
LOINC Mapping Experiences in Italy: The Case of Friuli-Venezia
Giulia Region
Maria Teresa Chiaravalloti
1 a
, Grazia Serratore
1 b
, Fabio Del Ben
2 c
and Agostino Steffan
2 d
1
Institute of Informatics and Telematics, National Research Council, Rende (CS), Italy
2
CRO Aviano National Cancer Institute IRCCS, Aviano (PN), Italy
Keywords: LOINC, Mapping, Coding System, EHR, Semantic Interoperability.
Abstract: Interoperability in healthcare requires accurate data exchange and interpretation across systems, making
standard terminologies essential for achieving semantic interoperability. This paper presents the approach
adopted by the Friuli Venezia Giulia Region in Italy to implement LOINC, the most widely used standardized
coding system for laboratory tests, into the electronic Laboratory Reports of five hospitals. Mapping was
conducted manually by physicians using RELMA, supported by training and guidance from LOINC Italy
experts. The validation process involved a dual-review procedure to ensure semantic accuracy but also to face
issues, such as implicit or incorrect information in local catalogues and the complexity of some specialties.
Collaboration among clinical staff, LOINC experts, and IT professionals proved essential in overcoming these
issues. As a result, over 7,000 local tests were mapped to LOINC, and 675 new codes for unrepresented
concepts were requested, thus creating a regional LOINC knowledge base. This experience highlights the
importance of training, support, and integrated management in adopting LOINC, as these elements are crucial
for a standardization process that enhances data traceability, minimizes errors, and supports semantic
interoperability. Additionally, this experience could be an example for other healthcare systems aiming to
standardize laboratory tests and achieve meaningful data exchange.
1 INTRODUCTION
Interoperability is defined as the ability of different
information and communications technology systems
and software applications to communicate, exchange
data consistently and reuse the information that has
been exchanged. In the clinical context,
interoperability enables the correct interpretation of
data across systems, allowing healthcare
professionals, patients, and other actors to understand
and act on health-related information and knowledge,
even across linguistic and cultural barriers (European
Commission: Directorate-General for the Information
Society and Media, 2009; Iroju et al., 2013).
Clinical data interoperability is a non-trivial issue
as it consists of technical, technological and semantic
interoperability. It is not sufficient to have an
information system or to adopt shared
a
https://orcid.org/0000-0003-4695-2026
b
https://orcid.org/0009-0009-9481-2213
c
https://orcid.org/0000-0002-1880-0669
d
https://orcid.org/0000-0002-6320-3054
communication protocols, but it is necessary that the
meaning of what is exchanged is not ambiguous so
that it can be understood and above all reused. This
translates into a single word: semantic
interoperability. To this aim, the implementation of
standardized terminologies is critical for effective
knowledge management in healthcare domain.
The use of specialized vocabularies and
terminologies addresses the challenges posed by the
lexical complexity and the high level of specificity of
the “medical jargon”(Gotlieb et al., 2022). Medical
standardized terminologies not only facilitate the
seamless sharing of information among different
healthcare institutions, but also ensure that intended
meanings are preserved throughout the entire clinical
workflow, eliminating ambiguity, controlling
synonyms or equivalents, and establishing explicit
semantic relationships. These systems serve as
Chiaravalloti, M. T., Serratore, G., Del Ben, F. and Steffan, A.
LOINC Mapping Experiences in Italy: The Case of Friuli-Venezia Giulia Region.
DOI: 10.5220/0013380800003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 2: HEALTHINF, pages 959-967
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
959

semantic roadmap, providing a shared framework for
both information specialists and users to navigate and
interpret data consistently (Tudhope et al., 2006). The
increasingly extensive use of Electronic Health
Record (EHR) systems requires full semantic
interoperability in order to achieve and pursue the
objective of a comprehensive and reliable record of
an individual’s health history (Aminpour et al., 2014).
In Italy, the Fascicolo Sanitario Elettronico (FSE),
which is the conceptual equivalent of the EHR, was
enacted with the Legislative Decree No. 179/2012. It
is based on a national federated and interoperable
technological infrastructure, which supports patient’s
access to healthcare services throughout the country,
by facilitating the exchange of clinical documents and
data among healthcare providers and patients.
Subsequently, the Prime Minister Decree No.
178/2015 regulated further aspects of the FSE, such
as the data structure of some types of clinical
documents. It then raised the question regarding the
use of classification and coding systems to
standardize and represent health and social-health
data in the clinical documents of the FSE, in order to
ensure, eventually recurring to transcoding, semantic
interoperability at regional, national and international
level. Specifically, the Technical Specifications
attached to the Decree No. 178/2015 indicate the
coding systems to be used within the FSE (Cardillo et
al., 2016), including LOINC (Logical Observation
Identifiers Names and Codes) for laboratory tests
encoding into the Laboratory Report document type.
