
Consent Understanding and Verification for Personalized Assistive
Systems
Ismael Jaggi
1 a
, Rachele Carli
2 b
, Berk Buzcu
1,3 c
, Michael Schumacher
1 d
and Davide Calvaresi
1 e
1
University of Applied Sciences and Arts Western Switzerland (HES-SO Valais/Wallis), Sierre, Switzerland
2
UMEA University, UMEA, Sweden
3
The Sense Innovation and Research Center, Lausanne, Switzerland
{ismael.jaggi, berk.buzcu, davide.calvaresi, michael.schumacher}@hevs.ch, rachele.carli@umea.se
Keywords:
Dynamic Informed Consent, Agent-Based Virtual Assistants, Healthcare, Data Protection.
Abstract:
The rapid adoption of personalized systems, driven by advancements in natural language processing, sensor
technologies, and AI, has transformed the role of virtual personal assistants (VPAs), particularly in healthcare.
While VPAs promise to enhance patient experiences through tailored support and adaptive workflows, their
complexity often results in opaque functionalities hindering user understanding. This lack of transparency
poses significant challenges, particularly in the context of informed consent, where users must comprehend
the implications of sharing sensitive personal data. Existing consent systems often rely on static declarations
and extensive documentation, which overwhelm users and fail to ensure informed decision-making. To address
this problem, this paper presents a novel consent management approach integrated into the EREBOTSv3.0, an
agent-based GDPR-compliant explainable framework for virtual assistants. The proposed solution introduces
(i) an interactive method that structures consent into clear sections with summaries and examples to improve
user comprehension and (ii) a question-based verification mechanism that assesses understanding and rein-
forces knowledge when needed. By leveraging EREBOTS’ modular architecture, real-time feedback, and
secure data management, the proposed approach enhances transparency, fosters trust, and simplifies the con-
sent understanding for dialog-based healthcare systems. This work lays the foundation for addressing critical
challenges at the intersection of personalized AI, healthcare, and data protection.
1 INTRODUCTION
The adoption of personalized systems providing tai-
lored support is rapidly increasing, driven by ad-
vances in natural language processing Eguia et al.
(2024), sensor technologies Cusack et al. (2024), and
artificial intelligence Wang et al. (2021). Virtual
personal assistants (VPAs) are particularly promis-
ing in delivering impactful, customized outcomes. In
healthcare, they hold the potential to enhance pa-
tient experiences and improve outcomes by address-
ing individual needs and supporting complex medical
workflows Fang et al. (2024).
Despite these advancements, VPAs are often so-
a
https://orcid.org/0009-0005-0092-5446
b
https://orcid.org/0000-0002-8689-285X
c
https://orcid.org/0000-0003-1320-8006
d
https://orcid.org/0000-0002-5123-5075
e
https://orcid.org/0000-0001-9816-7439
phisticated systems with opaque functionalities and
decision-making processes that most users struggle to
understand. This lack of transparency is especially
concerning healthcare, where AI systems are increas-
ingly employed to assist or replace human operators
for less safety-critical tasks. Examples include tools
promoting healthier habits Cruz Casados et al. (2024),
monitoring therapy adherence Ma et al. (2024), pro-
viding medication reminders Corbett et al. (2021),
and suggesting intervention strategies Sezgin et al.
(2020).
These intelligent systems depend on collecting
and analyzing personal data provided by patients,
with outcomes that can significantly impact users’
health and safety. This highlights the critical role of
informed consent, a cornerstone of the professional-
patient relationship. Informed consent ensures auton-
omy and addresses the inherent information asymme-
try where the professional or service provider holds
greater knowledge and authority.
694
Jaggi, I., Carli, R., Buzcu, B., Schumacher, M. and Calvaresi, D.
Consent Understanding and Verification for Personalized Assistive Systems.
DOI: 10.5220/0013376300003890
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Conference on Agents and Artificial Intelligence (ICAART 2025) - Volume 1, pages 694-701
ISBN: 978-989-758-737-5; ISSN: 2184-433X
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

When intelligent systems mediate this relation-
ship, it becomes vital to implement mechanisms that
not only disseminate legal documentation but also
ensure users comprehend the provided information.
These mechanisms mitigate potential adverse conse-
quences arising from misunderstanding or insufficient
awareness and reduce developers’ liability by demon-
strating efforts to inform users effectively. This is es-
pecially the case for consent given by patients in clin-
ical settings.
Current consent systems typically rely on static
declarations and detailed descriptions Kaye et al.
(2014). However, these approaches often over-
whelm users with complexity and information over-
load Schermer et al. (2014), leaving them unable to
make informed decisions. As a result, users may inad-
vertently agree to terms they do not fully understand,
risking unintended uses of their data. For instance,
sensitive medical data could be shared with third par-
ties without the user’s explicit awareness Falagas et al.
