
Multimorbidity in Heart Failure Patients: Application of Machine
Learning Algorithms to Predict Imminent Health Outcomes
Jorge Cerejo
1,2 a
, Rui Lopes Baeta
2 b
, Sim
˜
ao Gonc¸alves
1 c
, Bernardo Neves
1,3 d
,
Pedro Morais Sarmento
3 e
, Jos
´
e Maria Moreira
1 f
, Nuno Andr
´
e da Silva
1 g
, Francisca Leite
1 h
,
Bruno Martins
2 i
and M
´
ario J. Silva
2 j
1
Hospital da Luz Learning Health, Luz Sa
´
ude, Lisboa, Portugal
2
INESC-ID, Instituto Superior T
´
ecnico, Universidade de Lisboa, Lisboa, Portugal
3
Hospital da Luz Lisboa, Luz Sa
´
ude, Lisboa, Portugal
fi
{bernardo.neves, psarmento}@hospitaldaluz.pt, {jose.maria.moreira, nuno.asilva, francisca.leite}@luzsaude.pt,
Keywords:
Multimorbidity, Heart Failure, Laboratory Tests, Health Outcomes Prediction.
Abstract:
As populations age and life expectancy increases, multimorbidity, which is the simultaneous presence of two
or more chronic conditions, has become increasingly common, especially among older adults. Heart failure,
a widespread and heterogeneous syndrome, has sparked research into multimorbidity to deepen our under-
standing of its pathophysiology and improve clinical management approaches. This paper offers a detailed
characterization of a heart failure patient cohort, utilizing clinical data from a Portuguese tertiary hospital.
Based on this characterization, we developed a clinical tool for identification of high-risk patients and pre-
diction of imminent hospital admissions based on laboratory tests. Our models for predicting imminent hos-
pitalization showed reasonable effectiveness (AUROC of 0.79 with lab test prescriptions and 0.72 with lab
test results). These findings emphasize the significant predictive value of laboratory tests in the context of
HF. Additionally, we investigated the explainability of our models using SHAP values, in collaboration with
clinical experts, providing insights into factors influencing the models’ predictions. These results highlight the
importance of secondary clinical data analysis assisting healthcare professionals in identifying patients at high
risk of adverse events, and improving patient care and outcomes.
1 INTRODUCTION
As life expectancy increases and populations age,
multimorbidity, defined as the presence of two or
more chronic conditions, has become increasingly
prevalent in healthcare systems worldwide (WHO,
2016). Multimorbidity poses significant challenges
for patients, clinicians, and healthcare systems, un-
a
https://orcid.org/0009-0004-5221-4964
b
https://orcid.org/0009-0006-8350-8302
c
https://orcid.org/0009-0004-6565-8599
d
https://orcid.org/0000-0002-1559-7482
e
https://orcid.org/0000-0002-5970-2707
f
https://orcid.org/0000-0003-2420-7930
g
https://orcid.org/0000-0003-4216-2107
h
https://orcid.org/0000-0003-2550-2616
i
https://orcid.org/0000-0002-3856-2936
j
https://orcid.org/0000-0002-5452-6185
derscoring the urgent need for innovative tools to bet-
ter understand and improve clinical outcomes (Maj-
nari
´
c et al., 2021). In Portugal, the prevalence of mul-
timorbidity is especially high, affecting 78.3% of the
elderly population (aged 65 and older) and 38.3% of
those between the ages of 24 and 75 (Rodrigues et al.,
2018; Romana et al., 2019).
Heart Failure (HF), a clinical syndrome charac-
terized by structural and/or functional cardiac abnor-
malities, is particularly associated with multimorbid-
ity (Bozkurt et al., 2021). Among HF patients, 86%
suffer from at least two chronic conditions, and 42%
live with five or more (Chamberlain et al., 2015). The
coexistence of conditions complicates treatment de-
cisions and increases the risk of adverse outcomes,
emphasizing the need for improved risk stratification
methods (Navickas et al., 2016).
Electronic Health Records (EHRs) offer a valu-
able opportunity for advanced multimorbidity char-
330
Cerejo, J., Baeta, R. L., Gonçalves, S., Neves, B., Sarmento, P. M., Moreira, J. M., da Silva, N. A., Leite, F., Martins, B. and Silva, M. J.
Multimorbidity in Heart Failure Patients: Application of Machine Learning Algorithms to Predict Imminent Health Outcomes.
DOI: 10.5220/0013381800003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 2: HEALTHINF, pages 330-339
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

acterization and enhanced patient outcome predic-
tions (Williams et al., 2022). Prior studies suggest
that the secondary use of EHR data can improve care
quality, reduce medical errors, and generate cost sav-
ings (CMS, 2021). However, translating this data into
actionable insights remains a critical challenge.
In this work, we focus on the development of a
predictive tool designed to forecast imminent hospi-
talizations among HF patients with multimorbidity
using pseudonymized EHR data from a Portuguese
tertiary hospital. As part of the broader Intelligent-
Care project, this study aims to identify high-risk pa-
tients and support clinicians in making timely and in-
formed decisions in the emergency department (ED).
We introduce the ICIHO (IntelligentCare Imminent
Health Outcomes) predictive tool, which leverages
classification algorithms to predict imminent health
outcomes (IHO) using routine laboratory test results
collected during ED visits. By utilizing observational
data, the ICIHO tool enhances the early identification
of HF patients at heightened risk for hospital admis-
sion, thereby facilitating personalized treatment plan-
ning and potentially improving patient outcomes.
The paper is organized as follows: Section 2 pro-
vides an overview of key concepts and related work
relevant to the study. Section 3 describes the method-
ology used for data processing and prediction model-
ing. The results are presented in Section 4, followed
by a discussion in Section 5. Finally, Section 6 offers
concluding remarks and highlights the clinical impli-
cations of the study.
