
Investigation of the Relational Strength Between Suspected Atrial
Fibrillation Triggers and Detector-Based Arrhythmia Episode
Occurrence
Vilma Plu
ˇ
s
ˇ
ciauskait
˙
e
1 a
and Andrius Petr
˙
enas
1,2 b
1
Biomedical Engineering Institute, Kaunas University of Technology, Kaunas, Lithuania
2
Faculty of Electrical and Electronics Engineering, Kaunas University of Technology, Kaunas, Lithuania
{vilma.plusciauskaite, andrius.petrenas}@ktu.lt
Keywords:
Wearables, Remote Monitoring, Relation Assessment, Electrocardiogram, Photoplethysmogram, Arrhythmia
Detection, Personalized Arrhythmia Management.
Abstract:
Atrial fibrillation (AF) treatment remains challenging, with current options often limited to anticoagulants
and antiarrhythmic medications. Growing evidence suggests that acute exposures, referred to as AF triggers,
can initiate AF in some patients. Therefore, identifying and managing personal triggers may serve as an
effective strategy to complement conventional treatment. This study explores the utility of wearable-based
biosignals to assess the relational strength between the suspected triggers and AF occurrence when episodes
are detected using electrocardiogram (ECG) and photoplethysmogram (PPG). Biosignals from 33 patients
with paroxysmal AF (mean age 61 ± 13 years), who wore an ECG patch and a wrist-worn PPG device
during a 7.0 ± 0.7 day observation period, were used in the study. Suspected triggers due to physical exertion,
psychophysiological stress, and lying on the left side were identified based on a detection parameter calculated
over successive segments of the ECG and/or acceleration signals. The relational strength between a suspected
trigger and AF episodes is quantified based on AF burden, defined as the ratio of time spent in AF to the total
analysis time interval, assuming that the post-trigger AF burden is greater than the pre-trigger AF burden. The
results indicate that the relational strength between suspected triggers and AF episode occurrence, as detected
using ECG- and PPG-based AF detectors, differs from manual annotation by an average of 0.03±0.15 and
-0.21±0.21, respectively. This study demonstrates the potential of wearable-based biosignals in providing
personalized identification of suspected AF triggers. However, challenges such as non-wear periods and poor
PPG signal quality remain to be addressed for practical applications.
1 INTRODUCTION
The management of atrial fibrillation (AF) remains
a complex challenge (Lippi et al., 2021), with treat-
ment options often limited to anticoagulants and an-
tiarrhythmic medications, carrying serious side ef-
fects (Mani and Lindhoff-Last, 2014). Since acute ex-
posures, referred to as AF triggers, may initiate AF in
some patients (Hansson et al., 2004; Groh et al., 2019;
Severino et al., 2019), an effective approach could
involve addressing lifestyle and risk factors together
with conventional treatment (Chung et al., 2020).
Identifying triggers on a patient-specific basis
could become a key component of personalized AF
management, allowing clinicians to address the root
a
https://orcid.org/0000-0002-2949-912X
b
https://orcid.org/0000-0002-5700-7196
causes of AF episodes in individual cases. Mean-
while, patients could take an active role in managing
their AF through targeted lifestyle adjustments.
Among suspected AF triggers (Hansson et al.,
2004; Groh et al., 2019), alcohol has been the most
extensively studied, with consistent evidence link-
ing to the onset of AF episodes (Marcus et al.,
2022). Other triggers associated with the occurrence
of AF episodes include physical exertion (Abdulla
and Nielsen, 2009; Guasch and Mont, 2017), lying on
the left side (Gottlieb et al., 2021), and psychophysi-
ological stress (Leo et al., 2023).
A major limitation of previous studies is their re-
liance on self-reported AF triggers, typically gathered
through questionnaires (Hansson et al., 2004; Groh
et al., 2019; Marcus et al., 2022), which are suscep-
tible to bias. For instance, patients often identified
multiple triggers (Groh et al., 2019), suggesting con-
Pluš
ˇ
ciauskait
˙
e, V. and Petr
˙
enas, A.
Investigation of the Relational Strength Between Suspected Atrial Fibrillation Triggers and Detector-Based Arrhythmia Episode Occurrence.