(2009). This underscores the urgent need for innova-
tive consent solutions that simplify processes while
enhancing user comprehension and competence.
To address these challenges, this paper proposes
two key contributions: (i) an interactive method for
structuring consent into distinct sections with sum-
maries and examples to convey their meaning effec-
tively, particularly in scenarios lacking professional
support; and (ii) a question-based verification mecha-
nism to confirm user comprehension, supplemented
by reinforcement measures to address gaps in un-
derstanding. To test the overall system architec-
ture, we integrate it into the EREBOTSv3.0 frame-
work, designed to ensure users are thoroughly in-
formed about data usage and to promote long-term
understanding of consent. The EREBOTSv3.0 frame-
work is a sophisticated multi-agent system for de-
veloping modular and explainable virtual assistants.
It employs a multi-agent architecture, where each
user is represented by a personal agent managing in-
dividual interactions for personalized, adaptive ex-
periences. Additional agents, such as a gateway
agent, facilitate seamless communication between
front-end interfaces (e.g., mobile apps) and back-end
systems. EREBOTS adheres to principles of modu-
larity and data protection, supporting plug-and-play
components for customizable workflows and employ-
ing GDPR-compliant databases like Pryv to securely
manage sensitive data. This ensures transparency in
data processing and robust data protection.
By leveraging EREBOTS’ modularity and real-
time feedback mechanisms, the proposed consent sys-
tem offers a seamless and customizable user experi-
ence. It addresses critical challenges in healthcare,
such as ensuring transparency in data usage and fos-
tering trust. This approach simplifies the consent pro-
cess while laying the foundation for user-friendly and
reliable solutions in dialog-based healthcare systems.
The remainder of this paper is organized as fol-
lows. Section 2 reviews the relevant state-of-the-art.
Section 3 describes the system architecture and mod-
ule behaviors. Finally, Section 4 discusses the contri-
butions and their potential impact and outlines future
works concluding the paper.
2 STATE OF THE ART
The increasing reliance on digital systems for data
management has heightened the importance of ro-
bust consent mechanisms, particularly in sensitive do-
mains such as healthcare and conversational AI. This
section reviews advancements in consent manage-
ment systems, their application in assistive technolo-
gies, and ongoing challenges in ensuring informed
and verifiable consent.
2.1 Consent Information in Modern
Systems
Consent management has emerged as a central com-
ponent of online systems, driven by the latent value
of user data and the increasing diversity of its ap-
plications. A key regulatory framework underpin-
ning consent management is the General Data Pro-
tection Regulation (GDPR)Robol et al. (2022). The
advent of digitalization has catalyzed research into
Dynamic Consent Management Systems (DCMS).
These systems enable users to continually manage
and update their consent preferences, offering them
greater control over their personal data in real time Al-
banese et al. (2020). By leveraging online and mo-
bile platforms, consent forms can be stored elec-
tronically, allowing users to review and modify their
preferences at any time. These platforms also fa-
cilitate direct communication with researchers, en-
abling users to ask questions, request additional
information, or specify preferences for future re-
search projects. Furthermore, DCMS can accom-
modate diverse consent modalities—such as broad
consent, specific consent, or meta-consent—tailored
to the research context Budin-Ljøsne et al. (2017).
Technological advancements have significantly en-
hanced the usability and security of consent man-
agement systems. Blockchain technology, for ex-
ample, is increasingly employed to ensure the im-
mutability, traceability, and accessibility of consent
and data records Albanese et al. (2020). Simulta-
Consent Understanding and Verification for Personalized Assistive Systems
695

neously, privacy-preserving techniques such as dif-
ferential privacy and zero-knowledge proofs are be-
ing utilized to safeguard data confidentiality Khalid
et al. (2023a). Research has demonstrated that inter-
active platforms and multimedia tools improve user
satisfaction and comprehension of the consent pro-
cess. Moreover, user-centered and transparent design
methodologies have been shown to strengthen trust in
digital services Gesualdo et al. (2021). The health-
care sector represents a critical application domain for
DCMS, where robust confidentiality measures are es-
sential to safeguard patient privacy. These systems
ensure that data is utilized strictly within the scope
of the granted consent, thereby mitigating risks such
as privacy breaches and identity theft Khalid et al.
(2023b).
2.2 Consent in Assistive Conversational
Systems
Conversational systems, including voice assistants
and chatbots, are increasingly utilized across vari-
ous healthcare applications, such as telerehabilita-
tion and accessibility. These AI-driven systems offer
unique opportunities to enhance the consent process
by addressing comprehension challenges during user
interactions. AI-powered conversational agents can
present the consent process in incremental steps, al-
lowing users to digest information at their own pace.