2 RELATED WORK
EHRs serve as a rich source of patient data, enabling
applications in learning healthcare systems and preci-
sion medicine (Aronson and Rehm, 2015). However,
analyzing EHR data presents significant challenges,
including missing data, inaccuracies, and data hetero-
geneity (Hripcsak et al., 2011). To address these is-
sues, data standardization initiatives like the Observa-
tional Medical Outcomes Partnership (OMOP) Com-
mon Data Model (CDM) have emerged, providing
a framework for uniform data extraction and analy-
sis (Sciences and Informatics(OHDSI), 2023). The
OMOP CDM facilitates global data sharing for com-
parative longitudinal studies, making it an essential
tool for analyzing complex patient data (Dixon et al.,
2020; Liyanage et al., 2018).
Laboratory tests are essential in healthcare, serv-
ing roles in diagnosis, monitoring, screening, and re-
search. They play a crucial role in reducing diag-
nostic errors and facilitating informed clinical deci-
sions (Wians, 2009; Plebani and Lippi, 2016). Ab-
normal test values are often early indicators of ad-
verse events, such as increased morbidity and mortal-
ity (Asadollahi et al., 2007). Despite their critical im-
portance, analyzing laboratory data is challenging due
to its inherent heterogeneity. To ensure consistency
and improve interoperability, the healthcare industry
employs the LOINC (Logical Observation Identifiers,
Names, and Codes) system, which standardizes the
identification and representation of laboratory mea-
surements (Loinc® Indianapolis, IN: Regenstrief In-
stitute, Inc, ).
In recent years, the use of supervised machine
learning (ML) models to predict adverse clinical out-
comes from EHR data has grown substantially (Lee
et al., 2020; Nwanosike et al., 2022). Logistic Re-
gression (LR) models remain widely used due to their
simplicity, interpretability, and effectiveness in pre-
dicting key outcomes such as mortality and hospi-
tal admissions, thereby influencing clinical decisions
and improving healthcare delivery (Alanazi, 2022).
Meanwhile, more advanced techniques, such as deep
learning models, have gained prominence for their
ability to handle large datasets and extract complex
patterns (Shamout et al., 2020).
An important application of ML in healthcare is
the use of laboratory data to predict IHO. For ex-
ample, Loekito et al. developed a multivariate LR
model utilizing 30 laboratory variables to predict
IHOs (Loekito et al., 2013). Their model demon-
strated its effectiveness by accurately identifying key
outcomes such as Medical Emergency Team (MET)
calls (AUROC = 0.69), ICU admissions (AUROC
= 0.82), and in-hospital mortality (AUROC = 0.90).
Similarly, Mueller et al. employed a comparable ap-
proach to predict in-hospital mortality, achieving high
performance (AUROC = 0.88). Another model in-
tegrating both demographic and laboratory data also
demonstrated strong predictive ability for hospitaliza-
tions (AUROC = 0.80) (Mueller et al., 2021).
3 MATERIAL AND METHODS
Using a combination of data mining and ML tech-
niques, we developed a pipeline for characteriza-
tion of HF patterns with multimorbidity from EHRs.
The pipeline enables the stratification of HF patients
based on their risk of adverse events, such as immi-
nent (≤ 24h) hospitalizations. With the developed
pipeline, we processed the clinical records of Hos-
pital da Luz Lisboa (HLL), an institution that pro-
vides comprehensive medical services across all med-
ical specialties.
Multimorbidity in Heart Failure Patients: Application of Machine Learning Algorithms to Predict Imminent Health Outcomes
331
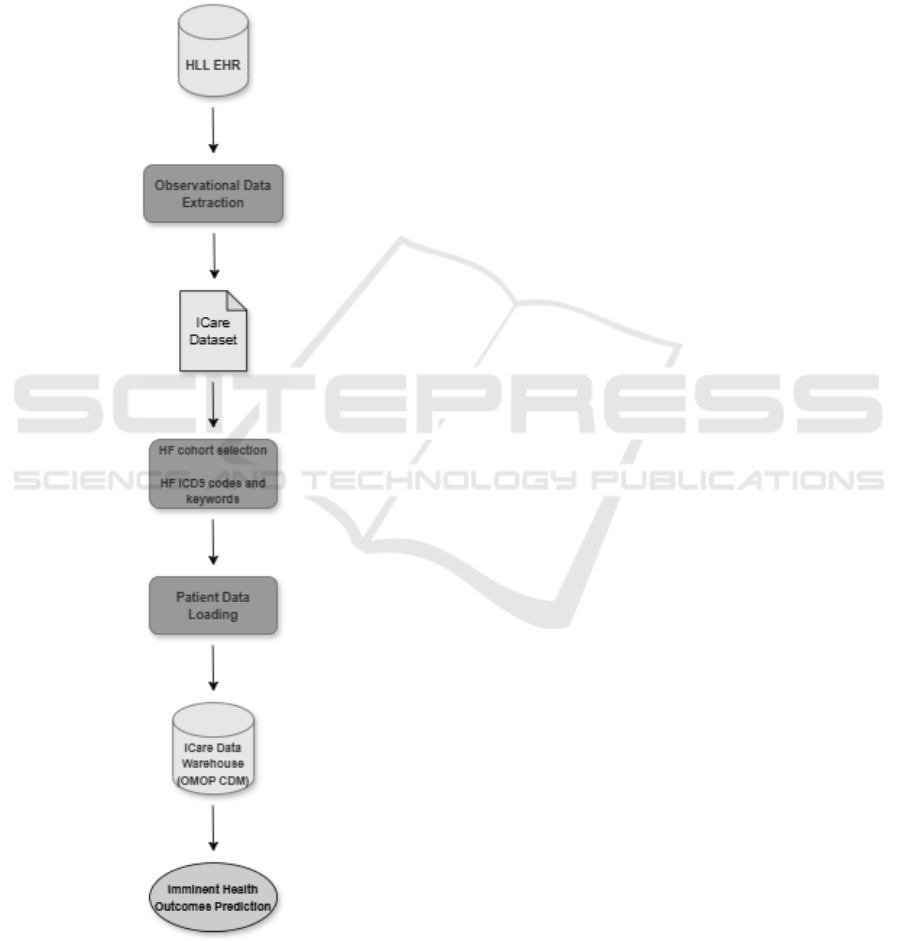
Figure 1 provides a visual overview of the devel-
oped workflow, which processes the clinical data in
four main stages:
1. Observational Data Extraction
2. HF Cohort Selection
3. Patient Data Loading
4. Imminent Health Outcomes Prediction
Figure 1: Overview of the developed workflow.