LOINC is a clinical terminology and the first
universal pre-coordinated code system for laboratory
test names, measurements, and observations (Forrey et
al., 1996). LOINC has been developed by the
Regenstrief Institute (RI) as an open standard and made
available at no cost worldwide. In addition to the
LOINC database, the RI also develops and distributes
a mapping tool called the Regenstrief LOINC Mapping
Assistant (RELMA). This tool facilitates research
through the LOINC database and assists during the
mapping operations between local tests and LOINC
codes. Today LOINC is increasingly widespread all
over the world, de facto becoming the reference
standard for these medical concepts. It is currently used
in more than 196 countries and translated into 15
languages and 20 linguistic variants (consult
https://loinc.org/international/ for continuous updates
on these numbers).
To address the local peculiarities of different
countries, LOINC International has recognized a
network of national partners around the world
(Vreeman et al., 2012). As the LOINC purpose is to
be integrated with local systems and not to substitute
them, it was necessary to collaborate with local
partners responsible for the translation of the standard
and its implementation in their respective national
contexts. Over time, central coordination has revealed
essential for having a common reference point to
address questions, support users, maintain
relationships with governmental bodies and third
parties, keep updated the standard and consider
international updates and challenges in the domain.
This role in Italy is played by the Institute of
Informatics and Telematics of the National Research
Council (IIT-CNR), which established the LOINC
Italy working group, recognized as the official partner
for Italy through a Memorandum of Understanding
signed with the RI in 2014.
LOINC Italy’s activities include biannual updates
to the Italian translation of the LOINC database,
translation of the LOINC Users’ Guide into Italian,
development of tutorials, provision of training
courses, and mapping validation services.
Additionally, an online helpdesk is offered on LOINC
Italy website (www.loinc.it) for information requests
and inquiries, along with the management of new
LOINC codes submissions as needed.
This paper aims to present the approach chosen by
the Friuli-Venezia Giulia Region for the
implementation of LOINC codes into the electronic
Laboratory Reports and, specifically, the mapping
process underway in five large hospitals in the
Region, highlighting the strengths and weaknesses of
this experience and drawing lessons from it to
systematize this practice.
2 LOINC MAPPING
Standardizing laboratory test requires using unique
identifiers for each concept and clinical investigation
to ensure consistent information exchange among
laboratories. For effective semantic interoperability,
each laboratory test needs to have a distinct
representation of its specificities. Mapping local
laboratory catalogues to LOINC deals with finding
semantic equivalence of the clinical meaning of each
test and assigning to it a unique code. The structure of
each LOINC code is composed of six fundamental
axes, which represent the pieces of information
needed to detail the performed test with high level of
granularity and specificity.
Nonetheless, mapping local terminologies to
LOINC presents significant challenges, because local
test names are idiosyncratic, often full of acronyms
and abbreviations, and not always explicit with all the
information necessary to uniquely identify the test.
HEALTHINF 2025 - 18th International Conference on Health Informatics
960

This makes them understandable within the
laboratory or hospital that created them but
ambiguous outside them. The name alone is not
sufficient to fully understand the examination
performed, as information such as the execution
method and the reporting unit of measurement are
essential to distinguish its clinical meaning from
others that may appear similar. At the same time, not
everything labeled in a different way necessarily
corresponds to substantively different tests. For
example, the concept “level of glycosylated
haemoglobin in blood” might appear as “HbA1C” in
some systems, while others might refer to it as
“Haemoglobin A1C” or “Glycohemoglobin”
(Parcero et al., 2013). Therefore, making all the
characteristics of a test explicit helps to quickly
identify the correct LOINC code to map.
Additionally, this reduce misinterpretations that can
impact also the laboratory workflow, from the pre-
analytical phase, through the analytical phase, to the
post-analytical phase (Yusof & Arifin, 2016).
Mapping local catalogues to LOINC helps to reduce
these errors because of the need to remove ambiguity
and provide a clear and consistent way to identify
laboratory tests.
Implementing a robust LOINC mapping requires
substantial planning, focused execution, and ongoing
maintenance to keep it updated with the biannual
releases of the standard. Even if it could not be easy
to introduce in realities with already consolidated
functioning, this standardization process is vital for
enabling meaningful data exchange. By adopting a
common reference terminology, hospitals can ensure
that identical tests are recognized consistently,
reducing errors and misinterpretations, and enhancing
communication among healthcare providers.
After the entry into force of the aforementioned
Prime Minister Decree No. 178/2015, there have been
several regional initiatives and those of individual
hospital structures that have chosen different
approaches to the implementation of LOINC in
Laboratory Reports, requesting or not the support of
LOINC Italy. Even if this initiative aims to facilitate
interoperability, improve patient care, and streamline
data exchange among the laboratories, the lack of
national coordination on the use of coding systems in
clinical documents has caused fragmentation in the
development and implementation of solutions that
ensure efficient management of these systems.