Moreover, the ability to interact with these systems
and pose questions in real time helps resolve ambigu-
ities. By designing these chatbots as friendly research
assistants that (pro)actively engage users, patients feel
more involved and are encouraged to participate ac-
tively Xiao et al. (2023). These systems provide pa-
tients with continuous access to detailed information,
eliminating the reliance on time-constrained human
interactions. Additionally, conversational agents can
adapt their responses to the patient’s level of lan-
guage comprehension, thereby alleviating the burden
on medical personnel while empowering patients to
make informed decisions Allen et al. (2024). Ethi-
cal and law-compliant considerations remain essen-
tial in the design and implementation of such systems.
Concerns surrounding privacy, algorithmic fairness,
and the potential for misunderstandings in agent-user
interactions necessitate the adoption of transparent,
user-centered design processes. This is particularly
critical when developing assistive technologies for
vulnerable populations, such as older adults or indi-
viduals with cognitive impairments. To address these
challenges, iterative development and active involve-
ment of end users are essential Wangmo et al. (2019).
2.3 Challenges in Consent
Comprehension and Verification
Both clinical and non-clinical domains face signifi-
cant challenges in ensuring the comprehension and
verification of informed consent Manson and O’Neill
(2007), a cornerstone of patient autonomy Harish
et al. (2015). Despite its importance, numerous stud-
ies reveal persistent deficiencies in the informed con-
sent process (ICP), undermining its legal validity and
ethical integrity Delany (2005).
A primary issue is the limited comprehension of
consent information by participants. Research indi-
cates that many individuals fail to grasp critical as-
pects of consent, such as the purpose of a study, as-
sociated risks, and the concept of randomization. A
systematic review by Pietrzykowski and Smilowska
(2021) found that fewer than half of the partici-
pants could recall essential study details, including
risks and the voluntary nature of participation. Sim-
ilarly, Wisgalla and Hasford (2022) highlighted that
consent documents are often excessively lengthy and
written in complex language. This complexity makes
such documents challenging to understand even for
individuals with advanced education, such as those
holding PhDs. Consequently, such structures can ob-
scure critical information for laypersons or individu-
als with limited literacy, contributing to widespread
misunderstandings. Nearly 45% of participants in
clinical research, for instance, are unable to identify
a single risk associated with the studies in which they
participate. Another critical challenge is cognitive
and informational overload. The extensive and in-
tricate nature of consent information can overwhelm
patients, hindering their ability to provide truly in-
formed consent. This phenomenon, termed “informa-
tional overload”, is well-documented by Bester et al.
(2016), who emphasize that excessive details can ren-
der the ICP ineffective.
To address these challenges, strategies such as the
teach-back method have been proposed. This tech-
nique involves asking patients to reiterate the infor-
mation they have received in their own words, allow-
ing misunderstandings to be identified and corrected
proactively. A study on surgical education demon-
strated that the teach-back method significantly im-
proved patients’ understanding of risks and benefits
while enhancing their trust in medical professionals.
These findings underscore the positive impact of ac-
tive engagement and repetition on the informed con-
sent process Seely et al. (2022).
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
696

3 ARCHITECTURE AND
IMPLEMENTATION
Figure 1 illustrates the extension of the EREBOTS
framework with a dynamic consent management sys-
tem. This modular, agent-based platform supports
the creation of GDPR-compliant virtual assistants, in-
corporating both core modules (which facilitate the
underlying communication mechanisms) and custom
modules (such as integrating an Explainable AI (XAI)
engine for user-oriented explanations Buzcu et al.
(2024)). The framework’s customization and modu-
larity allow for plug-and-play extensions, including
finite state machines (FSMs), user profiling systems,
and feedback management. Its adaptability, trans-
parency, and one-to-one user-agent mapping make it
an ideal candidate for integrating a customized con-
sent management system.
3.1 Pipeline
Consent management is integrated as an optional
module into the core functionality of the personal
agents (PAs) within the EREBOTS architecture. This
pipeline maintains consistency across all PAs to en-
sure fairness and observability. Figure 2 depicts the
entire consent management process, which can be di-
vided into four main stages based on user interaction.
1. Initialization:
• The user connects to the PA, creating a new en-
try with private information in Pryv.
• The user receives an explanation of the chat-
bot’s purpose and interaction instructions.
2. Consent Process:
• If no consent process is defined (see [Consent
process not defined]), the interaction proceeds
without user input. Otherwise, the consent pro-
cess begins following the welcome message
(explained in Section 3.2).