3.1 Observational Data Extraction
We utilized the IntelligentCare (ICare) dataset
from Hospital da Luz Lisboa (HLL), containing
anonymized medical histories of 834,529 patients,
spanning January 2007 to August 2021. This dataset
was approved by the hospital’s Institutional Review
Board (IRB) for research on multimorbidity. Data
included patient visits, diagnoses, and laboratory re-
sults, essential for predicting imminent health out-
comes (IHO).
3.2 HF and Comorbidities Phenotyping
We utilized a locally validated phenotyping algorithm
that combined ICD-9 codes and HF-related keywords
in clinical text, as detailed in a prior publication by
our research group (Martins et al., 2024). Comorbidi-
ties were selected based on their prevalence and clin-
ical significance, guided by clinical expertise. The
phenotyping rules applied included checks for both
ICD-9 codes and relevant clinical text to ensure accu-
rate cohort identification.
3.3 Patient Data Loading
To ensure interoperability, we harmonized the ICare
dataset and uploaded it into an OMOP CDM (version
5.3) data warehouse using tools from the Observa-
tional Health Data Sciences and Informatics (OHDSI)
community. The data warehouse serves as the back-
bone of our analytical processes, enabling standard-
ized analysis through tools like White Rabbit for
ETL design (OHDSI, 2021), Athena for concept map-
ping (Athena, 2022), and Achilles for generating stan-
dard metrics (OHDSI, 2014).
We populated six OMOP CDM tables: Per-
son (demographics), Visit Occurrence (patient vis-
its), Condition Occurrence (diagnoses and comorbidi-
ties), Measurement (laboratory values), Death (mor-
tality), and Observation Period (timeline of observa-
tions). We extracted the medical histories of HF co-
hort patients, preserving the chronological order of di-
agnoses, lab tests, and hospital interactions.
Conditions coded using ICD-9 were translated to
standardized concept IDs via the Condition Relation-
ship table to ensure accurate mapping within the Con-
dition Occurrence table. Laboratory data required ex-
tensive preprocessing due to non-uniform coding. For
unmatched lab codes, we applied heuristics such as
removing the last digit for mapping LOINC standards.
Of the nearly 40 million lab records, 11.4% could not
be mapped and were excluded.
Lab results, which varied from quantitative to
HEALTHINF 2025 - 18th International Conference on Health Informatics
332

nominal or free-text entries, were harmonized using
regular expressions and keywords. Numerical data
were standardized, and categorical results (e.g., ”Pos-
itive”, ”POS”) were unified under consistent labels.
Finally, we mapped the processed data to OMOP
CDM fields and loaded them into the data ware-
house using Pentaho Data Integration (Hitachi Ven-
tara, ), completing the ETL process and enabling ro-
bust, standardized analysis for subsequent predictive
modeling.
3.4 Imminent Health Outcome
Prediction
We developed a methodology to predict imminent
hospitalizations of HF patients admitted to the ED us-
ing laboratory data from the ICare dataset. The pri-
mary aim was to evaluate the predictive power of lab
tests in forecasting IHO for HF patients. Only pa-
tients with at least one recorded laboratory test were
included in the analysis.
To predict imminent hospitalizations, we linked
laboratory measurements to subsequent clinical
episodes in each patient’s history. Clinical episodes,
defined as healthcare visits (e.g., hospitalizations, ED
visits, consultations), were recorded in the Visit Oc-
currence table. The algorithm identified the next clin-
ical episode following each lab measurement and cal-
culated the time difference. Episodes followed by
hospitalization within 24 hours were labeled as posi-
tive (1), while those leading to discharge were labeled
as negative (0).
We first trained a multivariate LR model using
binary indicators of lab test prescriptions (1 if pre-
scribed, 0 otherwise). This approach eliminated the
need for actual lab results, mitigating issues with
missing data. By applying the Chi-square test, we
identified statistically significant lab tests for pre-
dicting imminent hospitalizations. The transformed
dataset, in categorical format, enabled the LR model
to capture patterns in physician decision-making.
To evaluate the predictive value of actual lab test
results, we compared LR and neural networks (NN)
models. LR was chosen for interpretability, while
NNs were evaluated for potential performance im-
provements. Statistically significant lab tests were
used as features, and NT-proBNP, a critical biomarker
for HF management (Bozkurt et al., 2021), was im-
puted where missing. Class imbalance was addressed
through weight correction. Elastic Net regularization
prevented overfitting in the LR model, with hyper-
parameters tuned via 5-fold cross-validation (train-
ing set size = 80%), optimizing the F1-score. The
NN architecture consisted of two hidden layers with
four neurons each (Dervishi, 2020), trained using bi-
nary cross-entropy loss and stochastic gradient de-
scent with backpropagation.