DOI: 10.5220/0013343000003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 805-810
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
805

firmation bias, while others may have been reluctant
to disclose certain triggers, such as those related to
unhealthy behaviors.
With advancements in technology, wearable de-
vices are now equipped with biosensors capable of
acquiring various biosignals, potentially enabling the
detection of AF triggers and self-terminating AF
episodes using the same device. In our previous work,
we introduced and examined an approach to quantify-
ing the relation between suspected triggers detected
in long-term biosignals and the occurrence of AF
episodes (Plu
ˇ
s
ˇ
ciauskait
˙
e et al., 2024a; Plu
ˇ
s
ˇ
ciauskait
˙
e
et al., 2024b). The previous study demonstrated the
potential of the proposed approach using clinician-
annotated AF episodes. However, applying this in
a clinical practice is particularly challenging, as an-
notating long-term signals is a time-consuming pro-
cess that requires enormous effort from cardiologists.
Therefore, the present study focuses on the utility
of wearable devices to assess the relational strength
when AF episodes are automatically detected using
electrocardiogram (ECG) and photoplethysmogram
(PPG) biosignals.
2 METHODS
2.1 Database
One hundred eighty-two patients diagnosed with
paroxysmal AF were recruited from the Cardiol-
ogy Department at Vilnius University Hospital San-
taros Klinikos. Only those who experienced at
least one AF episode during the observation period
(7.0 ± 0.7 days) were included, resulting in a sub-
set of 33 patients (19 women) with a mean age of
61 ± 13 years. The study was approved by the Vil-
nius Regional Bioethics Committee (reference num-
ber 158200-18/7-1052-557). All patients provided
written informed consent before participation in the
study and were fully informed of the research objec-
tives and procedures.
The database includes biosignals acquired dur-
ing daily activities using a Bittium OmegaSnap™
one-channel ECG patch (Bittium, Finland) and a
wrist-worn device developed at the Biomedical En-
gineering Institute of Kaunas University of Technol-
ogy (Bacevi
ˇ
cius et al., 2022). The ECG patch, po-
sitioned directly on the sternum, acquired continuous
ECG at 500 Hz and tri-axial acceleration signals at
25 Hz, while the wrist-worn device acquired contin-
uous PPG at 100 Hz. AF episodes were annotated
by cardiology residents who reviewed the long-term
ECGs and consulted an experienced cardiologist in
uncertain cases. The biosignal database can be ac-
cessed on Zenodo (Bacevi
ˇ
cius et al., 2024).
2.2 Detection of AF Episodes
Two detectors are used to detect AF episodes: one
based on the analysis of the ECG (Petr
˙
enas et al.,
2015) and the other on the PPG (Solo
ˇ
senko et al.,
2019). Both detectors rely on the irregularity of beat-
to-beat intervals and elevated heart rate during AF.
Additionally, the detectors include blocks for ectopic
beat removal and suppression of bigeminy and sinus
arrhythmia to reduce the number of false positives.
The PPG-based AF detector also incorporates sig-
nal quality assessment to ensure that detection is not
performed on low-quality pulses, whereas the ECG-
based detector does not include signal quality assess-
ment. Both detectors are configured to detect AF
episodes as short as 60 beats.
2.3 Detection of Suspected Triggers
The detection of suspected triggers in biosignals is
explained in detail in (Plu
ˇ
s
ˇ
ciauskait
˙
e et al., 2024b).
Each type of suspected trigger, ie., physical exertion,
psychophysiological stress, and lying on the left side,
is detected based on a detection parameter calculated
over successive segments of the ECG and/or acceler-
ation signals, producing a time series that undergoes
threshold-based detection.
Poor-quality ECG segments were excluded based
on the bsqi index, which assesses the agreement of
two QRS detectors (Behar et al., 2013), with an agree-
ment threshold of 90%. Only segments free of prema-
ture atrial contractions, atrial flutter, and atrial tachy-
cardia were considered for analysis.
Physical exertion is detected using the metabolic
equivalent of task (MET), a measure of energy ex-
penditure relative to the resting metabolic rate. The
METs are estimated from acceleration and heart
rate, accounting for patient-specific variability using
a regression equation derived in (Moeyersons et al.,
2019). A suspected trigger due to physical exertion is
detected if the mean MET, computed over 1-minute
intervals with non-AF rhythm, exceeds 5 METs.
