
Preliminary Usability Evaluation of a Virtual Reality (VR)
Application for Quitting Nicotine Vaping
Bethany K. Bracken
1
, Phillip C. Desrochers
1
, Ian McAbee
1
, Nicolette M. McGeorge
1
, Susan Latiff
1
,
Bradly T. Stone
1
, Dan T. Duggan
1
, Corinne Cather
2
and A. Eden Evins
2
1
Charles River Analytics, Inc., Cambridge, MA, U.S.A.
2
Center for Addiction Medicine, Massachusetts General Hospital, Boston, MA, U.S.A.
Keywords: Virtual Reality (VR), Cognitive Behavioral Therapy (CBT), Vaping Cessation, Usability Study, Human
Factors, Patient Safety, Digital Therapeutics (DTx).
Abstract: Nicotine vaping is a global problem. Limited vaping cessation interventions are available; and current
treatments have limited accessibility due to systemic barriers to care (e.g., scarcity of treaters). Digital
therapeutics (DTx) can reduce these barriers. We have embedded standard cognitive behavioral therapy
(CBT) content into virtual reality (VR) to create a VR-based app focused on vaping cessation: Novel, On-
demand VR for Accessible, Practical, and Engaging therapy (NO VAPE). NO VAPE allows users to practice
CBT skills gained in traditional therapy through an accessible, immersive, and engaging platform. Our
ultimate goal is to conduct a full clinical trial to test whether NO VAPE motivates greater intervention
adherence and satisfaction. To prepare, we conducted a usability study with N = 6 young adults who currently
vape, aiming to evaluate safety, usability, and overall enjoyment of NO VAPE. We categorized errors into
categories in ascending severity from minor usability errors to safety violations. There were no safety
violations by any participants providing evidence that the app is low-risk and safe (from a software use
perspective, not a substance use perspective). Participant reported high levels of enjoyment, said they would
like to use NO VAPE again, and did not experience symptoms of simulator sickness. We also identified
multiple software bugs we are now addressing.
1 INTRODUCTION
Vaping is an increasing problem around the world. In
2017-2018, the prevalence of vaping was 2.4% across
Europe. The highest prevalences were 7.2% in
England, 4.3% in France, and 4.1% in Greece (Gallus
et al., 2023). In 2019 in Asia, the highest prevalence
was 32.2% in Indonesia (Ko et al., 2024). In 2022, in
the US, an estimated 2.5M youths reported vaping.
Vaping is increasingly being used to assist in smoking
cessation (McNeill et al., 2021); however, vaping—
although likely less harmful than smoking (Abafalvi
et al., 2019; Levy et al., 2021)—is associated with
multiple adverse reactions, such as oral health
problems, cardiac disorders, lung injury, respiratory
disorders, and gastrointestinal disorders (Hammond,
2019; Irusa et al., 2020; McNeill et al., 2021;
Traboulsi et al., 2020). To help individuals attempt to
quit or maintain abstinence from vaping, in addition
to drug therapy, multiple psychological therapies
exist. Cognitive behavioral therapy (CBT), teaches
individuals to recognize the events that trigger
craving; their mental, physical, and behavioral
reactions to the events; and provides training on
strategies to resist cues and handle stressful situations
without vaping.
Traditional vaping cessation interventions have
limited accessibility due to systemic barriers to care,
including scarcity of treaters and personal factors
(e.g., lack of transportation). There are several
potential routes to maximize reach and efficacy of
current therapies. Growing evidence suggests that
digital and technology-based therapies improve
various mental health and substance use disorder
outcomes, as a standalone treatment or augmentation
strategy for in-person treatment (Graham et al., 2021,
2024). VR technology in particular may allow
individuals to practice CBT techniques to cope with
symptoms (e.g., cravings) and situations (e.g., stress
at work) in an immersive environment that may be
more evocative and effective than standard CBT for a
wide range of mental health and substance use
882
Bracken, B. K., Desrochers, P. C., McAbee, I., McGeorge, N. M., Latiff, S., Stone, B. T., Duggan, D. T., Cather, C. and Evins, A. E.
Preliminary Usability Evaluation of a Virtual Reality (VR) Application for Quitting Nicotine Vaping.
DOI: 10.5220/0013350000003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 2: HEALTHINF, pages 882-889
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

disorders (Gao et al., 2013). VR-delivered substance
use disorder treatments have been developed to
address multiple substances (e.g., nicotine, alcohol,
and marijuana (Loria, 2016)). People who
successfully develop relapse-avoidance strategies in
VR do translate those strategies to the real world
(Bellum, 2014). Presentation of smoking cues in VR
(García-Rodríguez et al., 2012) or smoking a virtual
cigarette (García-Rodríguez et al., 2013) increases
craving and heart rate similar to real-world stimuli
(Ferrer-Garcia et al., 2012). The use of VR may also
make interventions more engaging (Gao et al., 2013);
participants in one study reported enjoying a VR
game designed for quitting vaping and said they
would recommend it to friends (Weser & Hieftje,
2020), demonstrating its feasibility, satisfaction, and
salience. Technological interventions to aid quitting
can embed therapeutic content into VR (Lee et al.,
2004; Lee et al., 2003), thereby allowing participants
to practice resisting cues to use substances in a safe
environment (Metcalf et al., 2018).