To address the trade-off between the number of
features and the size of the training dataset, we de-
signed variations of LR and NN models with different
numbers of laboratory test features. Missing values in
the lab test results, which are not missing at random,
made imputation not recommended. Instead, rows
with missing values were excluded, meaning mod-
els with more features had smaller training datasets.
This approach aimed to evaluate how feature count
and training data size impacted model performance.
Model performance was assessed using balanced
accuracy, precision, recall, F1-score, AUROC, and
AUPRC (Han et al., 2012). Confidence intervals were
calculated using bootstrapping. To enhance inter-
pretability, we analyzed the LR model’s coefficients
using odds ratios (ORs) and SHAP (SHapley Addi-
tive exPlanations) values (Kasza and Wolfe, 2014;
Lundberg and Lee, 2017). We used SHAP summary
plots to visualize the overall importance of features
and SHAP force plots to analyze individual predic-
tions, offering insights into how specific lab results
contributed to imminent hospitalization risk (Lund-
berg et al., 2018).
To support real-time interpretation, we developed
the interactive ICIHO predictive tool
1
. This tool vi-
sualizes predicted hospitalization risk and highlights
the influence of key lab tests, enhancing clinicians’
ability to make informed decisions based on SHAP-
derived insights.
4 RESULTS
The study population included 3907 patients with HF
(53.4% women) with median age of 81 years (in-
terquartile range 72-88 years old) as depicted in Fig-
ures 2a and 2b. Comorbidities such as cardiovascular
conditions, CKD and Diabetes were highly prevalent
(Table 1).
We analyzed 3,407 patients for imminent outcome
prediction after excluding those without available lab-
oratory test data. These patients contributed to 46,922
distinct episodes, including 27,744 outpatient admis-
sions, 12,686 ED admissions, and 6,488 hospitaliza-
tions. From the ED admissions, 4,693 (37.0% of the
total amount of ED admissions) led to hospital admis-
sions within 24 hours, and were labeled as positives.
A total of 437,683 laboratory tests were conducted
during these ED visits, comprising 252 unique tests.
1
Available at ICIHO predictive tool
Multimorbidity in Heart Failure Patients: Application of Machine Learning Algorithms to Predict Imminent Health Outcomes
333
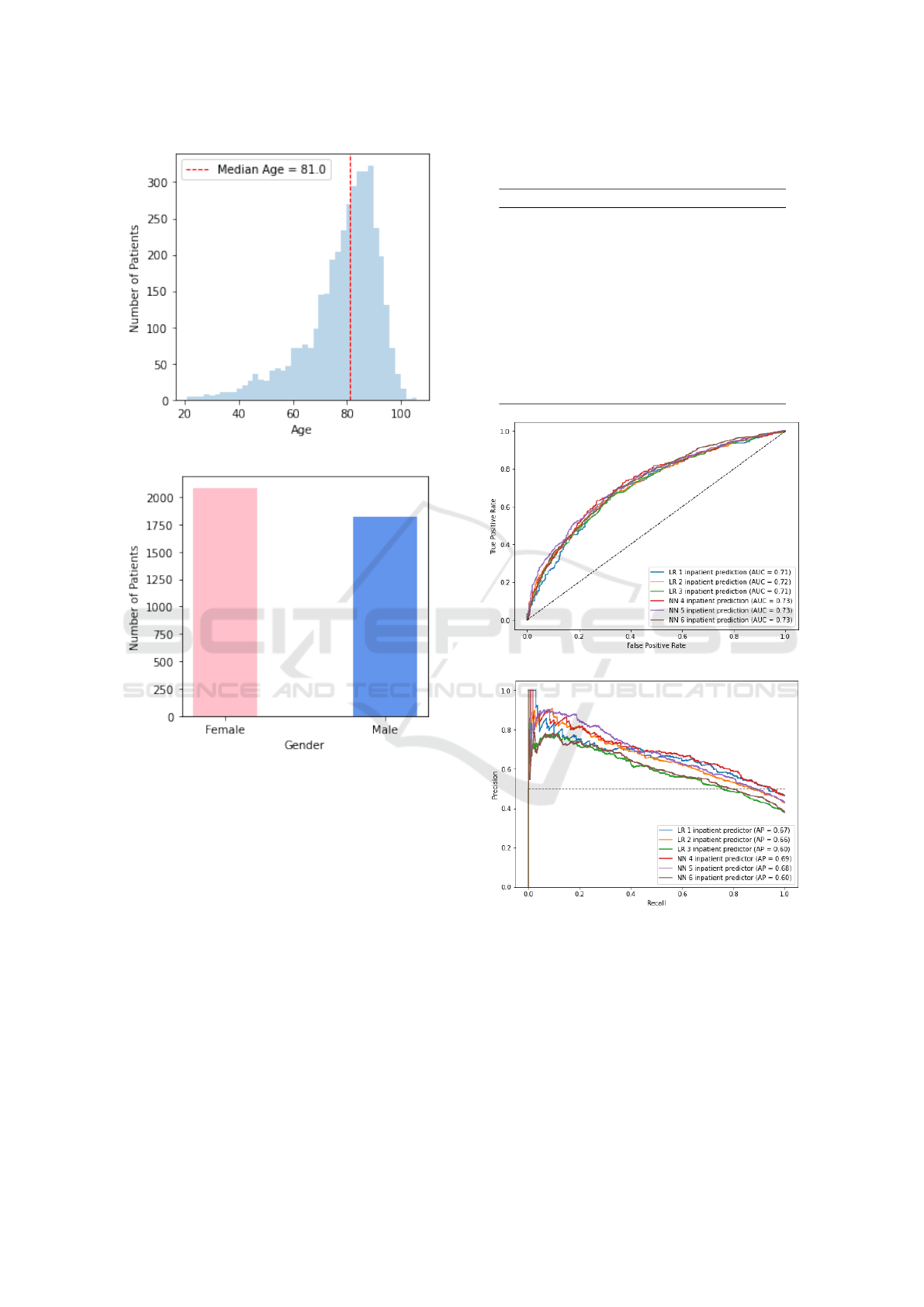
(a)
(b)
Figure 2: Gender and Age distributions of the HF cohort.