Psychophysiological stress is detected when heart
rate suddenly increases by more than 15 beats per
minute within a 1-minute interval, excluding eleva-
tions due to physical activity or arrhythmia (Brouwer
and Hogervorst, 2014). Physical activity is consid-
ered absent or negligible if the average mean absolute
deviation of the tri-axial raw acceleration signal in the
5-minute segment before and during the analyzed 1-
minute interval is below 22.5 mg (milligravity), indi-
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
806

cating sedentary behavior such as sitting or standing
still (V
¨
ah
¨
a-Ypy
¨
a et al., 2018).
Lying on the left side is detected when the medio-
lateral axis acceleration signal stays below −600 mg
for at least 1 hour. Since position changes may oc-
cur frequently during the night, only the first detected
trigger within the preceding 4 hours is considered.
2.4 Quantification of the Relational
Strength
The primary assumption for identifying a trigger is
that the actual trigger for a particular patient will in-
crease the AF burden B, defined as the ratio of time
the patient spends in AF relative to the total observa-
tion period.
The relational strength between suspected trig-
gers and AF episode occurrence in individual pa-
tients is assessed based on a cumulative princi-
ple (Plu
ˇ
s
ˇ
ciauskait
˙
e et al., 2024a), summing the events
where the post-trigger AF burden B
1,n
is greater than
the pre-trigger AF burden B
0,n
. The reason for invok-
ing the cumulative principle is based on the fact that it
is unlikely a trigger will consistently influence B, even
if a patient is prone to that particular trigger. Instead,
triggers are more likely to contribute to the sporadic
initiation of AF due to their interaction with the ar-
rhythmogenic substrate and modulating factors (Vin-
centi et al., 2006; Nattel and Dobrev, 2016; Severino
et al., 2019). The relational strength γ is quantified as
follows (Plu
ˇ
s
ˇ
ciauskait
˙
e et al., 2024a):
γ =
N
t
∑
n=1
B
1,n
1 + B
0,n
H(B
1,n
− B
0,n
), (1)
where N
t
represents the number of suspected triggers
during the observation period, n is the number of de-
tected suspected triggers, and H(·) is the Heaviside
step function. The analysis time interval T , used to
compute B
1,n
and B
0,n
, is set to 4 hours (Marcus et al.,
2021). The relational strength computed for anno-
tated and detector-based AF episode occurrences is
denoted as γ
a
and γ
d
, respectively.
3 RESULTS
Fig. 1 illustrates the annotated occurrence of episodes
in a patient with paroxysmal AF, along with episodes
detected using ECG- and PPG-based AF detectors,
and their effect on the relational strength. For this par-
ticular example, lying on the left side is only shown,
which was detected four times over a 7-day observa-
tion period. For an annotated AF episode occurrence,
the relational strength γ is 2.21, suggesting a strong
relation between the suspected trigger and increased
post-trigger B. Short-duration false positives from
the ECG-based AF detector increase the pre-trigger
AF burden B
0
, causing a slight decrease in the re-
lational strength (γ = 2.17). Conversely, poor PPG
signal quality and non-wear time of a wrist-worn de-
vice affect both the pre-trigger B
0
and post-trigger B
1
burdens, leading to a substantial reduction in the rela-
tional strength (γ = 1.08).
To provide further insight into the impact of AF
episode detection errors on the relational strength,
Fig. 2 shows how changes in B affect γ. The results in-
dicate that an increase in B, caused by falsely detected
AF episodes from the ECG-based detector, tends to
increase γ. This tendency is observed across all types
of suspected triggers. Conversely, due to non-wear
time, PPG-based detection almost always results in a
lower B, which leads to a decrease in γ.
Figure 3 illustrates the error in the relational
strength computed for detector-based and annotated
AF episodes. The results show that PPG-based
AF detection results in a markedly underestimated γ
(mean = -0.21), while the error for ECG-based AF
detection is low (mean = -0.03).