• User consent is gathered in incremental steps
(see [Process consent steps]). At each stage, the
user is prompted to indicate their understanding
of the system’s implications.
• If the user declines any part of the consent (see
[Consent part not accepted]), the process termi-
nates, and the PA stops processing messages.
• Upon completion, the consent is stored in Pryv
for compliance and future interactions.
3. User Profiling & Task Execution:
• The system transitions to the profiling state,
where ProfilingFSM is used to create a custom
user profile stored in Pryv.
• The PA switches to the customized Conversa-
tional State Machine (CSM) defined by the de-
veloper, marking the start of the PA’s main task.
3.2 The Consent FSM
The Consent FSM manages user interactions
throughout the consent process. Each state represents
a segment of the consent form or a related question.
Configuration is managed via a web interface and
stored in two YAML files: one for consent segments
and one for questions. These files are validated by
experts to ensure accuracy. The FSM loads all states
at startup, and user responses determine the transition
to the next state. Consent is gathered in two key
moments: (i) before account registration, where
consent segments are proposed alongside examples
and brief explanations, and (ii) during usage of the
VPa, where follow-up questions further elaborate on
the initial consent.
Consent Segmentation. The user is presented with
sections of the consent form that can be accepted or
rejected, as per legal requirements European Parlia-
ment and European Council (2016). Upon accep-
tance, the FSM proceeds to the next section or begins
providing services. Rejection at any point terminates
the process, and the decision is stored in the privacy-
preserving database, Pryv
1
. If consent is withdrawn
at any point, the chatbot stops, ceasing all operations.
If new consent is provided (the consent process can
be restarted at any time), the services are reactivated.
Informed consent is a critical legal principle
in the EU, safeguarding autonomy and personal
data European Parliament and European Council
(2016). It must be given freely, explicitly, and with
clear information, ensuring the user comprehends
the terms European Commission (2018); European
Parliament and European Council (2016). This is
especially important in AI systems, where ensuring
real understanding—not just acceptance to pro-
ceed—poses a significant challenge. To enhance
comprehension, the consent information is organized
into discrete blocks (e.g., data collected, purpose,
risks). The moment of consent should be clearly
distinct from other interactions, and design elements
such as text segmentation and visual cues can aid
in clarity. Insights from cognitive science and
psychology can inform the design, ensuring users
are not overwhelmed with technical language. A
summary of key concepts may also be provided if
necessary. The FSM validates user responses: correct
1
https://www.pryv.com/