Our multivariate LR model for the prediction of
imminent hospitalization using only the lab test pre-
scriptions demonstrated a reasonable performance,
with a recall of 0.654, a precision of 0.616, and an
F1-score of 0.634, with an AUROC of 0.785 and an
AUPRC of 0.707. Models trained on lab test re-
sults demonstrated similar performance, summarized
in Table 2. A comparative analysis on model perfor-
mance based, on the ROC curve and precision-recall
curve, is provided in Figure 3. Overall, the NN mod-
els slightly outperformed the LR models. Models
that included more features, namely LR 1 and NN
4, achieved higher performance, while models with
fewer features, namely LR 3 and NN 6, exhibited a
decrease in performance. This may indicate a lower
significance power of these features in predicting im-
minent hospitalizations among patients admitted to
Table 1: Prevalence of the 10 most frequent chronic condi-
tions identified in the population of HF patients.
Condition Prevalence
1 Essential hypertension 56%
2 Atrial fibrillation 33%
3 Dyslipidemia 27%
4 Chronic kidney disease 24%
5
Ischemic congestive
cardiomyopathy
23%
6 Obesity 18%
7 Heart valve disorder 16%
8 Diabetes mellitus 14%
9 Allergic disposition 13%
10 Bacterial pneumonia 12%
(a)
(b)
Figure 3: Comparison of (a) ROC curves and (b) precision-
recall curves for the different models that were trained. All
models perform similarly, with AUROC ranging between
0.71 and 0.73 and AUPRC ranging between 0.60 and 0.69.
the ED. We selected the LR 2 model, which is high-
lighted in Table 2, as the optimal approach consider-
ing the trade-off between model complexity and per-
formance. This model demonstrated reasonable per-
formance, with AUROC and AUPRC values of 0.718
and 0.663, respectively.
HEALTHINF 2025 - 18th International Conference on Health Informatics
334

Table 2: Summary of the performance metrics computed to evaluate the models trained for predicting imminent hospitaliza-
tions of HF patients admitted to the ED. The model variations (LR 1, LR 2, LR 3, NN 1, NN 2, NN 3) evaluate the trade-off
between the number of features and the size of the training dataset.
Model LR 1 LR 2 LR 3 NN 4 NN 5 NN 6
Proportion 50% 75% 90% 50% 75% 90%
Nr. features 19 16 9 19 16 9
Nr. samples 3685 8599 12014 3685 8599 12014
Bal accuracy
[95% CI]
0.660
[0.627-0.692]
0.664
[0.640-0.688]
0.659
[0.640-0.679]
0.672
[0.638-0.704]
0.673
[0.652-0.697]
0.664
[0.646-0.685]
Precision
[95% CI]
0.643
[0.593-0.693]
0.610
[0.575-0.646]
0.555
[0.526-0.586]
0.643
[0.594-0.692]
0.619
[0.578-0.644]
0.558
[0.529-0.588]
Recall
[95% CI]
0.609
[0.556-0.658]
0.624
[0.589-0.659]
0.624
[0.591-0.654]
0.656
[0.610-0.704]
0.653
[0.630-0.698]
0.636
[0.606-0.668]
F1-score
[95% CI]
0.625
[0.584-0.667]
0.617
[0.575-0.646]
0.587
[0.561-0.613]
0.649
[0.610-0.690]
0.635
[0.608-0.664]
0.595
[0.569-0.620]
AUROC
[95% CI]
0.709
[0.673-0.743]
0.718
[0.693-0.743]
0.711
[0.691-0.733]
0.726
[0.690-0.761]
0.732
[0.708-0.756]
0.726
[0.706-0.747]
AUPRC
[95% CI]
0.668
[0.614-0.720]
0.663
[0.630-0.701]
0.596
[0.564-0.631]
0.691
[0.634-0.744]
0.682
[0.649-0.719]
0.605
[0.571-0.640]
The odds ratio values corresponding to the coeffi-
cients of model LR2 are displayed in Table 3. These
values reveal that C-reactive protein (CRP) and NT-
proBNP are the laboratory tests that most significantly
influence the prediction of imminent hospitalizations
of patients admitted to the ED. The laboratory tests of
Erythrocytes and Lymphocytes exhibited lower odds
values, indicating a negative contribution to imminent
hospitalizations. Despite age being considered a risk
factor for multimorbidity, its influence in predicting
imminent hospitalizations of HF patients is reduced.
Table 3 lists the P-values associated with each
coefficient of the multivariate model. Certain vari-
ables, such as Hematocrit, Creatinine, Sodium, and
Monocytes, seem to be statistically insignificant in
predicting imminent hospitalization in a multivariate
approach.
The SHAP summary plot shown in Figure 4a fur-
ther reinforces these findings. High values of CRP,
Urea, Leukocytes, and NT-proBNP are associated
with higher SHAP values, indicating their strong posi-
tive influence on the model’s predictions. Conversely,
low values of Erythrocytes are associated with higher
contributions to the prediction of imminent hospital-
izations.
