maintain. It is also crucial to continually update the
models with new data to ensure they remain relevant
and accurate. Addressing these challenges requires
robust data governance practices and collaboration
between data scientists and domain experts (Aguirre,
2019).
3 IMPLEMENTATION
APPROACH
This project is intended to be used in a commercial
setting by hospital providers, so that they can comply
with the requirements of patient registries with less
burden to hospital staff. Therefore, we wanted to only
use commercially available and respected software
products which have been approved to handle
protected health information (PHI) under the United
States’s HIPAA (Health Insurance Portability and
Accountability Act of 1996) (Moore, 2019).
Therefore, we chose to implement our project
using software available from Microsoft including
Azure, Azure Machine Learning (AML) (Barga,
2015) (Barnes, 2015) and OpenAI.
Azure Machine Learning is a cloud service from
Microsoft designed to streamline the machine
learning process. It provides a variety of tools for
building, training, and deploying machine learning
models, catering to data scientists, developers, and
organizations. The platform supports integration with
popular frameworks and offers features such as
automated machine learning (AutoML), model
versioning, and deployment in a secure, scalable
environment. Notable capabilities include automated
hyperparameter tuning, experiment tracking, and
seamless integration with Azure’s cloud
infrastructure for efficient model management. Azure
Machine Learning also supports collaborative
development through integrated notebooks and offers
monitoring and management tools post-deployment.
It accommodates both code-based and low-code
development, making it accessible for users with
different skill levels. This flexibility enables
businesses to advance their AI projects while
ensuring governance, security, and scalability in
production environments. (Barnes, 2015).
The department uses two source systems to
manage participant records: Inspire and MedQuest.
Inspire is a custom solution built in Microsoft
Dynamics. Med-Quest is a custom solution built to
manage Medicaid claims.
Our evaluation determined that adverse events
were overwhelmingly under-reported in Inspire.
Augmenting the participate record with
corresponding claims data allows us to identify any
adverse event that resulted in a medical claim. Figure
1 shows the data flow diagram for both the data
abstraction and the machine learning.
HEALTHINF 2025 - 18th International Conference on Health Informatics
846
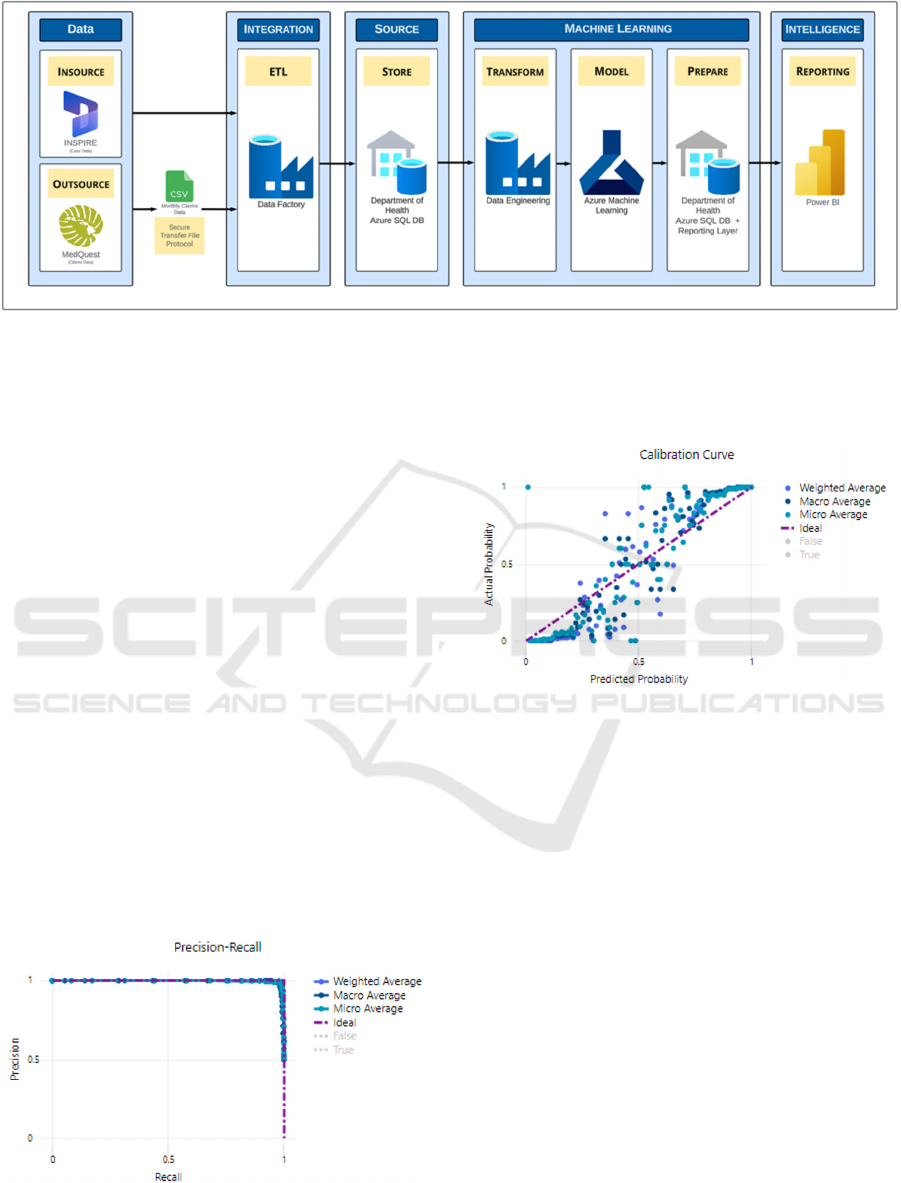
Figure 1: Architectural data flow diagram.