Even though this research is encouraging,
currently, no engaging, immersive intervention exists
for vaping cessation therapy, and there are no
published controlled clinical trials to our knowledge
testing the efficacy of embedding CBT content into
VR to increase success at achieving vaping
abstinence. To address this need, we built Novel, On-
demand VR for Accessible, Practical, and Engaging
therapy (NO VAPE). In the next Phase of the project
we will conduct a pilot controlled clinical trial of its
efficacy for vaping abstinence outcomes. The project
presented here is thus a usability study, NOT an
efficacy study or clinical trial. In preparation for this
clinical trial, we conducted a preliminary human
factors usability study to assess its ease of use,
engagement, and safety, including not inducing
simulator sickness (FDA, 2016). The usability
study’s goals were to:
1 Determine if there are usability issues that could
increase risks to users (e.g., walking into a wall)
to unacceptable levels, and test whether the NO
VAPE system can be used by representative
users under simulated conditions without
producing patterns of failure that could result in
negative impact to the user.
2 Evaluate effectiveness of instructional materials.
3 Determine if the potential for critical errors that
would or could result in high-severity outcomes
to the user (from a software-use perspective, not
a substance use perspective) have been
mitigated.
4 Provide recommendations for the device,
instructional materials, and labeling that may
mitigate the probability of use errors (usability-
and safety-related).
5 Assess the navigation of the interface and
identify areas for improvement.
2 METHODS
Participants evaluated NO VAPE during one-on-one
sessions lasting up to three hours. Participants were
paid $25 per hour in gift cards (e.g., Amazon, Visa)
to encourage participants not to rush and to take their
time in the VR environments. Each participant
completed 11 scenarios (a tutorial and all 10
simulated vaping scenarios). First, they completed the
tutorial, which provided instruction on how to move
around the VR environment and interact with items
around them. Then they completed the 10 vaping
scenarios in randomized order, including scripted
activities and CBT content. The environments
included: (1) a bedroom in the morning where they
decide whether to take their vape with them when
they leave, (2) a kitchen where they get ready for the
day, (3) a coffee shop where someone asks them to
watch their bag then the person goes into the
bathroom and vapes, (4) a classroom where friends
ask them to go to lunch (where they will be vaping),
(5) a work or school bathroom where someone in the
next stall is vaping, (6) a car ride with a friend who is
vaping, (7) a stressful day at work in an office
environment, (8) a party where people are vaping and
they must navigate an awkward conversation, (9) a
corner store where they previously bought vapes, and
(10) a living room at home alone at night. See Figure
1 for a screenshot of one of the scenarios (the
classroom scenario). Participants were encouraged to
“think aloud” during their interactions in each
environment to express what they were thinking,
doing, had questions about, etc.
Figure 1: Screenshot from the classroom scenario.
As participants completed tasks within the VR
environment, we mirrored the screen in the VR
Preliminary Usability Evaluation of a Virtual Reality (VR) Application for Quitting Nicotine Vaping
883
headset onto a laptop to allow experimenters to view
and record (screen capture recording) what the
participant was seeing in the VR environment. The
video recording allowed review and coding of user
interactions and activities in the VR environment (e.g.,
what task they were struggling to complete and why),
and enabled coders to hear participants’ comments in
context. Two “raters” recorded completion of each task
in real time as the participant progressed through tasks
in the environment. In the case of a mismatch in ratings
between the two experimenters, a third rater reviewed
the video of the experimental session and served as the
“tie breaker.” Task performance was assessed based on
task completion success and the number and type of
use-based errors.
Table 1: Task Hierarchy for the bathroom scenario (a
breakdown of the tasks required to complete the scenario).
Task Task X Task X.X Task X.X.X
1
Read “Reach for
Va
p
e”
Respond
2 Read “Plan” Respon
d
3 Read “Sit Down”
Navigate to
Toilet
4
Read “Check
Phone”
Respond
5 Read “Res
p
onse” Res
p
on
d
6 Read “Thin
k
” Click Next
7
Read “How to
Rewar
d
”
Respond
7.1
Deep
Breathing
Respond
7.2 Meditation
Listen to
exercise
7.3
Muscle
Relaxation
Respond
8 Read “Check In” Res
p
on
d
9
Read “Deal with
Boredom”
Respond
10
Read “Wash
Hands”
Navigate to
Sin
k
10.1
Place Hands
Under Sin
k
Participants who had previously expressed
interest in studies about reducing or quitting vaping
were contacted to determine whether they were
interested in learning more about this study.