Figure 4b showcases a SHAP force plot example,
illustrating the prediction of imminent hospitalization
of a HF patient from the test dataset who was cor-
rectly classified. The model identified that values of
Erythrocytes (789-8=2.16 counts10
9
/L), Leukocytes
(6690-2=16.22 counts10
9
/L), and CRP (1988-5=8.65
mg/dL) had a significant impact on predicting immi-
nent hospitalizations, as indicated by the wider red
bars in the plot.
5 DISCUSSION
We developed a framework for processing clinical
data to gain insights on the multimorbidity popula-
tion with HF, uncovering patterns and risks associ-
ated to this condition. The ability to utilize healthcare
data for better characterization of complex patients
and the development of clinical strategies represents
a step forward in the management of HF. Compared
to related works, our model achieves similar perfor-
mance while uniquely incorporating feature contribu-
tion analysis using odds ratios and SHAP. Addition-
ally, we developed a user-friendly web interface to
visualize predictions and feature impacts, supporting
clinical decision-making.
Firstly, our multivariate analysis, which focused
on lab test prescriptions for HF patients admitted to
the ED, enables early identification of patients at an
increased risk of imminent hospitalization. We be-
lieve that by exploring the rationale behind each lab
test prescription, we can partially reveal the intricate
clinical judgments and organizational factors influ-
encing these decisions. This approach opens up new
research avenues for clinical and operational improve-
ments in high-demand settings, such as the ED.
In addition, we have shown the practical utility
of commonly available laboratory test results in con-
ducting risk stratification to predict short-term hospi-
tal admissions for HF patients. These models were
proficient in making reasonably accurate predictions
of hospital admission. We are optimistic that integrat-
ing additional information like demographics, vital
signs, and diagnoses can further enhance the models’
discrimination capabilities. Using these data, clini-
Multimorbidity in Heart Failure Patients: Application of Machine Learning Algorithms to Predict Imminent Health Outcomes
335

Table 3: Imminent Hospitalization odds ratio for each coefficient of the prediction named model LR 2. The p-values for the
null hypothesis that a coefficient is equal to zero (i.e., the odds ratio is equal to one), were computed with a Wald test (Wald,
1943).
Variable Component odds ratio (P > |z|)
1988-5 C-reactive protein 1.4689 (< 0.05)
33762-6 NT-proBNP 1.2919 (< 0.05)
6690-2 Leukocytes 1.2359 (< 0.05)
22664-7 Urea 1.2339 (< 0.05)
788-0 Erythrocyte distribution width 1.1745 (< 0.05)
4544-3 Hematocrit 1.1118 (0.068)
Age Age 1.0597 (< 0.05)
2823-3 Potassium 1.0581 (< 0.05)
40248-7 Creatinineˆbaseline 1.0414 (0.353)
2951-2 Sodium 1.0110 (0.805)
5905-5 Monocytes/100 leukocytes 0.9764 (0.337)
785-6 Erythrocyte mean corpuscular hemoglobin 0.9037 (< 0.05)
713-8 Eosinophils/100 leukocytes 0.8829 (< 0.05)
2075-0 Chloride 0.8816 (< 0.05)
736-9 Lymphocytes/100 leukocytes 0.8463 (< 0.05)
789-8 Erythrocytes 0.7980 (< 0.05)
cians can more accurately gauge the need for hospital
admission of these patients, and hospital staff can ob-
tain early estimates of admission rates that can, for
instance, lead to improved efficiency in hospital bed
planning and resource allocation.
Our machine learning models prioritized inter-
pretability, thereby enhancing trust and clinical ap-
plicability. Rigorous evaluation using logistic re-
gression weights and SHAP values ensured transpar-
ent and practically relevant outcomes, vital for real-
world applications and future research (Lundberg and
Lee, 2017). This methodology permits detailed in-
terpretability, allowing clinicians to perform individ-
ualized patient risk assessments, significantly improv-
ing clinical utility. Our findings indicate that ele-
vated levels of NT-proBNP and CRP are positively
correlated with imminent hospital admission, consis-
tent with prior studies (Bozkurt et al., 2021; Anand
et al., 2005). NT-proBNP is a well-established marker
of HF severity, while the link between CRP and HF
prognosis, suggesting possible concurrent infections
or unaddressed inflammatory diseases, warrants fur-
ther investigation. Moreover, we identified a neg-
ative correlation between erythrocyte concentration
and patient outcomes, reinforcing existing evidence
of anemia’s adverse impact on HF prognoses, includ-
ing higher hospitalization rates (Anand et al., 2004).
Intriguingly, our analysis revealed that when labora-
tory data is included, age becomes a less significant
predictor of imminent hospitalization.
Furthermore, the study highlights the utility of the
SHAP force plot in assessing individual patient risks,
offering a detailed insight into the specific impacts of
features on model predictions. This analytical tool
increases the model’s clinical relevance by elucidat-
ing not just the direction but also the magnitude of a
feature’s impact on predictions of imminent hospital-
ization. Such precise feature-level interpretability is
invaluable for predicting heightened risks in scenar-
ios with interacting factors, rendering it a potent in-
strument for handling complex clinical scenarios like
multimorbidity.