Consent Understanding and Verification for Personalized Assistive Systems
697
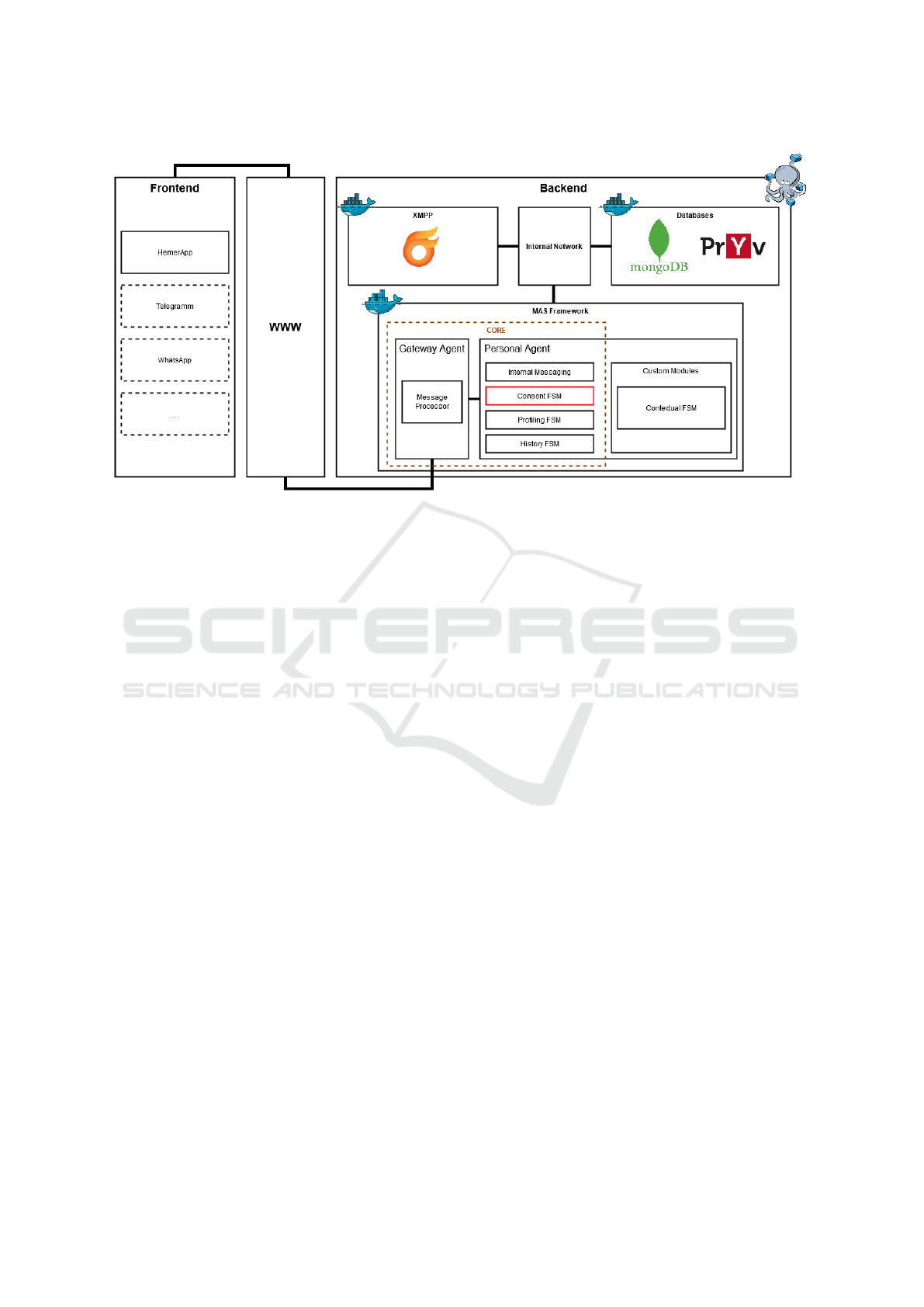
Figure 1: Architecture of the EREBOTS Framework.
answers transition to the next state, while incorrect
ones prompt the user to review and provide a new
response. Once all consent sections are accepted, the
user’s consent is stored in Pryv. If consent is modified
or new segments are added or changed, they will be
presented to the user upon reconnecting. The user
must review and either accept or reject the updates. If
rejected, the chatbot stops operating.
Consent Verification. After consent is given, peri-
odic checks will be conducted to ensure the user’s
understanding remains (or is) accurate. To do so,
throughout the system use, users will be prompted
with questions to verify their understanding of the
consented terms. If any misunderstandings are de-
tected, relevant information will be provided to re-
solve them. Once clarified, users can modify or re-
confirm their consent. The consent validation pro-
cess includes multiple question types: (i) True/False,
to verify correctness; (ii) numerical answers, such as
time periods; and (iii) open-ended responses, such as
specific terms. Future work will address the timing
and frequency of questions, as well as which ones to
prompt and when to re-prompt given users or cate-
gories of users.
4 DISCUSSION & CONCLUSIONS
This paper presents a dynamic approach to consent
management that addresses key challenges, including
mitigating the information overload inherent in tradi-
tional models, verifying the actual understanding of
consent, and reinforcing correct understanding over
time. Central to the proposed system is a stepwise
approach to presenting consent, which breaks down
complex and monolithic terms into smaller, more di-
gestible sections. By segmenting the consent process
into manageable parts, users are more likely to under-
stand the information they are consenting to. Addi-
tionally, alongside a straightforward presentation of
the terms (to comply with legal requirements), the
system guides users step-by-step and offers simplified
explanations, examples, and aggregated information
for those less familiar with formal terminology. This
approach reduces the risk of misunderstanding or un-
intentional, uninformed consent.
The proposed system is integrated within the
EREBOTS v3.0 framework, a modular, agent-based
platform that allows for flexible adaptation to dy-
namic consent management needs. By default, this
system adheres to GDPR-compliant standards, ensur-
ing that privacy is maintained throughout the process.
The modularity of the system facilitates not only the
development and configuration of the consent com-
munication process but also enables personalized ver-
ification of users’ understanding of consent. This is
achieved through periodic follow-up questions, rein-
forcing comprehension and ensuring that consent is
granted only after users fully understand its implica-
tions.