The Friuli-Venezia Giulia Region decided to
approach the mapping process starting from the
laboratories of five large hospitals: CRO Aviano
National Cancer Institute IRCCS of Aviano (PN), the
Burlo children’s hospital of Trieste, and the three-city
hospital of Pordenone (ASFO), Udine (ASUFC) and
Trieste (ASUGI). The work was coordinated by the
in-house company, named Insiel, which manages all
the health informatics process of the Region. The
“mapping team” was composed by informaticians,
MDs from CRO, and LOINC Italia experts. This has
allowed different professionals gathered around the
same table, who have contributed their expertise and
their point of view to achieving a complex objective,
in which the cooperation of IT, medical and specialist
skills on the standard is essential to achieve an
effective and efficient result. Preliminary meetings
were held to analyze the situation and plan the work
phases, as well as numerous meetings to monitor the
progress of the activities.
Despite laboratories working with the same
Laboratory Information System (LIS), they don’t
share the same tests catalogue. This means that
laboratories could perform the same test but call it
differently. Since carrying out a preliminary
reconciliation of the local catalogues was deemed
inconvenient for several reasons, the consequent
decision was to map each hospital’s catalogue to
LOINC, although aware that this would have
necessarily implied the duplication of mapping
efforts on some tests. On the other hand, as a long-
term objective, this approach would have allowed us
to align hospitals’ catalogues by reconciling multiple
names for the same test and allowing to differentiate
identical names that actually conceal clinically
different tests in practice.
Considering the tests catalogues of the five
mentioned hospitals, the total amount of tests to map to
LOINC was 10,619. In accordance with Insiel, we
decided to consider single tests only, and to postpone
the panel’s mapping. In LOINC a panel is a common
name for groups of single tests that are usually ordered
and/or reported together. In Italy it is also called
multiple or battery. It could be more challenging
because mapping a panel code means there must be
matching in their respective child elements.
3 METHODS
The methodology defined for the mapping process
involved several structured phases aimed at ensuring
full semantic correspondence between local tests and
LOINC codes and an effective validation process.
In January 2023, LOINC Italy experts delivered a
comprehensive six-hour webinar to the MDs from the
five involved laboratories, focusing on LOINC,
RELMA and the mapping process. The education
session was followed by a training session aimed to
LOINC Mapping Experiences in Italy: The Case of Friuli-Venezia Giulia Region
961

familiarize laboratory staff with the LOINC coding
system. After that, from February to June 2023,
LOINC Italy experts provided dedicated mapping
assistance on-site at each laboratory. This phase was
crucial in facilitating hands-on support as laboratory
MDs began to implement the mapping process.
Throughout the training process, experts from
LOINC Italy collaborated with laboratory teams to
address any challenges and provide guidance tailored
to their specific contexts and needs. The local
laboratory catalogues were divided according to the
different clinical specialties so the mapping could
have been performed by the MD competent for the
specific sector. This is a very important aspect, as
local test catalogues contain a lot of implicit
information, and sometimes even the information
present is not always correct. This makes it clear that
mapping is not purely an IT matter, as specific
domain expertise is required. RELMA was used as a
tool to support the mapping of local laboratory
catalogues to LOINC.
Starting from July 2023, the laboratories have sent
their locally mapped test catalogues to LOINC Italy,
and the experts have started the third phase of the
activity, which is the validation of the mappings. This
phase includes a dual review process: at first, LOINC
Italy experts verify the mappings based on the
information in the local catalogues. When there are
questionable mappings, they highlight the test in red
and indicate the reason for not validating it. If there
are tests that cannot be mapped because there is no
LOINC code that represents them in a semantically
equivalent way, they submit a request for the creation
of a new LOINC code to LOINC International;
subsequently, mapping MDs are involved for a direct
discussion on questionable mapping cases in order to
find together the right LOINC code or to model a new
code submission. The involvement of clinical domain
experts ensures that the terminology used aligns with
medical practices and ensure that the test performed
is correctly and semantically identified. On the other
hand, LOINC Italy experts are responsible for
verifying that there is conceptual correspondence
between source and target codes and that the
modelling of requests for new codes should be done
according to the formalisms of the standard.
Once the mappings have been validated, it was
then possible to compare them through the chosen
LOINC code to detect any inconsistencies.
At the conclusion of this experience, it was
considered essential to gather feedback from the
clinical users involved in the mapping process. To this
end, one of the participating physicians was asked to
evaluate the experience with the RELMA software and
to provide his insights into the application of the
LOINC standard. This included identifying any
challenges encountered, suggesting areas for
improvement, and highlighting potential benefits.
Additionally, the physician was invited to offer his
perspective as a clinical expert on the
representativeness of LOINC codes across different
laboratory specialties, as well as his thoughts on
potential future applications of the standard and how
the results achieved in this work could be expanded
and reused.
4 RESULTS
The total amount of tests mapped to LOINC is
10,619: 1,013 are from CRO; 2,615 are from ASFO;
3,670 are from ASUFC; 1,693 are from ASUGI, and
1,628 are from Burlo children hospital. The first three
have already completed mapping the tests from their
local catalogues to LOINC, while for the remaining
two, the work is still in progress. Below the results of
the mappings realized by CRO, ASFO, and ASUFC
are presented, in particular describing percentages of
correct mappings, submissions to LOINC for
requesting new codes, and tests identified as non-
mappable because they are either obsolete or only
used for internal calculations and therefore not
reportable into the Laboratory Reports.