4 DISCUSSION
Considering the practical applicability of the pro-
posed approach for detecting AF triggers in biosig-
nals, it is unrealistic to rely on manually annotated
occurrences of AF episodes for every patient; thus,
automated AF detection becomes essential. To ad-
dress this, we examined both a chest ECG patch and
a wrist-worn device capable of acquiring PPG sig-
nals. Since reliable detection of AF episodes re-
quires long-term monitoring and robust AF detec-
tors (Butkuvien
˙
e et al., 2024), understanding how de-
tection errors affect episode occurrence – and, in turn,
relational strength – is a crucial area of investigation.
The main finding of the study is that misdetec-
tions and non-wear periods considerably reduce the
relational strength between suspected triggers and AF
episode occurrence. However, the limitations of AF
detectors do not render the proposed method imprac-
tical, due to the cumulative principle used in calcu-
lating relational strength. This principle ensures that
the relational strength increases as the number of sus-
pected triggers grows, indicating that a longer obser-
vation period is needed to achieve the same effect on
γ. While clinical studies are still needed to determine
how γ should be interpreted, we suggest using γ val-
ues greater than 1.5 as a starting point for considering
Investigation of the Relational Strength Between Suspected Atrial Fibrillation Triggers and Detector-Based Arrhythmia Episode Occurrence
807
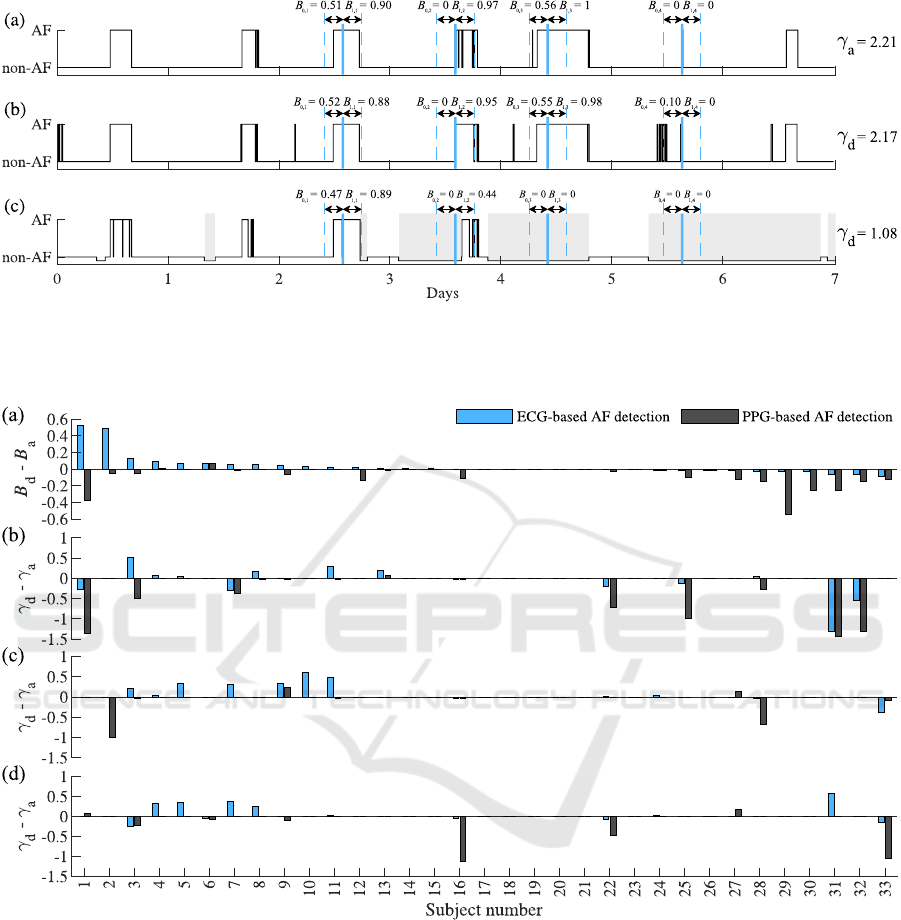
Figure 1: Illustration of the relational strength γ for AF episodes identified by (a) annotation, (b) ECG-based detection, and (c)
PPG-based detection. The onsets of a suspected trigger, attributed to lying on the left side, are shown in blue. Grey rectangles
indicate periods of non-wear or poor PPG quality. Note that the ECG-based AF detector prioritizes sensitivity, resulting in
more false positives, while the PPG-based detector emphasizes specificity, leading to missed or truncated episodes.