Interested individuals were screened by phone for
eligibility. Inclusion criteria were: age 18 years or
older, reported vaping nicotine daily or near daily in
the prior ≥ 3 months, nicotine dependence
operationalized by a score of ≥4 (at least mild
dependence) on the 10-item E-cigarette Dependence
Inventory (ECDI) (Piper et al., 2020; Vogel et al.,
2020), self-reported interest in quitting vaping, at
least one prior experience with using a VR program,
ability to understand study procedures and read and
write in English, and vision corrected to within
20/500 bilaterally.
To identify critical tasks, we conducted a
Hierarchical Task Analysis (HTA), a task
decomposition method that produces a hierarchy of
activities users must do within NO VAPE and the
associated necessary conditions (i.e., required
subtasks to meet goals) (Diaper & Stanton, 2003).
HTA establishes conditions when sub-tasks should be
carried out to meet goals. See Table 1 for an example
Task Hierarchy required to complete the bathroom
scenario. We used the result of this HTA to identify
errors (e., inability to complete a task).
For each scenario, we categorized errors into the
following categories: (1) Slips: occur as the result of
minor errors of execution, but the participant was able
to recover without help from the experimenter, (2)
Lapses: occur when a person could not complete a
task without a hint by the experimenter (we let them
fail 3 times before providing a hint), (3) Mistakes:
occur when participants did not complete the task
even with the help of the experimenter (note that these
were mostly software errors when we had to restart
the software), and (4) Violations: occur when actions
deviate from safe procedures, standards, or rules,
whether deliberate or erroneous. Importantly, there
were not Violations by any participants in any
scenario providing evidence that the app is low-risk
and safe to use.
Average prevalence of each error was calculated
as a percentage of all tasks and averaged across
participants. We counted errors for top-level tasks.
For example, if there are multiple steps to complete a
task, we count the entire procedure as a single task.
As a specific example, in the bathroom scenario, to
fully complete Task 8, the participant had to complete
the top-level task (8) as well as at least one of the
second level tasks (8.1, 8.2, or 8.3) (see Table 1).
Participants completed the following
questionnaires: Demographics, E-cigarette
Dependence Inventory (ECDI)) (Piper et al., 2020;
Vogel et al., 2020), Vaping History, Presence (Witmer
& Singer, 1998), Simulator Sickness (Lin et al., 2002),
Engagement, Enjoyment, Interactivity, and Immersion
(E
2
I) (Lin et al., 2002), Post Experience (adapted from
(Usoh et al., 2000)), Post Evaluation Interview.
3 RESULTS
Participants included 6 adults (3 female; we did not
collect race/ethnicity information) aged 20-31
HEALTHINF 2025 - 18th International Conference on Health Informatics
884
(average age = 24). All reported having some
experience with VR (i.e., previously used VR at least
five times for five minutes). 50% of participants (3/6)
reported also currently smoking tobacco.
Table 2 shows prevalence of each error type in
each scenario. For example, of the 8 tasks in the
tutorial, participants slipped an average of 10% of the
time, ranging from 0 slips to 2 slips per participant
across the full tutorial level. Participants committed
lapses 8% of the time, ranging from 0-1 lapse per
participant. There were no mistakes in the Tutorial.
Table 2: For each scenario, we calculated the percentage of
each type of error for each participant, then averaged across
participants. We show the number of tasks as a reference as
each scenario had differing numbers of major tasks to
complete (see Table 1 for an example of a task hierarchy).
Scenario
#
Tasks
% Slips
(Range)
% Lapses
(Range)
%
Mistakes
(Range)
Tutorial 8 10%
(
0-2
)
8%
(
0-1
)
0%
Bathroo
m
17 2%
(
0-1
)
0% 0%
Bedroo
m
22 7%
(
0-3
)
0% 0%
Café 21 2% (0-2) 0% 1% (0-1)
Ca
r
19 5% (0-1) 0% 5% (0-1)
Classroom 21 5%
(
0-1
)
0% 0%
Kitchen 16 6%
(
0-2
)
0% 1%
(
0-1
)
Living
Room
32 4% (0-3) 1% (0-1) 2% (0-1)
Part
y
26 3%
(
0-2
)
0% 1%
(
0-1
)
Store 29 5%
(
0-2
)
3%
(
0-2
)
0%
Wor
k
36 2%
(
0-2
)
0% 1%
(
0-1
)
E-Cigarette Dependence Index scores ranged
from 8 to 16 (mean = 11.8) consistent with moderate
dependence (Foulds et al., 2015). Daily use varied
from 0 to 5-9 times (50-90 mins). All participants
reported vaping within 60 minutes of waking when
able to vape freely. Half of participants reported
awakening at night to vape at least twice a week. All
participants reported finding it very hard to quit and
finding it hard to keep from vaping in places they are
not supposed to. With all participants having strong
cravings to vape, all noted that they become more
irritable when they are unable to use an electronic
cigarette and 4/6 felt nervous, restless or anxious
when they could not use an electronic cigarette.