CRO mapped 1,013 test codes, belonging to
clinical pathology, clinical biochemistry and clinical
and experimental oncohematology. The mapping was
performed by 1 MD, who collected the necessary
information from his colleagues. For 865 of the tests, it
was possible to identify an existing LOINC code, even
after several clarification meetings between LOINC
Italia experts and the MD. The tests for which it was
not possible to identify an existing LOINC code
amounted to 94. LOINC Italy started the submission
process to request the creation of new LOINC codes to
semantically represent them. This process has a median
processing time of approximately 45 days. The created
terms are then published in the subsequent LOINC
release; however, once developed, they can be viewed
on the pre-release term webpage
(https://loinc.org/prerelease/). Furthermore, the
mapping process enabled the MD to identify
inconsistencies in the catalogue, consisting of 5 tests
non-mappable because they are either not
representative of unique results or used for internal
calculations that do not generate reportable outcomes,
and 54 tests that are no longer performed. So mapping
was also the chance to clean up the local catalogue.
Figure 1 shows the percentage distribution of
HEALTHINF 2025 - 18th International Conference on Health Informatics
962
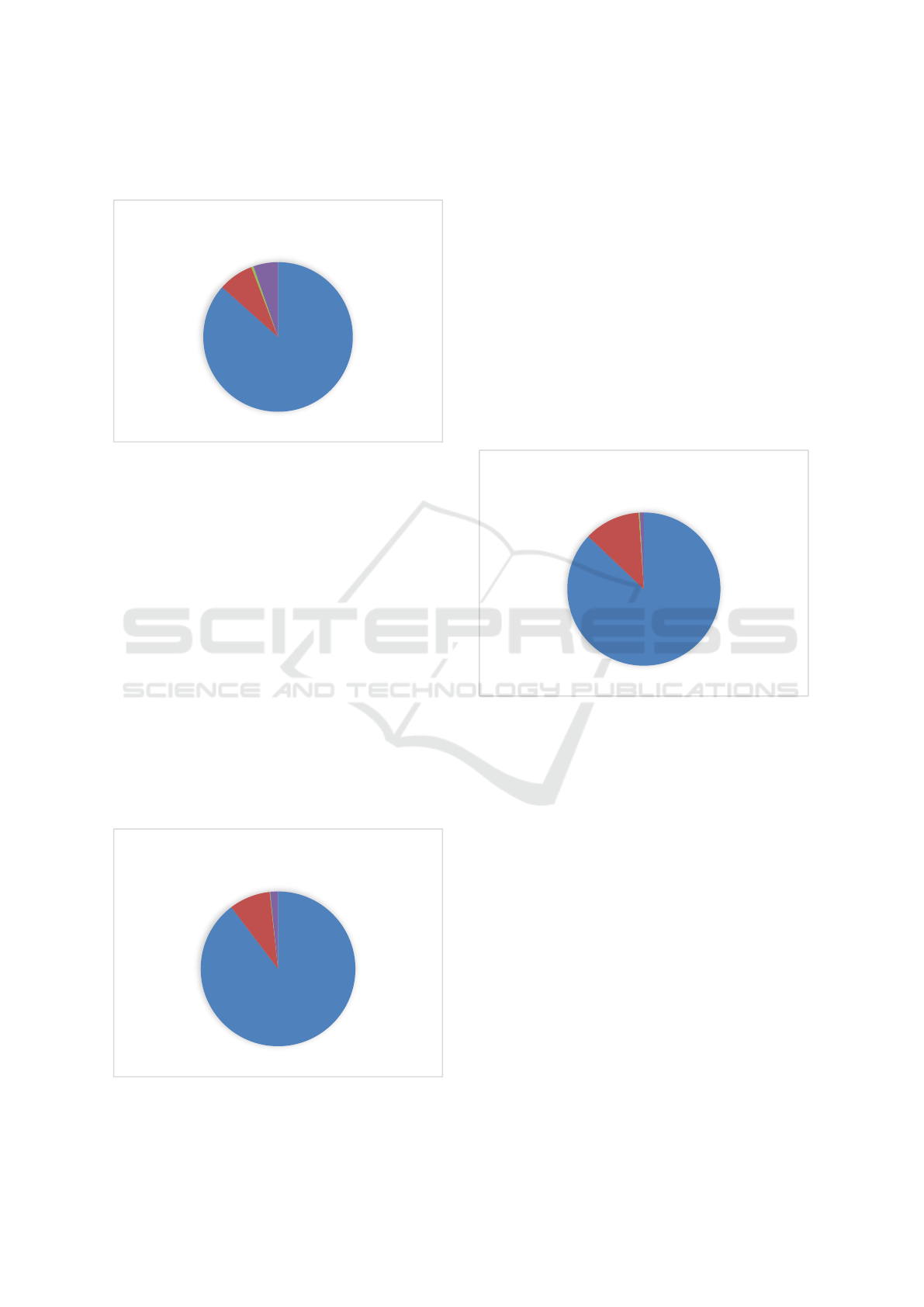
mappings completed by CRO across the described
categories.