A key feature of this model is its balance between
offering users adequate time to review the consent
process and avoiding information overload. By break-
ing consent into smaller, digestible steps and incor-
porating regular checks on user understanding, the
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
698
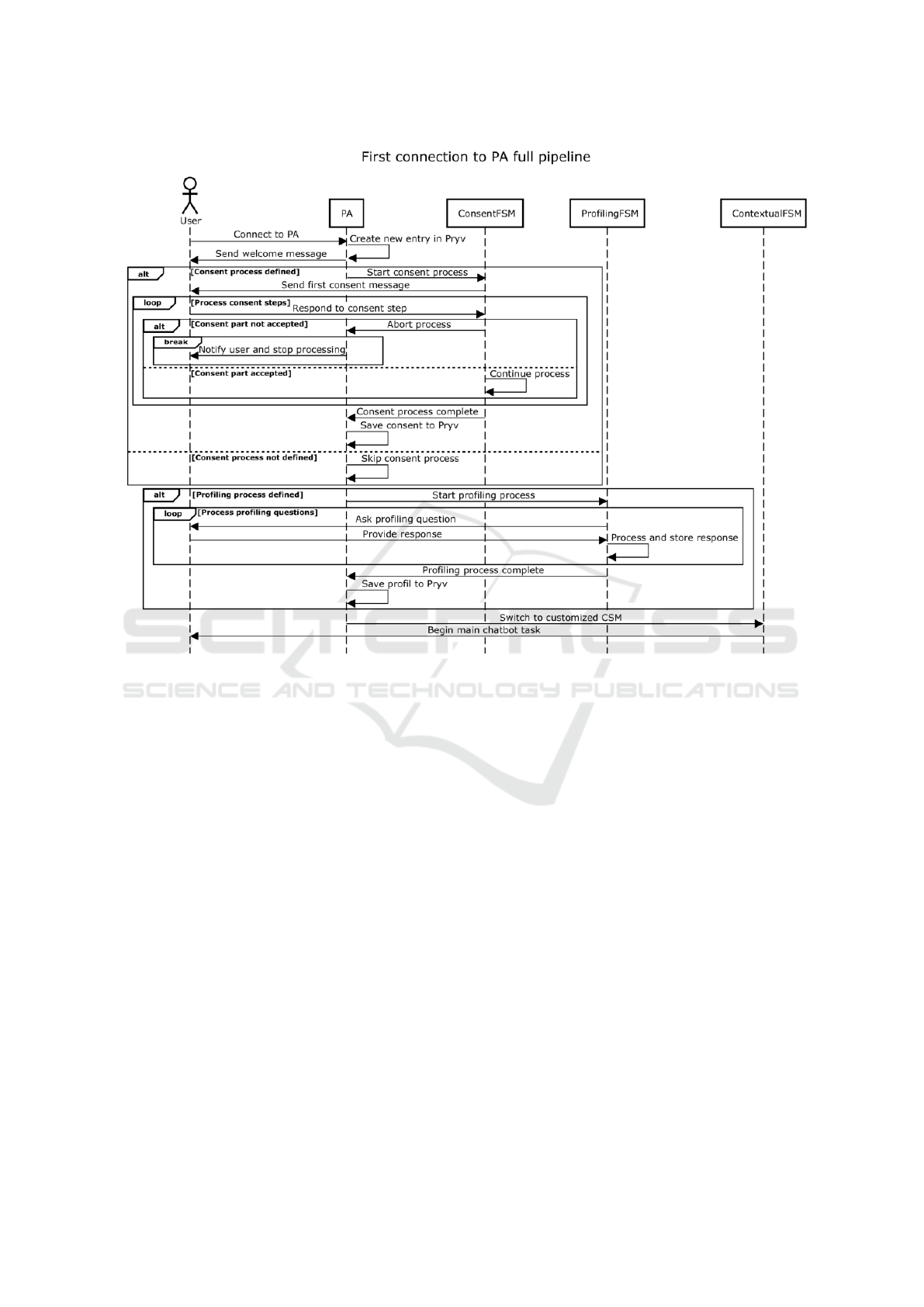
Figure 2: Sequence Diagram of the Pipeline Flow.
system significantly reduces the risk of uninformed
consent, a common issue in many existing systems.
This iterative review process enables the early iden-
tification of gaps in understanding, preventing misin-
terpretations before they lead to uninformed decision-
making.
Despite its advantages, the use of AI-based con-
sent management raises concerns regarding potential
persuasive techniques that could influence user de-
cisions. It is critical to ensure that the iterative na-
ture of the consent process does not inadvertently co-
erce users into accepting terms they do not fully com-
prehend. Maintaining the integrity of informed con-
sent requires that the process remains free from undue
influence. To address this, the system incorporates
transparent, well-defined mechanisms that track both
the information provided and the manner in which it is
delivered. This transparency ensures that the system
is subject to rigorous oversight, reducing the trust gap
that might arise in the absence of human-human inter-
actions (such as a doctor explaining terms in simpler
language). Furthermore, the system enhances human-
human interactions by mitigating the risk of coercive
behaviors—often difficult to detect and prove in tra-
ditional settings. In this way, the AI system offers a
more efficient means of ensuring the reliability and le-
gitimacy of consent, promoting ethical standards and
user autonomy.