Average age to begin vaping was 20.1 years. Most
common reasons for initiation was peer pressure and
having family/friends who vape. Participants had
vaped 3.5 years on average, between 0.14 and 1
cartridge per day. The Crave brand was used by 4/6
participants. The most common reason for wanting to
quit were concerns about future health problems
followed by financial reasons. All participants had
tried to quit vaping within the past year; half had quit
vaping for at least 24 hours with an average cessation
period of 27.1 days. Two participants reported having
used nicotine gum, 1 participant reported use of
bupropion, and 1 participant reported using
medication other than bupropion or nicotine and/or
herbal treatments.
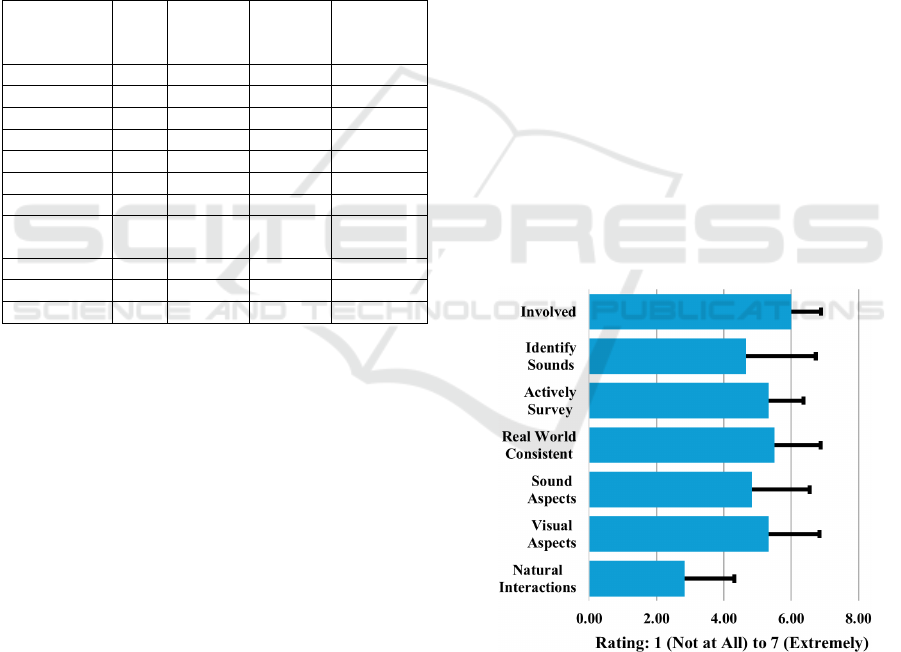
In the Presence Questionnaire, participants
characterized their experience in the virtual
environment, rating their experience on a scale of 1-
7, from 1 (bounds related to low presence) to 7
(bounds related to high presence), across seven
questions (see Figure 2). Participants noted their
interactions in the environment felt less natural (see
discussion), with a mean of 2.83 (1 – “Completely
artificial” to 7 – “Completely natural”). However,
they felt somewhat involved in the visual
(mean=5.33) and auditory (mean=4.83) aspects of the
environment on a scale of 1 being “Not at all” to 7
being “Completely”. Experiences in the simulation
were consistent with those in the real world (mean =
5.5 (1 – “Not at all” to 7 – “Completely”)).
Completeness of the ability to visually survey the
environment was good (mean=5.33) (1 – “Not at all”
to 7 – “Completely”). Participants were somewhat
able to successfully identify sounds (mean = 4.67 (1
– “Not at all” to 7 – “Completely”)). Participants felt
involved in the virtual experience with a mean of 6 (1
– “Not involved” to 7 – “Completely engrossed”).
Figure 2: Presence questionnaire results.