Figure 1: The percentage distribution of mappings
completed by CRO according to the described categories.
In the ASFO hospital the mapping process
covered a total of 2,615 test codes. The local
catalogue was divided among 4 MDs, according to
the laboratory specialties of their specific expertise.
The tests belong to the following sectors: allergy,
autoimmunity, bacteriology, biochemistry,
hematology, endocrinology, gastroenterology, HLA,
injury markers, nephrology, POCT, serology,
toxicology, and virology. Overall, the tests mapped to
a LOINC code amount to 2,113; those for which a
submission process has been initiated to request the
creation of a specific LOINC code are 228; 3 have
been classified as non-mappable for the same reasons
stated for the CRO; while 43 refers to tests no longer
performed. Figure 2 shows the percentage
distribution of mappings completed by ASFO across
the categories described.
Figure 2: The percentage distribution of mappings
completed by ASFO according to the described categories.
ASUFC mapped a total of 3,670 codes from
multiple laboratory sectors, such as allergy,
chemistry, autoimmunity, molecular biology,
coagulation, electrophoresis, hematology,
toxicology, gastroenterology, inhibition,
cerebrospinal fluid, cardiac markers, injury markers,
microbiology, hormones, POCT, urine,
uroporphyrins, and virology. The mapping was
performed by 2 MDs, who gathered necessary
information from other laboratory specialties’ MDs.
Of them, 3,347 tests were mapped to an existing
LOINC code; 283 were formally modeled to request
a new LOINC code; 9 are non-mappable codes and
31 no longer realized. Figure 3 shows the percentage
distribution of mappings completed by ASUFC
across the categories described.
Figure 3: The percentage distribution of mappings
completed by ASUFC according to the described
categories.
4.1 Mapping Peculiarities
In this paragraph, we would like to present some
peculiarities observed during the validation of
mappings. First and foremost, it is important to
specify that the effort required to verify the
correctness of the semantic association between the
source code and the target code is not uniform across
all laboratory specialties. There are, in fact, highly
structured and consolidated sectors, either because
they consist of common and recently defined tests,
such as clinical chemistry, or because they are
internally standardized, such as allergology. On the
other hand, there are specialties characterized by
continuous and rapid evolution, where new tests are
frequently formalized, such as genetics, as well as
sectors with recognized intrinsic complexity, such as
microbiology.
Mapped
90%
Submissions
8%
No more realized
2%
ASFO
Total number of tests performed: 2,653
Mapped
87%
Submissions
12%
No more realized
1%
ASUFC
Total number of tests performed: 3,670
Mapped
87%
Submissions
8%
Issues
0%
No more realized
5%
CRO OF AVIANO (PN)
Total number of tests performed: 1,013
LOINC Mapping Experiences in Italy: The Case of Friuli-Venezia Giulia Region
963
In Allergology, the use of Allergen International
Codes as synonyms for the Latin name of the allergen
reported in the LOINC component helps quickly
identify the correct code to map. Nonetheless, even if
international codes are used to identify allergens, it
was necessary to pay close attention to the test
description. For example, the sole label "sunflower"
in the tests “w204 sunflower serum” and “k84
sunflower serum” is not sufficient to distinguish
between tests on pollen or seeds. However, thanks to
the presence of the international codes w204 and k84,
it was possible to assign the correct LOINC code to
each test. Always verifying the correct semantic
interpretation of the test remains crucial to identifying
the most accurate LOINC code. For instance, in the
case of the local test “t45 North American elm serum”
the international code was misleading because it
corresponds to another species of elm, namely Ulmus
Crassifolia. In this case, it was necessary to consult
the competent physician to clarify whether the
international code or the allergen name had been
incorrectly indicated.
In multiple cases we found idiosyncratic local test
names to describe substantially the same test. For
example, the LOINC code 1756-6 Albumin in
CSF/Albumin in Serum or Plasma was assigned to
both the tests named “Barrier Permeability of CSF”
and “Albumin Quotient of CSF”. This is actually the
reason why going to international standards such as
LOINC is so crucial, and it shows how the correct
interpretation of the test semantics is the only way to
identify the most accurate LOINC code. In other
cases, the level of granularity required by LOINC in
the test description, compared to the lack of
descriptive detail in local test catalogues, makes the
mapping validation difficult, as much of the
information is implicit. This often leads also the
physician to map to a more general LOINC code,
even though a more specific one would exist.
Representativeness and granularity issues emerged
mainly for the System axis of virology and
bacteriology tests. For example, the tendency is to use
LOINC codes with System Respiratory system
specimen.lower even if specifying it in Bronchial or
BAL would be possible. In the case of the ASFO
hospital, 41 new LOINC codes with BAL in the
System axis were requested. It was necessary to
ensure an accurate representation of the test
performed. The guiding principle is to always request
new codes if they need to disambiguate and uniquely
identify a test with a greater level of detail.