One of the most important factors for successful
model is to determine which features are valuable to
the prediction and have a true causal effect. Data
fields which we evaluated and leveraged include
participant age, race, use of medical devices, care
setting, level of participation in program, history of
trauma, medications and history of schizophrenia,
4 RESULTS
This research is in early stages of development and
test.
Our model made 24,146 predictions. The
algorithm predicted adverse events in 6,181
instances. In 6,156 instances the adverse event
occurred. Therefore, when an adverse event is
predicted, there is a 99.6% chance the adverse event
will occur. There were only 25 false positives,
representing only 0.1% of the preductions. There
were 246 false negatives, where the model failed to
predict a adverse event. This represents 1.0% percent
of predictions. The predictions were correct overall
98.9% of the time.
Figure 2: Precision and Recall.
Figure 2 shows the precision and recall for our model.
Figure 3 the calibiration curve and predicted
probability.
Figure 3: Calibration Curve.
5 NEXT STEPS
Currently, our research predicts the occurrence of any
adverse event in a given month. It would be
preferable if we could predict exactly which adverse
event will occur. For a next step, we will attempt to
model each adverse event separately. This will also
allow for a distinct set of features per event, instead
of forcing a common set of prediction features across
all adverse events.
Additionally, we realize that more model tuning,
training and rigor is needed to show the statistical
significance of our algorithm and process over time.
There is a large amount of free text data collected
for the program participants. As a future long term
step, we would like to edit the records management
software to utilize drop down lists and not allow free
text. In order to get value out of the vast amount of
text data already collected, we plan to use generative
AI to summarize the data.
Predicting Adverse Events in Developmental Disabilities Population
847

As stated in our introduction, the third and final
goal of the project is to utilize these prediction to take
prescriptive action and prevent adverse events. This
will require making suggestions for interventions and
tracking the input of these interventions on participant
outcomes. This is left for future work and has not yet
been attempted. To do this properly, we will need to
provide integrated data into the case management
system enabling our case managers to take
appropriate action.
6 CONCLUSIONS
It is vital that both case workers and researchers know
when participants have adverse events. By
augmenting the participant records with claims data,
we were able to almost double the number of known
adverse events.
The primary purpose of this initiative is to predict
adverse events before they happen. While this is a
preliminary evaluation, our early results show an
exceptional 98.9% accuracy across all predictions.
This shows the promise of AI to help these
participants.
REFERENCES
Aguirre, S., Mahr, D., & Rivas, M. (2019). Data-driven
predictive analytics in business decision-making.
Springer.
Berrar, D. (2019). Predictive modeling applications in
business and industry. Wiley-IEEE Press.
Chung, W. Y., Park, J. H., & Kim, H. (2018). Applications
of predictive analytics in healthcare. Springer.
Mahesh, B. (2020). Machine learning algorithms-a
review. International Journal of Science and Research
(IJSR).[Internet], 9(1), 381-386.
Jordan, M. I., & Mitchell, T. M. (2015). Machine
learning: Trends, perspectives, and prospects.
Science, 349(6245), 255-260.
Bi, Q., Goodman, K. E., Kaminsky, J., & Lessler, J. (2019).
What is machine learning? A primer for the
epidemiologist. American journal of
epidemiology, 188(12), 2222-2239.
Panch, T., Szolovits, P., & Atun, R. (2018). Artificial
intelligence, machine learning and health
systems. Journal of global health, 8(2).
Ravì, D., Wong, C., Deligianni, F., Berthelot, M., Andreu-
Perez, J., Lo, B., & Yang, G. Z. (2016). Deep learning
for health informatics. IEEE journal of biomedical and
health informatics, 21(1), 4-21.
Johnson, A. E., Pollard, T. J., & Mark, R. G. (2017,
November). Reproducibility in critical care: a mortality
prediction case study. In Machine learning for
healthcare conference (pp. 361-376). PMLR.
Shen, D., Wu, G., & Suk, H. I. (2017). Deep learning in
medical image analysis. Annual review of biomedical
engineering, 19(1), 221-248.