For the Simulator Sickness questionnaire,
participants were asked to rate on a scale of 1-3 (1-
“Not at all” to 3 – “a lot”) the degree to which they
experienced sixteen conditions: general discomfort,
fatigue, headache, eyestrain, difficulty focusing,
increased salivation, sweating, nausea, difficulty
Preliminary Usability Evaluation of a Virtual Reality (VR) Application for Quitting Nicotine Vaping
885
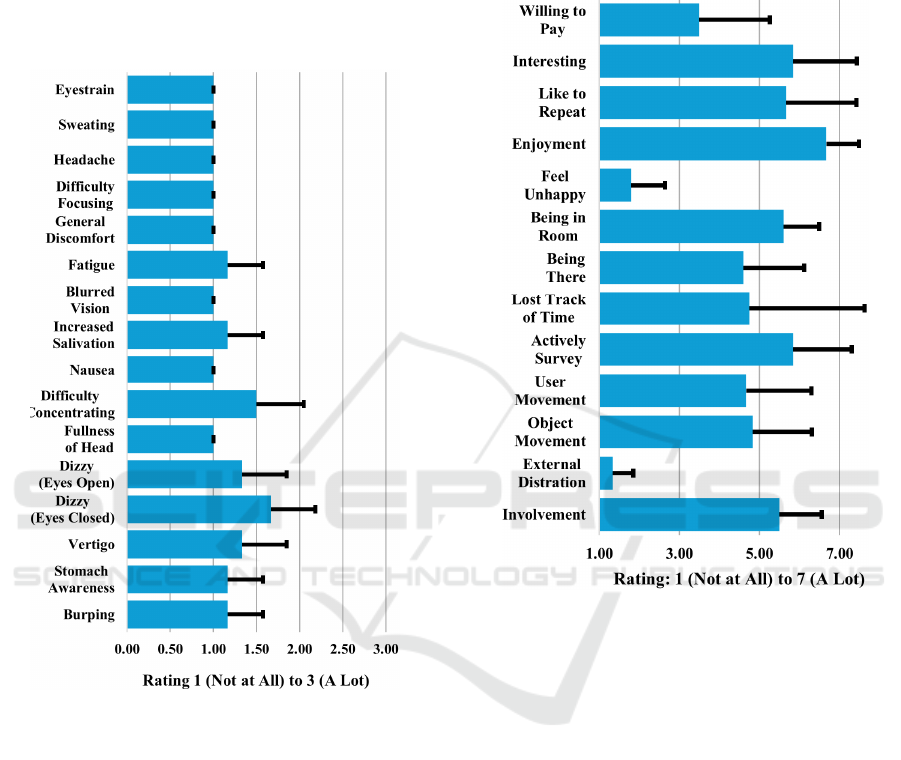
concentrating, fullness of head, dizzy (eyes open),
dizzy (eyes closed), vertigo, stomach awareness, and
burping. The mean across all conditions was 1.15.
The only negative symptom that participants
experienced from the simulation was increased
eyestrain (mean=1.7). Sweating (mean=1.5),
headaches (mean=1.33) and difficulty focusing
(mean=1.33) were felt but not strongly. See Figure 3.
Figure 3: Simulator Sickness questionnaire results.
For the Engagement, enjoyment, interactivity,
and immersion questionnaire, participants were asked
to respond to 12 questions on a scale of 1-7 (1- “Not
at all” to 7 – “A lot”). Most participants were attracted
to the visual scenes within the application with a
mean of 5.5 and noise outside the simulation was not
an issue for most (mean=1.33). Feelings were mixed
about the matching of the real world to the virtual
environment with participants feeling an average of
somewhat "being there" in the virtual environment
(mean=4.6). Overall, moving objects within the
virtual space was somewhat compelling (mean=4.83)
as was moving oneself through the space
(mean=4.67). Time tracking varied across
participants with two participants losing track of time
entirely, and one not at all (mean=4.75). All
participants were not unhappy when the simulation
was over (mean=1.8). They would likely repeat the
experience (mean=5.6) and found it interesting
(mean=5.83), however they would not be likely to
pay for it. Results are summarized in Figure 4.
Figure 4: Engagement, enjoyment, interactivity, and
immersion questionnaire (E2I) results.
Participants answered a Post Experience
Questionnaire to probe for additional factors related
to VR presence and physiological effects on a scale
of 1 (seemingly artificial) to 7 (like being in the real
world) For presence-related questions, participants
felt mixed about the simulation accurately
representing their normal experiences of being in a
place (mean=5). The virtual environment did not feel
completely like a "reality" to most (mean=4.17) and
their sense of being fully immersed was average.
Participants recalled simulated images both as images
they saw and places they visited (mean of 4.17 on a
scale of 1 - “Simulated images” to 7 - “Somewhere
that I visited”). Their sense of being in the simulated
environment was slightly greater than being
elsewhere with a mean of 5.17 (1 – “Being
elsewhere” to 7 – “Being in the simulated
environment”). Participant memory of the virtual
space was somewhat vivid as it related to places they
had visited that day (mean of 4 on a scale of 1 – “Not
HEALTHINF 2025 - 18th International Conference on Health Informatics
886

at all” to 7 – “Very much so”). Most participants did
not pay attention to events in the real world during
their time in the simulation (mean=2.33) and most
were completely focused on the tasks (mean=6.33).
Participants also rated the degree to which they
experienced physiological effects during NO VAPE
use, on a scale from 1 (“not at all”) to 7 (“almost all
the time”). None of the participants experienced
strong feelings of nausea (mean=1.2), dizziness
(mean=1.2) or headaches (mean=1.3). However,
there was some degree of mild eye strain noted
(mean=2.2).
After the session, we asked open-ended questions
to understand the opinions of participants.