4.2 New LOINC Codes Submissions
The validation of the mappings realized by the five
hospitals inevitably required requesting the LOINC
Committee to create new LOINC codes for concepts
that were not represented by the existing ones. These
are not always tests recently introduced in the
scientific reference domain; sometimes, they are
requests to narrow the scope of an existing code,
while other times, they are tests specific to a context
different from the North American operational
setting. New LOINC code submissions require a
thorough understanding of the standard's formalism,
as the local test must be "translated" into the six
fundamental LOINC axes, potentially providing
supporting documentation necessary to better
understand what is effectively tested. For this reason,
the submission process is always carried out by
LOINC Italy.
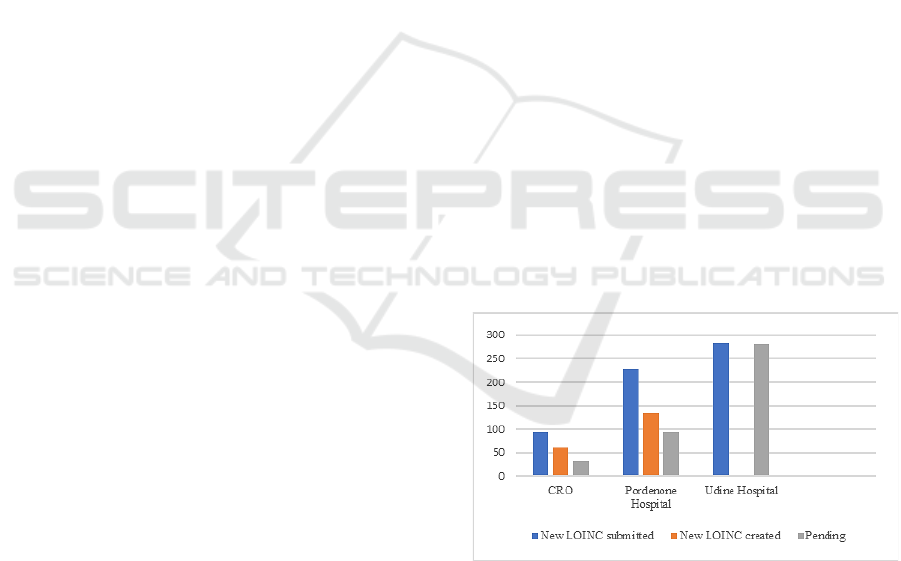
The chart in Figure 4 shows the distribution of
submissions across the CRO, ASFO, and ASUFC
hospitals, for a total of 605 submissions, specifying
those that have already led to the creation of new
LOINC codes (194 out of 605) and those that are still
under review of the LOINC content developers (411
out of 605). The process of creating a new LOINC
code does not stop at the submission, as interactions
with LOINC content developers are often necessary
to precisely identify the clinical meaning of the test
and the semantics to be conveyed through the six
LOINC axes.
Figure 4: New LOINC codes submissions in the CRO,
ASFO and ASUFC hospitals.
Regarding the laboratory specialties for which the
highest number of new codes have been requested,
also considering the observations presented in the
previous paragraph, it is not surprising that the
highest number of new LOINC codes submissions
came from virology and bacteriology, respectively
with 83 and 137 submissions.
HEALTHINF 2025 - 18th International Conference on Health Informatics
964

4.3 User Experience
Since we believe that user experience is not a
marginal aspect in the implementation of the LOINC
standard, we asked one of the doctors who actively
participated in the mappings to share his impressions
regarding this activity. He started considering the
challenges posed by the complex and often non-
intuitive structure of the LOINC lexicon but
recognizing that before engaging in any meaningful
mapping task, it is firstly necessary that users
familiarize themselves with the six fundamental
LOINC axes, which serve as the foundation for
understanding the LOINC coding structure. Without
this knowledge, navigating the code system becomes
significantly more difficult, making the mapping
process less effective and efficient. About RELMA
he highlights that it has its own complexity because
of its relatively unfamiliar user interface, although
acknowledging that uploading local databases,
mapping, and exporting results is relatively
straightforward. Challenges lie in mastering the
technical language, understanding the user interface,
and filtering algorithms used by the software.
According to his mapping experience, finding the
semantically correspondent LOINC code depends
heavily on the precision of the search queries. If user
does not get any result, criteria used to filter results in
the “search limits” should be considered as they can
drastically alter the outcomes. This highlights the
importance of a deeper understanding of search
algorithms, a skill set not typically possessed by
medical professionals. As a result, non-technical
users may struggle to achieve the most accurate
mappings without additional training or support. The
high level of granularity in the description of tests
often multiplies the descriptive strings, and therefore,
even when the user performs a search using a single
term and expects a direct result, he/she has to deal
with multiple strings. In these cases, users have to
consider factors such as the ranking of results or the
number of institutions that have chosen a particular
code, opting for what appears to be the most
commonly accepted option. This, however, does not
eliminate the need for LOINC expert validation,
particularly in areas where there is a high degree of
ambiguity. Furthermore, users operating in
unfamiliar domains are often required to consult with
domain experts. This adds both time and complexity
to the task, increasing the potential for human error.