In summary, the proposed dynamic consent man-
agement system, with its step-by-step design and con-
tinuous verification of understanding, holds promise
for enhancing both user trust and the security of the
consent process. The system’s modular architecture
enables a flexible and adaptable approach that pre-
vents cognitive overload while supporting informed
decision-making. This approach not only addresses
the limitations of traditional consent systems, particu-
larly in complex environments such as healthcare, but
also complies with legal and ethical standards, ensur-
ing that consent is fully informed and free from coer-
cion.
The development of this system within the ERE-
BOTS v3.0 framework represents a significant ad-
vancement in consent management. The interactive,
user-centered design, coupled with continuous com-
prehension checks, provides a comprehensive solu-
tion to the challenges posed by conventional consent
systems. Future work will focus on refining the indi-
Consent Understanding and Verification for Personalized Assistive Systems
699

vidual components of the consent process in collabo-
ration with legal AI experts, creating testable consent-
related questions and examples, and conducting initial
trials in the healthcare sector as part of a European
project. These trials will offer valuable insights into
the system’s real-world effectiveness and its potential
for broader adoption.
ACKNOWLEDGEMENTS
Partially supported by CHIST-ERA project EXPEC-
TATION (EU grant CHIST-ERA-19-XAI-005 and na-
tional grant 20CH21
195530).
REFERENCES
Albanese, G., Calbimonte, J.-P., Schumacher, M., and Cal-
varesi, D. (2020). Dynamic consent management for
clinical trials via private blockchain technology. Jour-
nal of ambient intelligence and humanized computing,
11(11):4909–4926.
Allen, J. W., Earp, B. D., Koplin, J., and Wilkinson, D.
(2024). Consent-gpt: is it ethical to delegate procedu-
ral consent to conversational ai? Journal of Medical
Ethics, 50(2):77–83.
Bester, J., Horsburgh, C., and Kodish, E. (2016). The lim-
its of informed consent for an overwhelmed patient:
Clinicians’ role in protecting patients and preventing
overwhelm. AMA journal of ethics, 18:869–886.
Budin-Ljøsne, I., Teare, H. J. A., Kaye, J., Beck, S.,
Bentzen, H. B., Caenazzo, L., Collett, C., D’Abramo,
F., Felzmann, H., Finlay, T., Javaid, M. K., Jones, E.,
Kati
´
c, V., Simpson, A., and Mascalzoni, D. (2017).
Dynamic consent: a potential solution to some of
the challenges of modern biomedical research. BMC
Medical Ethics, 18(1):4.
Buzcu, B., Pannatier, Y., Aydo
˘
gan, R., Ignaz Schumacher,
M., Calbimonte, J.-P., and Calvaresi, D. (2024). A
framework for explainable multi-purpose virtual as-
sistants: A nutrition-focused case study. In Inter-
national Workshop on Explainable, Transparent Au-
tonomous Agents and Multi-Agent Systems, pages 58–
78. Springer.
Corbett, C. F., Combs, E. M., Chandarana, P. S., Stringfel-
low, I., Worthy, K., Nguyen, T., Wright, P. J.,
and O’Kane, J. M. (2021). Medication adher-
ence reminder system for virtual home assistants:
Mixed methods evaluation study. JMIR Form Res,
5(7):e27327.
Cruz Casados, J., Cervantes L
´
opez, M. J., and Gil Herrera,
R. d. J. (2024). Promoting healthy eating habits via
intelligent virtual assistants, improving monitoring by
nutritional specialists: State of the art. In Xie, X.,
Styles, I., Powathil, G., and Ceccarelli, M., editors,
Artificial Intelligence in Healthcare, pages 170–184,
Cham. Springer Nature Switzerland.
Cusack, N. M., Venkatraman, P. D., Raza, U., and Faisal, A.
(2024). Review—smart wearable sensors for health
and lifestyle monitoring: Commercial and emerging
solutions. ECS Sensors Plus, 3(1):017001.
Delany, C. M. (2005). Respecting patient autonomy
and obtaining their informed consent: ethical the-
ory—missing in action. Physiotherapy, 91(4):197–
203.
Eguia, H., S
´
anchez-Bocanegra, C. L., Vinciarelli, F.,
Alvarez-Lopez, F., and Saig
´
ı-Rubi
´
o, F. (2024). Clin-
ical decision support and natural language processing
in medicine: Systematic literature review. J Med In-
ternet Res, 26:e55315.
European Commission (2018). How should my consent be
requested? Accessed: 2024-12-13.
European Parliament and European Council (2016). Gen-
eral data protection regulation (gdpr), regulation (eu)
2016/679 of the european parliament and of the coun-
cil of 27 april 2016 on the protection of natural per-
sons with regard to the processing of personal data and
on the free movement of such data, and repealing di-
rective 95/46/ec (2016).