Figure 2: (a) The difference in AF burden B between annotated episodes and those detected using ECG- and PPG-based AF
detection, along with its impact on the difference in relational strength γ for suspected triggers of (b) physical exertion, (c)
psychophysiological stress, and (d) lying on the left side. Subjects are arranged in descending order based on the difference
in B observed with ECG-based detection.
a suspected trigger as an actual trigger (Plu
ˇ
s
ˇ
ciauskait
˙
e
et al., 2024a). A γ value above 1.5 can be indicative
of a strong relation, as achieving such a value requires
at least two suspected triggers for which B
1,n
is much
greater than B
0,n
.
Understanding how triggers affect AF episodes
in individual patients remains an unresolved ques-
tion. In this study, we focused on three suspected
triggers based on their relations with AF occurrence
and their feasibility for detection in biosignals. High-
intensity exercise is a known trigger for AF episodes,
both in athletes and the general population (Sham-
loo et al., 2018). Psychophysiological stress releases
stress hormones, elevating heart rate and cardiac con-
tractions, which may also trigger AF (Leo et al.,
2023). Meanwhile, the left lying position is com-
monly self-reported as an AF trigger (Groh et al.,
2019), likely due to increased pressure on the atrial
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
808

Figure 3: The error in relational strength computed for
detector-based and annotated AF episodes across different
types of suspected triggers.
walls and pulmonary veins (Gottlieb et al., 2021; Got-
tlieb et al., 2023). In our previous work, we also in-
vestigated sleep disturbances as a suspected trigger,
quantified by the standard deviation of beat-to-beat
intervals, which serves as an indicator of the domi-
nant sympathetic and vagal activity components (Ma-
lik and Camm, 2004). However, we did not include
sleep disorders in this study due to the lack of differ-
ence when compared to the control γ.
The study has several limitations. Within the ini-
tial cohort of patients diagnosed with paroxysmal AF,
only 18% experienced at least one AF episode dur-
ing the one-week observation period. Consequently,
the findings, based on this relatively small dataset,
should be interpreted with caution. Another limita-
tion lies in the lack of a reference for triggers, leav-
ing it unclear whether the detected suspected triggers
were actual triggers. While lying on the left side can
be reliably detected when a chest sensor is used, vali-
dating physical exertion or psychophysiological stress
is more challenging. These triggers are mostly sub-
jective and may not consistently align with the effects
observed in biosignals.
5 CONCLUSIONS
This paper explores the potential of long-term ECG-
and PPG-based monitoring to identify suspected trig-
gers of AF episodes in patients with paroxysmal AF.
The relational strength between suspected triggers
and AF episode occurrences, as detected by ECG- and
PPG-based AF detectors, differs from manual anno-
tation by an average of 0.03 and -0.21, respectively.
While wearable biosignals show promise for person-
alized identification of suspected AF triggers, chal-
lenges such as non-wear periods and poor PPG signal
quality must be addressed to enable clinical applica-
tion.
ACKNOWLEDGEMENTS
This work was supported by the Research Council of
Lithuania under Agreement No. S-MIP-24-73.
REFERENCES
Abdulla, J. and Nielsen, J. R. (2009). Is the risk of atrial
fibrillation higher in athletes than in the general pop-
ulation? A systematic review and meta-analysis. Eu-
ropace, 11(9):1156–1159.
Bacevi
ˇ
cius, J., Abramikas,
ˇ
Z., Dvinelis, E., Audzijonien
˙
e,
D., Petrylait
˙
e, M., Marinskien
˙
e, J., Staigyt
˙
e, J.,
Karu
ˇ
zas, A., Juknevi
ˇ
cius, V., Jakait
˙
e, R., Basyt
˙
e-
Bacevi
ˇ
c
˙
e, V., Bilei
ˇ
sien
˙
e, N., Solo
ˇ
senko, A., Sokas, D.,
Petr
˙
enas, A., Butkuvien
˙
e, M., Paliakait
˙
e, B., Daukan-
tas, S., Rapalis, A., Marinskas, G., Jasi
¯
unas, E.,
Darma, A., Marozas, V., and Aidietis, A. (2022). High
specificity wearable device with photoplethysmogra-
phy and six-lead electrocardiography for atrial fib-
rillation detection challenged by frequent premature
contractions: DoubleCheck-AF. Front. Cardiovasc.