However, he is keen to point out that there are not
only negative aspects and that in fact once users have
mastered these technical aspects, the mapping process
tends to progress smoothly and efficiently. As
familiarity with the LOINC terminology and the
RELMA software grows, the system reveals its
strengths, particularly in its ability to filter results
effectively based on well-constructed queries.
Additionally, the use of standard units of
measurement significantly aids in narrowing down
the search results, ensuring greater and faster
accuracy in the mapping process. This functionality
proves particularly valuable in more established
domains, where consistency in test specifications
allows for quicker and more reliable mappings.
About representativeness, he noticed that LOINC
offers a robust and well-structured framework for
mature fields such as clinical chemistry, while newer
areas like molecular diagnostics are not yet as well-
represented. In these fields, LOINC codes may be
missing or lack the granularity required for detailed
mapping, indicating that the code system has not fully
caught up with advancements in these scientific areas
yet.
In conclusion, he thinks that LOINC holds
considerable promises for facilitating cross-national
interpretation of laboratory results, especially as the
number of mapped local catalogues increases. In the
recently launched European Health Data Space
LOINC could be instrumental in harmonizing inter-
laboratory data across borders, enhancing the
interoperability of health data. Moreover, LOINC
codes can contribute to the development of artificial
intelligence (AI) and machine learning models by
providing a standardized framework for similar tests,
thus bypassing the need for manual annotation and
transcoding. This still relies on the availability of tests
correctly mapped to LOINC through human effort or
at least validated by a human expert. However, the
main critical challenge he foresees might stem from
the lack of specificity regarding the method, as it is
the only axis with optional specification. This, in fact,
could make tests based on different methodologies
appear equivalent. This could introduce significant
variability, potentially skewing AI models.
5 DISCUSSIONS
Programming and implementing the mapping of
laboratory tests from three hospitals in the Friuli
Venezia Giulia region to the LOINC standard codes
enabled the analyses described in the previous
paragraph but also allowed for some reflections on
the mapping work in general. The mapping of local
laboratory catalogues to LOINC is an onerous but
essential process for achieving standardization.
Despite its complexity, efficient planning and
LOINC Mapping Experiences in Italy: The Case of Friuli-Venezia Giulia Region
965

programming can significantly reduce the workload,
ensuring that resources are utilized effectively. It is
crucial to place the right competencies in the right
place at the right time, relying on both domain experts
and LOINC specialists to ensure accurate mappings.
Anticipating the most frequently asked question
from doctors, we always recommend paying attention
to “false friend mappings”, that is the risk of relying
on mappings performed by other laboratories without
carefully reviewing all the descriptive parameters of
the tests before fully adopting their mappings. Tests
that may appear similar in name can differ in context
or clinical specificity and should therefore be mapped
differently. Conversely, not everything labeled
differently necessarily represents fundamentally
different tests. Explicitly stating the values
corresponding to the six fundamental LOINC axes is
the only way to uniquely identify a test.
As a result of what has been explained so far
emerges that it is not feasible to adopt a systematic
method for mapping the local catalogues of different
hospitals to LOINC. A deep understanding of the
information being represented at a semantic level is
essential and, even when two tests appear similar,
careful consideration is needed to distinguish
between them. Additionally, it is not only the naming
of the tests that matters, but also the way the results
are reported. This includes whether the findings are
presented as a laboratory report in natural language or
as evidence based on a specific scale. The way the
tests are documented significantly influences the
choice of the correct LOINC code. Therefore, it is not
possible to apply a uniform method across all
hospitals, as factors such as the form of reporting and
the specific context of each test must be taken into
account when selecting the appropriate LOINC code.
Additionally, if the search for a LOINC code to
map does not yield any results, one should not
immediately resort to requesting the creation of a new
code through submission, especially when dealing
with well-known tests. Often, the appropriate LOINC
code already exists and simply needs careful
identification, for example trying to search with
synonyms or to better focus on the core of the analyte.
Finally, conducting a reverse check of the
mappings at the end of the work is essential. This step
helps in identifying potential errors, such as incorrect
mappings, overlaps between local codes, and
duplications. By implementing this review, the
overall accuracy and consistency of the mapping are
greatly improved. The Friuli Venezia Giulia’s in-
house company was thus able to achieve a general
reorganization of the catalogues of the hospitals
involved, ensuring consistency, particularly in the
two (CRO and ASFO) that share the same test
catalogue. Additionally, it was possible to identify
codes that appeared to represent single tests but
produced in the Laboratory report a series of results
corresponding to multiple observable values, thus
effectively functioning as panel codes.
Finally, it was possible to draft a sort of ranking
of mapping based on difficulty, identifying the
specialties from which it would be advisable to start,
as they are simpler, e.g. allergology because of the
use of Allergen international codes to quickly identify
the right LOINC component to map to; clinical
chemistry is also among the sectors that can be easily
mapped, as the tests have been consolidated for a long
time and are well-structured in the values of the six
fundamental LOINC axes.
6 CONCLUSION AND FUTURE
WORK
This paper describes the approach chosen by the
Friuli Venezia Giulia Region in Italy to map
laboratory catalogues of five big hospitals to LOINC.