Bishop, C. M., & Nasrabadi, N. M. (2006). Pattern
recognition and machine learning (Vol. 4, No. 4, p.
738). New York: Springer.
Kotsiantis, S. B., Zaharakis, I., & Pintelas, P. (2007).
Supervised machine learning: A review of
classification techniques. Emerging artificial
intelligence applications in computer
engineering, 160(1), 3-24.
LeCun, Y., Bengio, Y., & Hinton, G. (2015). Deep learning.
Nature, 521(7553), 436-444.
Moore, W., & Frye, S. (2019). Review of HIPAA, part 1:
history, protected health information, and privacy and
security rules. Journal of nuclear medicine
technology, 47(4), 269-272.
Barga, R., Fontama, V., Tok, W. H., & Cabrera-Cordon, L.
(2015). Predictive analytics with Microsoft Azure
machine learning (pp. 221-241). Berkely, CA: Apress.
Barnes, J. (2015). Azure machine learning. Microsoft
Azure Essentials. 1st ed, Microsoft.
Siontis, G. C., Tzoulaki, I., Siontis, K. C., & Ioannidis, J. P.
(2012). Comparisons of established risk prediction
models for cardiovascular disease: systematic
review. Bmj, 344.
Collins, G. S., & Moons, K. G. (2012). Comparing risk
prediction models. Bmj, 344.
Ghosh, S., & Sharma, V. (2024). Tracking of Disease—A
Review of the State of the Art of Technology for Next
Generation Healthcare. Deep Learning in Internet of
Things for Next Generation Healthcare, 242-268.
Adegoke, B. O., Odugbose, T., & Adeyemi, C. (2024). Data
analytics for predicting disease outbreaks: A review of
models and tools. International journal of life science
research updates [online], 2(2), 1-9.
Van der Schaar, M., Alaa, A. M., Floto, A., Gimson, A.,
Scholtes, S., Wood, A., ... & Ercole, A. (2021). How
artificial intelligence and machine learning can help
healthcare systems respond to COVID-19. Machine
Learning, 110, 1-14.
Malik, Y. S., Sircar, S., Bhat, S., Ansari, M. I., Pande, T.,
Kumar, P., ... & Dhama, K. (2021). How artificial
intelligence may help the Covid‐19 pandemic: Pitfalls
and lessons for the future. Reviews in medical
virology, 31(5), 1-11.
Heidari, A., Jafari Navimipour, N., Unal, M., & Toumaj, S.
(2022). Machine learning applications for COVID-19
outbreak management. Neural Computing and
Applications, 34(18), 15313-15348.
Branda, F., & Scarpa, F. (2024). Implications of Artificial
Intelligence in Addressing Antimicrobial Resistance:
Innovations, Global Challenges, and Healthcare’s
Future. Antibiotics, 13(6), 502.
Rodríguez-González, A., Zanin, M., & Menasalvas-Ruiz,
E. (2019). Public health and epidemiology informatics:
can artificial intelligence help future global challenges?
An overview of antimicrobial resistance and impact of
HEALTHINF 2025 - 18th International Conference on Health Informatics
848

climate change in disease epidemiology. Yearbook of
medical informatics, 28(01), 224-231.
Srinivasaiah, B. (2024). The Power of Personalized
Healthcare: Harnessing the Potential of Machine
Learning in Precision Medicine. International Journal
of Science and Research, 426-429.
Rafique, R., Islam, S. R., & Kazi, J. U. (2021). Machine
learning in the prediction of cancer
therapy. Computational and Structural Biotechnology
Journal, 19, 4003-4017.
Quazi, S. (2022). Artificial intelligence and machine
learning in precision and genomic medicine. Medical
Oncology, 39(8), 120.
Osisanwo, F. Y., Akinsola, J. E. T., Awodele, O.,
Hinmikaiye, J. O., Olakanmi, O., & Akinjobi, J. (2017).
Supervised machine learning algorithms: classification
and comparison. International Journal of Computer
Trends and Technology (IJCTT), 48(3), 128-138.
Kotsiantis, S. B., Zaharakis, I. D., & Pintelas, P. E. (2006).
Machine learning: a review of classification and
combining techniques. Artificial Intelligence
Review, 26, 159-190.
Sharma, R., Kumar, N., & Sharma, V. (2025). Computer
Vision for Disease Detection—An Overview of How
Computer Vision Techniques Can Be Used to Detect
Diseases in Medical Images, Such as X‐Rays and
MRIs. AI in Disease Detection: Advancements and
Applications, 77-98.
Islam, R., Sultana, A., & Islam, M. R. (2024). A
comprehensive review for chronic disease prediction
using machine learning algorithms. Journal of Electrical
Systems and Information Technology, 11(1), 27.
Predicting Adverse Events in Developmental Disabilities Population
849