Med., 9.
Bacevi
ˇ
cius, J., Plu
ˇ
s
ˇ
ciauskait
˙
e, V., Abramikas,
ˇ
Z., Badaras,
I., Butkuvien
˙
e, M., Daukantas, S., Dvinelis, E., Gu-
dauskas, M., Jukna, E., Kiseli
¯
ut
˙
e, M., Kundelis, R.,
Marinskien
˙
e, J., Paliakait
˙
e, B., Petr
˙
enas, A., Petry-
lait
˙
e, M., Pilkien
˙
e, A., Pudinskait
˙
e, G., Radavi
ˇ
cius,
V., Rapalis, A., Sokas, D., Solo
ˇ
senko, A., Staigyt
˙
e, J.,
Stankevi
ˇ
ci
¯
ut
˙
e, G., Taparauskait
˙
e, N., Zarembait
˙
e, G.,
Aidietis, A., and Marozas, V. (2024). Long-term elec-
trocardiogram and wrist-based photoplethysmogram
recordings with annotated atrial fibrillation episodes.
Dataset on Zenodo.
Behar, J., Oster, J., Li, Q., and Clifford, G. D. (2013). ECG
signal quality during arrhythmia and its application
to false alarm reduction. IEEE Trans. Biomed. Eng.,
60(6):1660–1666.
Brouwer, A.-M. and Hogervorst, M. A. (2014). A new
paradigm to induce mental stress: the Sing-a-Song
Stress Test (SSST). Front. Neurosci., 8:224.
Investigation of the Relational Strength Between Suspected Atrial Fibrillation Triggers and Detector-Based Arrhythmia Episode Occurrence
809

Butkuvien
˙
e, M., Petr
˙
enas, A., Martin-Yebra, A., Marozas,
V., and S
¨
ornmo, L. (2024). Characterization of atrial
fibrillation episode patterns: A comparative study.
IEEE Trans. Biomed. Eng., 71(1):106–113.
Chung, M. K., Eckhardt, L. L., Chen, L. Y., Ahmed, H. M.,
Gopinathannair, R., Joglar, J. A., Noseworthy, P. A.,
Pack, Q. R., Sanders, P., Trulock, K. M., et al. (2020).
Lifestyle and risk factor modification for reduction
of atrial fibrillation: A scientific statement from the
American Heart Association. Circ., 141(16):e750–
e772.
Gottlieb, L. A., Coronel, R., and Dekker, L. R. (2023). Re-
duction in atrial and pulmonary vein stretch as a thera-
peutic target for prevention of atrial fibrillation. Heart
Rhythm, 20(2):291–298.
Gottlieb, L. A. et al. (2021). Self-reported onset of parox-
ysmal atrial fibrillation is related to sleeping body po-
sition. Front. Physiol., 12:708650.
Groh, C. A., Faulkner, M., Getabecha, S., Taffe, V., Nah,
G., Sigona, K., McCall, D., Hills, M. T., Sciarappa,
K., Pletcher, M. J., et al. (2019). Patient-reported trig-
gers of paroxysmal atrial fibrillation. Heart Rhythm,
16(7):996–1002.
Guasch, E. and Mont, L. (2017). Diagnosis, pathophysi-
ology, and management of exercise-induced arrhyth-
mias. Nat. Rev. Cardiol., 14(2):88–101.
Hansson, A., Madsen-H
¨
ardig, B., and B. Olsson, S. (2004).
Arrhythmia-provoking factors and symptoms at the
onset of paroxysmal atrial fibrillation: a study based
on interviews with 100 patients seeking hospital as-
sistance. BMC Cardiovasc. Disord., 4(1):1–9.
Leo, D. G., Ozdemir, H., Lane, D. A., Lip, G. Y., Keller,
S. S., and Proietti, R. (2023). At the heart of the
matter: How mental stress and negative emotions
affect atrial fibrillation. Front. Cardiovasc. Med.,
10:1171647.
Lippi, G., Sanchis-Gomar, F., and Cervellin, G. (2021).
Global epidemiology of atrial fibrillation: an increas-
ing epidemic and public health challenge. Int. J.