Participants did note several areas of improvement in
the simulation. The meditation room was not well-
received by several participants who noted it needed
visual and audio improvements (“Immersion breaks
for meditation”, “More detail in meditation room
would be nice.”). The placement of text messages in
the space were difficult for some users to view, with
the text being too close or running into the walls
during some scenarios. Some of the interactions
proved challenging for participants and they would
have liked to become more familiar with the controls
before they began the scenarios (“was looking at
hands for feedback on what each control did”,
“teleportation was sometimes hard”). Some of the
options were difficult to select and there was
confusion about which objects could be interacted
with. Inconsistent interaction mechanics pulled some
of the participants out of the experience and specific
issues with object interaction broke the immersion
(“immersion stronger in some parts than others”).
However, overall, feedback was positive.
Participants felt that scenarios were generally
accurate, immersive, relatable and valuable
(“Scenario content was accurate”, “Felt real/actual
situations that happen”). They really enjoyed the
interaction-based approach once they got used to the
controls and how to navigate the space. They noted
that the activities in the scenarios reinforced good
habits. The highlighting was a very effective means
of guiding users through tasks and when not present,
participants faltered. Participants liked the options
they were given for where they could place objects
(“multiple options to hide the vape was good”).
Participants felt that the system would be safe for use
at home, and some viewed it as an empowering
therapeutic tool for quitting (“more empowered to
quit”). They “could see it as a therapeutic tool.”, felt
"more empowered to quit", and “didn't think of it as
therapy until after.”
4 CONCLUSIONS
This preliminary study addressed all the initial goals
outlined in Section 1. First, we determined that there
are no usability issues that could increase risks to
users to unacceptable levels (from a software use
perspective, not a substance use perspective), and that
the NO VAPE System can be used by representative
users under simulated conditions without producing
patterns of failure that could result in negative impact
or injury to the user. Across all of our participants,
and in all of our scenarios, there were zero deviations
from safe procedures (i.e., Violations).
Second, we evaluated the effectiveness of the
instructional materials for teaching users how to
interact with the system without frustration. The
Tutorial scenario was always the first scenario that
participants completed, and the goal of this scenario
was to teach participants how to interact with the
application (e.g., how to navigate from one place to
another, open cupboards or drawers, pick up items
and put them into a bag, interact with non-player
characters (NPCs), eat and drink, etc.). As expected,
participants committed more errors in the tutorial
(slips an average of 10% of major tasks and lapses an
average of 8% of major tasks) than the later scenarios
as they were not yet familiar with how to navigate the
software. However, one weakness was that
participants had difficulty interacting with the
environment (found interactions to be unnatural).
This information allowed us to review videos and task
completion information to identify where interactions
were difficult for participants (e.g., interacting with
the phone), allowing us to fix these issues.
Third, we determined that the potential for
critical errors that would or could result in high-
severity outcomes to the user have been mitigated to
the extent reasonable or possible through the design
of the device and instructional materials. We
previously completed a related VR application
focused on smoking cessation (called Constructed
Environments for Successfully Sustaining
Abstinence Through Immersive and On-Demand
Treatment; CESSATION), and conducted a full set of
human factors studies on that app. During that work,
we discovered that the errors with high severity
outcomes were related to using the VR itself. These
included potentially walking physically throughout
the environment rather than virtual teleporting,
resulting in a potential to walk into a wall or other
furniture, and “forgetting” that they were in VR and
potentially trying to physically sit on a chair that was
not present outside of the VR world. That study
resulted in the production of a similar Tutorial
Preliminary Usability Evaluation of a Virtual Reality (VR) Application for Quitting Nicotine Vaping
887

environment in CESSATION. We used those lessons
when building NO VAPE. The results of the current
usability evaluation indicate that we were successful
in mitigating any potential high-severity outcomes
through this Tutorial material.
Fourth, by analyzing the errors that were made
whenever a participant had trouble with or could not
complete a task, we were able to collate information
about each error, and provide recommendations for
VR app refinement, including to improve
instructional materials (e.g., participants wanted to
receive additional information about the trivia
question content), and labeling to mitigate the
probability of use errors (usability- and safety-related
content). Most of the Mistakes were actually a result
of software errors that would not allow the participant
to play further through the level. For example in the
living room scenario, sometimes the participant
accidentally dropped the TV remote onto the couch
before they turned on the TV. The remote disappeared
and they could not retrieve it again. This prevented
them from turning on the TV, preventing them from
completing any task further into the scenario. As
planned, this usability study has allowed us to fix
these identified software errors.
Fifth, we assessed the navigation of the interface
and identify areas for improvement. During the study,
participants were encouraged to use the “think aloud”
method to talk about what they were doing in the
environment (the experimenter could also view
participant interactions on a mirrored screen). This
allowed us to collect subjective comments from
participants (e.g., “ooh, I like the nature sounds” and
“this room feels really sterile” during the meditation
practice in a separate meditation room). We collated
all of the participant comments that occurred
naturally during the experiment as well as the
responses to the post-evaluation interview, and
compiled a list of recommendations that will inform
refinement of the NO VAPE app prior to conduct of
the planned clinical study.