This study also has limitations that warrant discus-
sion. A primary limitation is the analyzed data com-
ing from a single hospital, which may be the source of
biases associated with the diversity of diseases treated
and the complexity of healthcare delivery at this facil-
ity. Although the hospital provides a range of care ser-
vices, the lack of universal primary healthcare could
limit the scope of our analysis, thus restricting the
depth of insights into disease complexity and nuances
in healthcare provision. Moreover, our focus on lab-
oratory test prescriptions might overlook essential as-
pects of patient histories and experiences prior to ad-
mission to the ED, which are captured in different
data types. Integration with alternative data sources,
such as clinical notes and drug prescriptions, could
bolster confidence in our findings. Our observed cor-
relations between prescription patterns and clinical
outcomes do suggest implicit clinical and organiza-
tional processes that are interesting research avenues
to explore. However, extra caution is advised in in-
terpreting their significance. Not only is further ex-
ternal validation necessary, but there is also a need
to be mindful of potential biases that this approach
may introduce and perpetuate, such as discrimination
HEALTHINF 2025 - 18th International Conference on Health Informatics
336
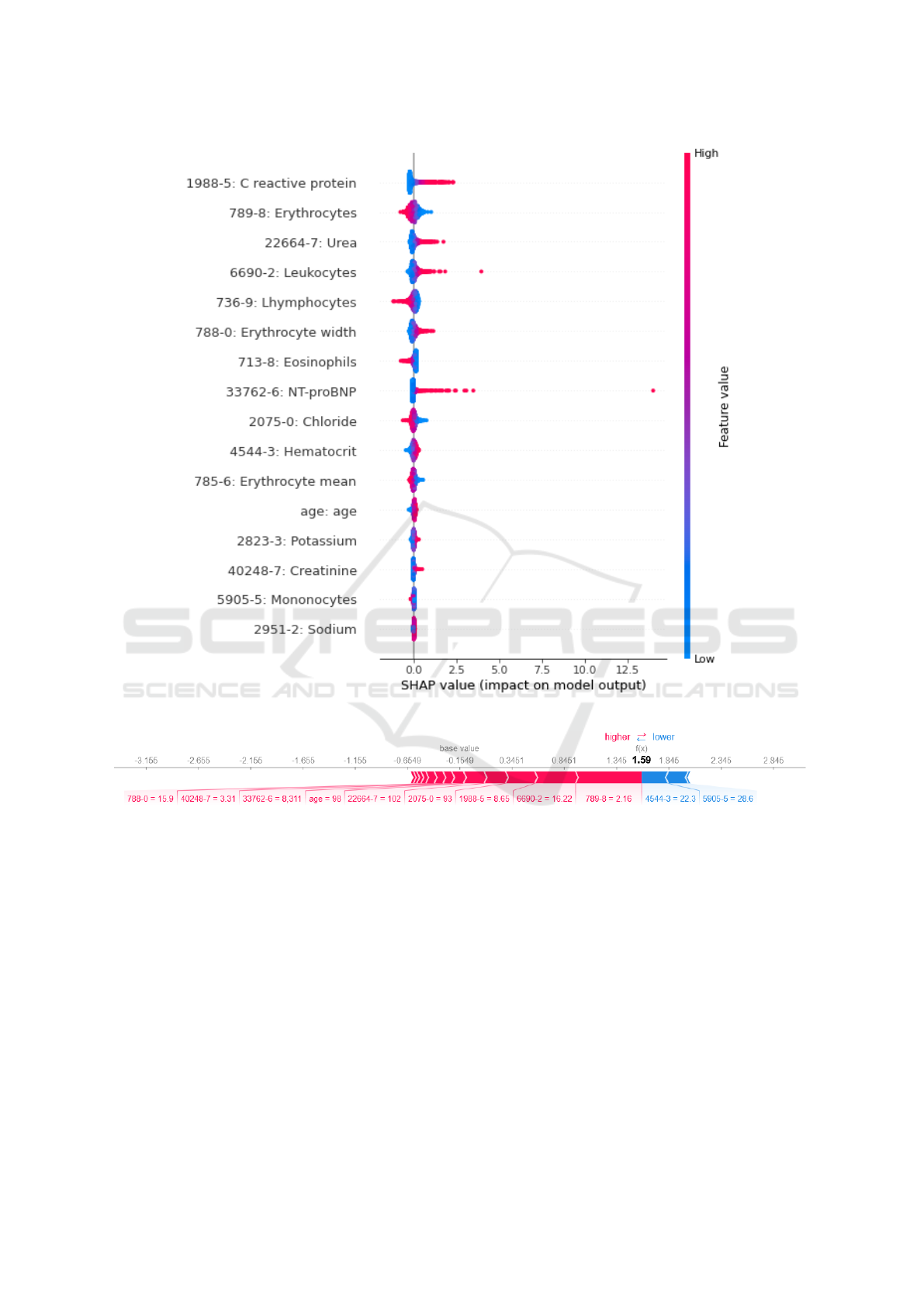
(a) SHAP summary plot
(b) SHAP force plot
Figure 4: (a) SHAP summary plot computed using laboratory results and age of all episodes of the test dataseet. (b) SHAP
force plot of a HF patient correctly classified as imminent hospitalization, in which colour red represents the lab tests results
(or age) that are increasing the chance of imminent hospitalization while colour blue represents the negative outcome.
against underrepresented subpopulations. This issue
has been increasingly recognized in the literature and
merits further investigation before any clinical imple-
mentation (Obermeyer et al., 2019).
Additionally, the inclusion of more comprehen-
sive clinical information could enhance data interpre-
tation and improve algorithm performance. For ex-
ample, ejection fraction, a key factor in heart failure
(HF), was not included in our study. Often recorded in
free text, this information presents challenges for sys-
tematic and reliable extraction and was consequently
not utilized. We intend to address this limitation in
future work.
Finally, it is essential to emphasize that the tools
developed in this study just went through an initial
trial stages. The utilization of AI systems to aid clin-
ical decision-making represents a significant innova-
tion in healthcare. However, their adoption requires
meticulous evaluation and strict adherence to regula-
tions, particularly in Europe. The tools and method-
ologies described in our research serve as illustrations
of potential approaches to enhance clinical decision-
making by leveraging existing clinical data with its
inherent limitations. Prior to their implementation in
Multimorbidity in Heart Failure Patients: Application of Machine Learning Algorithms to Predict Imminent Health Outcomes
337

real-world clinical settings, these tools must undergo
comprehensive regulatory processes to ensure their
safety, effectiveness, and ethical compliance.