The mapping was manually performed by medical
doctors using RELMA. Overall, over 7,000 local tests
have been mapped to LOINC and the creation of 675
new LOINC codes was requested to represent
concepts not included in the standard. Of these, 194
have already been created and are part of the official
LOINC releases. Thus, a sort of regional LOINC
knowledge base has been created.
Mapping local terms to a standardized vocabulary
is not only a matter of interoperable informative
systems, but it requires a deep knowledge of both the
source and target terminology structures, i.e. the
organization of tests in the local catalogue, which
usually reflects not only a scientific criterion but also
a functional one, and the structuring of a coding
standard such as LOINC. It is a demanding task the
first time, but it becomes easy to maintain afterward,
and the advantages it offers in terms of data
traceability and semantic interoperability are
countless. In our work, it was necessary to find
solutions to the multiple issues encountered during
the mapping and it was possible to address them
through a continuous collaboration among the clinical
staff, the LOINC experts and the informaticians
involved in the activity. The high percentages of
correct mappings and the low percentages of not
identified matches demonstrate that training activities
and mapping support play a fundamental role in
understanding the right way to approach this
HEALTHINF 2025 - 18th International Conference on Health Informatics
966

standard. An integrated management of a medical
terminology cannot be able to leave all those aspects
out of consideration, as they all contribute to make
effective and efficient the use of a standardized
system.
All the experiences and specific cases
encountered so far will serve in the future as a
valuable knowledge base for improvements and
efficiencies of the mapping process, potentially
streamlining and accelerating the mapping process
itself and enabling work in an AI-driven environment.
Future work prospects include the need to
complete the validation of mappings carried out by
the two remaining hospitals out of the five (ASUGI
and Burlo Children’s Hospital), covering a total of
3,321 local tests; finalize all pending submissions of
new LOINC terms; and, most importantly, in
collaboration with all stakeholders define
maintenance policy for the mappings performed and
establish procedures for mapping new tests that will
be introduced in the catalogues of the five hospitals
involved.
REFERENCES
Aminpour, F., Sadoughi, F., & Ahamdi, M. (2014).
Utilization of open source electronic health record
around the world: A systematic review. Journal of
Research in Medical Sciences: The Official Journal of
Isfahan University of Medical Sciences, 19(1), 57–64.
Cardillo, E., Chiaravalloti, M. T., & Pasceri, E. (2016).
Healthcare Terminology Management and Integration
in Italy: Where we are and What we need for Semantic
Interoperability. European Journal for Biomedical
Informatics, 12(01).
https://doi.org/10.24105/ejbi.2016.12.1.14
European Commission: Directorate-General for the
Information Society and Media. (2009). Semantic
interoperability for better health and safer healthcare:
Deployment and research roadmap for Europe.
Publications Office of the European Union.
https://data.europa.eu/doi/10.2759/38514
Forrey, A. W., McDonald, C. J., DeMoor, G., Huff, S. M.,
Leavelle, D., Leland, D., Fiers, T., Charles, L., Griffin,
B., Stalling, F., Tullis, A., Hutchins, K., & Baenziger,
J. (1996). Logical observation identifier names and
codes (LOINC) database: A public use set of codes and
names for electronic reporting of clinical laboratory test
results. Clinical Chemistry, 42(1), 81–90.
https://doi.org/10.1093/clinchem/42.1.81
Gotlieb, R., Praska, C., Hendrickson, M. A., Marmet, J.,
Charpentier, V., Hause, E., Allen, K. A., Lunos, S., &
Pitt, M. B. (2022). Accuracy in Patient Understanding
of Common Medical Phrases. JAMA Network Open,
5(11).
https://doi.org/10.1001/jamanetworkopen.2022.42972
Iroju, O., Soriyan, A., Gambo, I., & Olaleke, J. (2013).
Interoperability in Healthcare: Benefits, Challenges
and Resolutions. 3(1).
Parcero, E., Maldonado, J. A., Marco, L., Robles, M.,
Bérez, V., Más, T., & Rodríguez, M. (2013). Automatic
Mapping tool of local laboratory terminologies to
LOINC. Proceedings of the 26th IEEE International
Symposium on Computer-Based Medical Systems, 409–
412. https://doi.org/10.1109/CBMS.2013.6627828
Tudhope, D., Koch, T., & Heery, R. (2006). Terminology
Services and Technology.
Vreeman, D. J., Chiaravalloti, M. T., Hook, J., &
McDonald, C. J. (2012). Enabling international
adoption of LOINC through translation. Journal of
Biomedical Informatics, 45(4), 667–673.
https://doi.org/10.1016/j.jbi.2012.01.005
Yusof, M. M., & Arifin, A. (2016). Towards an evaluation
framework for Laboratory Information Systems.
Journal of Infection and Public Health, 9(6), 766–773.
https://doi.org/10.1016/j.jiph.2016.08.014
LOINC Mapping Experiences in Italy: The Case of Friuli-Venezia Giulia Region
967