One limitation to this work is the number of
participants, and the fact that all participants were
over 18 years old, even though we plan to use it with
participants 16+. We are now working to enroll
additional participants for a target of N=15 who are
18+ and N=15 who are 16-17.
We believe that the results of this preliminary
usability evaluation indicate that once we implement
the recommended software and scenario content
improvements, we will have a safe and user-friendly
VR application to use in our clinical study. The only
controlled trial to date of an intervention for cessation
of vaped nicotine is a parallel, two-group, double-
blind, individually randomized clinical trial of “This
is Quitting (TIQ)”, a free, anonymous texting app,
that incorporates messages from people who have
attempted to or successfully quit e-cigarettes. This
study included 2588 young adults aged 18-24.
Participants were significantly more likely to quit
vaping when receiving TIQ than without intervention
(24.1% versus 18.6%; p<0.001) (Graham et al., 2021,
2024). However, text messaging is not immersive,
provides no opportunity for ecologically valid
practice of resisting cravings, and may not be
engaging across continuous use. Therefore, in the
next phase of this work, we will conduct a single-
blind, parallel group, randomized clinical trial in 90
non-smoking, nicotine-dependent people, age 16+,
who want to quit vaping. We hypothesize that those
assigned to NO VAPE combined with a 12-session
vaping cessation CBT program (experimental group)
will have a higher rate of 4-week continuous
abstinence at the end of 12 weeks treatment than those
assigned to CBT alone (control group). If successful,
this will provide clinical evidence of real-world
outcomes and efficacy of NO VAPE.
ACKNOWLEDGEMENTS
We thank the participants in the trial for their time and
input. Research reported in this publication was
supported by the National Institute On Drug Abuse of
the National Institutes of Health under Award
Number R44DA059018. The content is solely the
responsibility of the authors and does not necessarily
represent the official views of the National Institutes
of Health.
REFERENCES
Abafalvi, L., Pénzes, M., Urbán, R., Foley, K. L., Kaán, R.,
Kispélyi, B., & Hermann, P. (2019). Perceived health
effects of vaping among Hungarian adult e-cigarette-
only and dual users: A cross-sectional internet survey.
BMC Public Health, 19(1), 1–10.
Bellum, S. (2014). Virtual Reality and Drug Abuse
Treatment. NIDA for Teens. https://teens.drugabuse.
gov/blog/post/virtual-reality-and-drug-abuse-treatment
Diaper, D., & Stanton, N. (2003). The handbook of
task analysis for human-computer interaction.
https://books.google.com/books?hl=en&lr=&id=Eudd
OAMeI5sC&oi=fnd&pg=PP1&dq=the+handbook+for
+task+analysis&ots=7M0qGFaefd&sig=HGOX2pLK
hUVXRFYBGsyZdhwKNWI
Ferrer-Garcia, M., Garcia-Rodriguez, O., Pericot-Valverde,
I., Yoon, J. H., Secades-Villa, R., & Gutierrez-
HEALTHINF 2025 - 18th International Conference on Health Informatics
888

Maldonado, J. (2012). Predictors of smoking craving
during virtual reality exposure. Presence: Teleoperators
and Virtual Environments, 21(4), 423–434.
Food and Drug Administration. (2016). Applying Human
Factors and Usability Engineering to Medical
Devices [Guidance]. https://www.fda.gov/regulatory-
information/search-fda-guidance-documents
Foulds, J., Veldheer, S., Yingst, J., Hrabovsky, S., Wilson,
S. J., Nichols, T. T., & Eissenberg, T. (2015).
Development of a questionnaire for assessing
dependence on electronic cigarettes among a large
sample of ex-smoking E-cigarette users. Nicotine &
Tobacco Research, 17(2), 186–192.
Gallus, S., Lugo, A., Stival, C., Cerrai, S., Clancy, L.,
Filippidis, F. T., Gorini, G., Lopez, M. J., López-Nicolás,
Á., & Molinaro, S. (2023). Electronic cigarette use in 12
European countries: Results from the TackSHS survey.
Journal of Epidemiology, 33(6), 276–284.
Gao, K., Wiederhold, M. D., Kong, L., & Wiederhold, B.
K. (2013). Clinical experiment to assess effectiveness of
virtual reality teen smoking cessation program.
García-Rodríguez, O., Pericot-Valverde, I., Gutiérrez-
Maldonado, J., Ferrer-García, M., & Secades-Villa, R.
(2012). Validation of smoking-related virtual
environments for cue exposure therapy. Addictive
Behaviors, 37(6), 703–708.