Falagas, M. E., Korbila, I. P., Giannopoulou, K. P., Kondilis,
B. K., and Peppas, G. (2009). Informed consent: how
much and what do patients understand? The American
Journal of Surgery, 198(3):420–435.
Fang, C. M., Danry, V., Whitmore, N., Bao, A., Hutchison,
A., Pierce, C., and Maes, P. (2024). Physiollm: Sup-
porting personalized health insights with wearables
and large language models.
Gesualdo, F., Daverio, M., Palazzani, L., Dimitriou, D.,
Diez-Domingo, J., Fons-Martinez, J., Jackson, S., Vi-
gnally, P., Rizzo, C., and Tozzi, A. E. (2021). Digital
tools in the informed consent process: a systematic
review. BMC Medical Ethics, 22(1):18.
Harish, D., Kumar, A., and Singh, A. (2015). Patient auton-
omy and informed consent: the core of modern day
ethical medical. Journal of Indian Academy of Foren-
sic Medicine, 37(4):410–414.
Kaye, J., Whitley, E., Lund, D., Morrison, M., Teare, H.,
and Melham, K. (2014). Dynamic consent: A patient
interface for twenty-first century research networks.
European journal of human genetics : EJHG, 23.
Khalid, M. I., Ahmed, M., Helfert, M., and Kim, J. (2023a).
Privacy-first paradigm for dynamic consent manage-
ment systems: Empowering data subjects through
decentralized data controllers and privacy-preserving
techniques. Electronics, 12(24).
Khalid, M. I., Ahmed, M., and Kim, J. (2023b). Enhanc-
ing data protection in dynamic consent management
systems: Formalizing privacy and security definitions
with differential privacy, decentralization, and zero-
knowledge proofs. Sensors, 23(17).
Ma, Y., Achiche, S., Tu, G., Vicente, S., Lessard, D., Engler,
K., Lemire, B., Laymouna, M., Pokomandy, A., Cox,
J., and Lebouche, B. (2024). The first ai-based chatbot
to promote hiv self-management: A mixed methods
usability study. HIV Medicine, pages n/a–n/a.
Manson, N. C. and O’Neill, O. (2007). Rethinking informed
consent in bioethics. Cambridge University Press.
ICAART 2025 - 17th International Conference on Agents and Artificial Intelligence
700

Pietrzykowski, T. and Smilowska, K. (2021). The real-
ity of informed consent: empirical studies on patient
comprehension—systematic review. Trials, 22(1):57.
Robol, M., Breaux, T. D., Paja, E., and Giorgini, P. (2022).
Consent verification monitoring.
Schermer, B. W., Custers, B., and van der Hof, S. (2014).
The crisis of consent: how stronger legal protection
may lead to weaker consent in data protection. Ethics
and Information Technology, 16(2):171–182.
Seely, K. D., Higgs, J. A., Butts, L., Roe, J. M., Merrill,
C. B., Zapata, I., and Nigh, A. (2022). The “teach-
back” method improves surgical informed consent and
shared decision-making: a proof of concept study. Pa-
tient Safety in Surgery, 16(1):33.
Sezgin, E., Militello, L. K., Huang, Y., and Lin, S. (2020).
A scoping review of patient-facing, behavioral health
interventions with voice assistant technology target-
ing self-management and healthy lifestyle behaviors.
Translational Behavioral Medicine, 10(3):606–628.
Wang, D., Wang, L., Zhang, Z., Wang, D., Zhu, H., Gao,
Y., Fan, X., and Tian, F. (2021). “brilliant ai doctor”
in rural clinics: Challenges in ai-powered clinical de-
cision support system deployment. In Proceedings of
the 2021 CHI Conference on Human Factors in Com-
puting Systems, CHI ’21, New York, NY, USA. Asso-
ciation for Computing Machinery.
Wangmo, T., Lipps, M., Kressig, R. W., and Ienca, M.
(2019). Ethical concerns with the use of intelligent
assistive technology: findings from a qualitative study
with professional stakeholders. BMC Medical Ethics,
20(1):98.
Wisgalla, A. and Hasford, J. (2022). Four reasons why too
many informed consents to clinical research are in-
valid: a critical analysis of current practices. BMJ
Open, 12(3).
Xiao, Z., Li, T. W., Karahalios, K., and Sundaram, H.
(2023). Inform the uninformed: Improving online in-
formed consent reading with an ai-powered chatbot.
In Proceedings of the 2023 CHI Conference on Hu-
man Factors in Computing Systems, CHI ’23, page
1–17. ACM.
Consent Understanding and Verification for Personalized Assistive Systems
701