Stroke, 16(2):217–221.
Malik, M. and Camm, A. J. (2004). Autonomic Balance,
chapter 6, pages 48–56. John Wiley & Sons, Ltd.
Mani, H. and Lindhoff-Last, E. (2014). New oral anticoag-
ulants in patients with nonvalvular atrial fibrillation:
a review of pharmacokinetics, safety, efficacy, quality
of life, and cost effectiveness. Drug Des. Devel. Ther.,
pages 789–798.
Marcus, G. M., Modrow, M. F., Schmid, C. H., Sigona, K.,
Nah, G., Yang, J., Chu, T.-C., Joyce, S., Gettabecha,
S., Ogomori, K., et al. (2022). Individualized stud-
ies of triggers of paroxysmal atrial fibrillation: the I-
STOP-AFib randomized clinical trial. JAMA Cardiol.,
7(2):167–174.
Marcus, G. M., Vittinghoff, E., Whitman, I. R., Joyce, S.,
Yang, V., Nah, G., Gerstenfeld, E. P., Moss, J. D., Lee,
R. J., Lee, B. K., et al. (2021). Acute consumption
of alcohol and discrete atrial fibrillation events. Ann.
Intern. Med., 174(11):1503–1509.
Moeyersons, J., Amoni, M., Van Huffel, S., Willems, R.,
and Varon, C. (2019). R-DECO: An open-source Mat-
lab based graphical user interface for the detection and
correction of R-peaks. Peerj Comput. Sci., 5:e226.
Nattel, S. and Dobrev, D. (2016). Electrophysiological and
molecular mechanisms of paroxysmal atrial fibrilla-
tion. Nat. Rev. Cardiol., 13(10):575–590.
Petr
˙
enas, A., Marozas, V., and S
¨
ornmo, L. (2015). Low-
complexity detection of atrial fibrillation in contin-
uous long-term monitoring. Comput. Biol. Med.,
65:184–191.
Plu
ˇ
s
ˇ
ciauskait
˙
e, V., Butkuvien
˙
e, M., Rapalis, A., Marozas,
V., S
¨
ornmo, L., and Petr
˙
enas, A. (2024a). An ob-
jective approach to identifying individual atrial fibril-
lation triggers: A simulation study. Biomed. Signal
Process. Control, 87:105369.
Plu
ˇ
s
ˇ
ciauskait
˙
e, V., Solo
ˇ
senko, A., Jan
ˇ
ciulevi
ˇ
ci
¯
ut
˙
e, K.,
Marozas, V., S
¨
ornmo, L., and Petr
˙
enas, A. (2024b).
Assessment of the relational strength between triggers
detected in physiological signals and the occurrence
of atrial fibrillation episodes. Physiological Measure-
ment, 45(9):095011.
Severino, P., Mariani, M. V., Maraone, A., Piro, A., Cec-
cacci, A., Tarsitani, L., Maestrini, V., Mancone, M.,
Lavalle, C., Pasquini, M., et al. (2019). Triggers for
atrial fibrillation: The role of anxiety. Cardiol. Res.
Pract., 2019.
Shamloo, A. S., Arya, A., Dagres, N., and Hindricks, G.
(2018). Exercise and atrial fibrillation: some good
news and some bad news. Galen Med. J., 7:e1401.
Solo
ˇ
senko, A., Petr
˙
enas, A., Paliakait
˙
e, B., S
¨
ornmo, L., and
Marozas, V. (2019). Detection of atrial fibrillation us-
ing a wrist-worn device. Physiol. Meas., 40:025003.
V
¨
ah
¨
a-Ypy
¨
a, H., Husu, P., Suni, J., Vasankari, T., and
Siev
¨
anen, H. (2018). Reliable recognition of lying,
sitting, and standing with a hip-worn accelerometer.
Scand. J. Med. Sci. Sports, 28(3):1092–1102.
Vincenti, A., Brambilla, R., Fumagalli, M. G., Merola, R.,
and Pedretti, S. (2006). Onset mechanism of parox-
ysmal atrial fibrillation detected by ambulatory Holter
monitoring. EP Europace, 8(3):204–210.
BIOSIGNALS 2025 - 18th International Conference on Bio-inspired Systems and Signal Processing
810