García-Rodríguez, O., Weidberg, S., Gutiérrez-Maldonado,
J., & Secades-Villa, R. (2013). Smoking a virtual
cigarette increases craving among smokers. Addictive
Behaviors, 38(10), 2551–2554.
Graham, A. L., Amato, M. S., Cha, S., Jacobs, M. A.,
Bottcher, M. M., & Papandonatos, G. D. (2021).
Effectiveness of a Vaping Cessation Text Message
Program Among Young Adult e-Cigarette Users:
A Randomized Clinical Trial. JAMA Internal
Medicine, 181(7), 923–930. https://doi.org/10.1001/
jamainternmed.2021.1793
Graham, A. L., Cha, S., Jacobs, M. A., Amato, M. S.,
Funsten, A. L., Edwards, G., & Papandonatos, G. D.
(2024). A vaping cessation text message program for
adolescent e-cigarette users: A randomized clinical
trial. Jama, 332(9), 713–721.
Hammond, D. (2019). Outbreak of pulmonary diseases
linked to vaping. In Bmj (Vol. 366). British Medical
Journal Publishing Group.
Irusa, K. F., Vence, B., & Donovan, T. (2020). Potential
oral health effects of e-cigarettes and vaping: A review
and case reports. Journal of Esthetic and Restorative
Dentistry, 32(3), 260–264.
Ko, K., Ting Wai Chu, J., & Bullen, C. (2024). A Scoping
Review of Vaping Among the Asian Adolescent
Population. Asia Pacific Journal of Public Health,
36(8), 664–675. https://doi.org/10.1177/1010539524
1275226
Lee, J. H., Ku, J., Kim, K., Kim, B., Kim, I. Y., Yang, B.-H.,
Kim, S. H., Wiederhold, B. K., Wiederhold, M. D., &
Park, D.-W. (2003). Experimental application of virtual
reality for nicotine craving through cue exposure.
CyberPsychology & Behavior, 6(3), 275–280.
Lee, J., Lim, Y., Graham, S. J., Kim, G., Wiederhold, B. K.,
Wiederhold, M. D., Kim, I. Y., & Kim, S. I. (2004).
Nicotine craving and cue exposure therapy by using
virtual environments. CyberPsychology & Behavior,
7(6), 705–713.
Levy, D. T., Tam, J., Sanchez-Romero, L. M., Li, Y., Yuan,
Z., Jeon, J., & Meza, R. (2021). Public health
implications of vaping in the USA: The smoking and
vaping simulation model. Population Health Metrics,
19(1), 1–18.
Lin, J.-W., Duh, H. B.-L., Parker, D. E., Abi-Rached, H., &
Furness, T. A. (2002). Effects of field of view on
presence, enjoyment, memory, and simulator sickness
in a virtual environment. Virtual Reality, 2002.
Proceedings. IEEE, 164–171.
Loria, K. (2016). Therapists have created a virtual reality
heroin cave in an attempt to help addicts. Tech Insider.
McNeill, A., Brose, L., Calder, R., Simonavicius, E., &
Robson, D. (2021). Vaping in England: An evidence
update including vaping for smoking cessation,
February 2021. Public Health England: London, UK,
1–247.
Metcalf, M., Rossie, K., Stokes, K., Tallman, C., & Tanner,
B. (2018). Virtual Reality Cue Refusal Video Game for
Alcohol and Cigarette Recovery Support: Summative
Study. JMIR Serious Games, 6(2), e7.
Piper, M. E., Baker, T. B., Benowitz, N. L., Smith, S. S., &
Jorenby, D. E. (2020). E-Cigarette dependence
measures in dual users: Reliability and relations with
dependence criteria and e-cigarette cessation. Nicotine
and Tobacco Research, 22(5), 756–763.
Traboulsi, H., Cherian, M., Abou Rjeili, M., Preteroti, M.,
Bourbeau, J., Smith, B. M., Eidelman, D. H., &
Baglole, C. J. (2020). Inhalation toxicology of vaping
products and implications for pulmonary health.
International Journal of Molecular Sciences, 21(10),
3495.
Usoh, M., Catena, E., Arman, S., & Slater, M. (2000). Using
presence questionnaires in reality. Presence:
Teleoperators and Virtual Environments, 9(5), 497–503.
Vogel, E. A., Prochaska, J. J., & Rubinstein, M. L. (2020).
Measuring e-cigarette addiction among adolescents.
Tobacco Control, 29(3), 258–262.
Weser, V. U., & Hieftje, K. D. (2020). Invite Only VR: A
Vaping Prevention Game: An Evidence-Based VR
Game for Health and Behavior Change. Special Interest
Group on Computer Graphics and Interactive
Techniques Conference Talks, 1–2.
Witmer, B. G., & Singer, M. J. (1998). Measuring presence
in virtual environments: A presence questionnaire.
Presence, 7(3), 225–240.
Preliminary Usability Evaluation of a Virtual Reality (VR) Application for Quitting Nicotine Vaping
889