6 CONCLUSION
In conclusion, this study has successfully developed a
comprehensive framework for analyzing clinical data,
particularly in the context of patients with HF with
concurrent multimorbidity. Our approach contributes
to more refined risk stratification and informed clin-
ical decision-making. This methodology showcases
the potential of healthcare data to improve clinical
insight and individualized risk assessment that can
eventually lead to better patient outcomes.
Looking ahead, our research lays the groundwork
for future investigations to enhance predictive mod-
els that make use of laboratory data and delve deeper
into the impacts of various comorbidities on HF out-
comes on long-term perspective. Prioritizing the ex-
pansion of data collection methods, will be essential
to enrich the quality and relevance of the data in fu-
ture studies. Furthermore, the versatility of our an-
alytical framework holds promise for broader appli-
cations, extending to diverse patient populations with
chronic conditions such as Diabetes Mellitus, Chronic
Kidney Disease, and Chronic Obstructive Pulmonary
Disease. We intend to address these in future work.
FUNDING
This work was developed under the IntelligentCare
project LISBOA-01-0247-FEDER-045948 which is
co-financed by the ERDF/LISBOA2020 and by FCT,
Portugal, under CMU-Portugal and by FCT, Por-
tugal, through the INESC-ID Research Unit, ref.
UIDB/00408/2020 and ref. UIDP/00408/2020.
ACKNOWLEDGEMENTS
We acknowledge Carlos Magalh
˜
aes and Jaime
Machado, from Hospital da Luz, for the data extrac-
tion.
REFERENCES
Alanazi, A. (2022). Using machine learning for healthcare
challenges and opportunities. Informatics in Medicine
Unlocked, 30:100924.
Anand, I., McMurray, J. J., Whitmore, J., Warren, M.,
Pham, A., McCamish, M. A., and Burton, P. B.
(2004). Anemia and Its Relationship to Clinical Out-
come in Heart Failure. Circulation, 110(2):149–154.
Anand, I. S., Latini, R., Florea, V. G., Kuskowski, M. A.,
Rector, T., Masson, S., Signorini, S., Mocarelli, P.,
Hester, A., Glazer, R., and Cohn, J. N. (2005).
C-Reactive Protein in Heart Failure. Circulation,
112(10):1428–1434.
Aronson, S. J. and Rehm, H. L. (2015). Building the foun-
dation for genomics in precision medicine. Nature,
526(7573):336–342.
Asadollahi, K., Hastings, I., Beeching, N., and Gill, G.
(2007). Laboratory risk factors for hospital mortality
in acutely admitted patients. QJM : monthly journal
of the Association of Physicians, 100:501–7.
Athena (2022). Ohdsi athena. (accessed: 13.01.2023).
Bozkurt, B., Coats, A. J., Tsutsui, H., and et al. (2021).
Universal Definition and Classification of Heart Fail-
ure: A Report of the Heart Failure Society of America,
Heart Failure Association of the European Society of
Cardiology, Japanese Heart Failure Society and Writ-
ing Committee of the Universal Definition of Heart
Failure. Journal of Cardiac Failure, 27(4):387–413.
Chamberlain, A. M., Sauver, J. L., Gerber, Y., Manemann,
S. M., Boyd, C. M., Dunlay, S. M., Rocca, W. A., Rut-
ten, L. J., Jiang, R., Weston, S. A., and Roger, V. L.
(2015). Multimorbidity in Heart Failure: A Commu-
nity Perspective. The American journal of medicine,
128(1):38.
CMS (2021). Electronic Health Records.
Dervishi, A. (2020). A deep learning backcasting approach
to the electrolyte, metabolite, and acid-base param-
eters that predict risk in icu patients. PLOS ONE,
15(12):1–19.
Dixon, B. E., Wen, C., French, T., Williams, J. L., Duke,
J. D., and Grannis, S. J. (2020). Observational Health
Data Science and Informatics (OHDSI). BMJ Health
Care Inform, 27:100054.
Han, J., Kamber, M., and Pei, J. (2012). Data mining: Data
mining concepts and techniques. Morgan Kaufmann,
3 edition.
Hitachi Ventara. Pentaho Data Integration & Analytics.
Hripcsak, G., Knirsch, C., Zhou, L., Wilcox, A., and
Melton, G. (2011). Bias associated with mining elec-
tronic health records. Journal of Biomedical Discov-
ery and Collaboration, 6:48–52.
Kasza, J. and Wolfe, R. (2014). Interpretation of com-
monly used statistical regression models. Respirology,
19(1):14–21.
Lee, T. C., Shah, N. H., Haack, A., and Baxter, S. L. (2020).
Clinical implementation of predictive models embed-
ded within electronic health record systems: A sys-
tematic review. Informatics, 7(3):25. Epub 2020 Jul
25.
Liyanage, H., Liaw, S. T., Jonnagaddala, J., Hinton, W.,
and De Lusignan, S. (2018). Common Data Models
(CDMs) to Enhance International Big Data Analytics:
A Diabetes Use Case to Compare Three CDMs. Stud-
ies in Health Technology and Informatics, 255:60–64.
HEALTHINF 2025 - 18th International Conference on Health Informatics
338

Loekito, E., Bailey, J., Bellomo, R., Hart, G. K., Hegarty,
C., Davey, P., Bain, C., Pilcher, D., and Schneider, H.
(2013). Common laboratory tests predict imminent
medical emergency team calls, intensive care unit ad-
mission or death in emergency department patients.
Emergency Medicine Australasia, 25(2):132–139.
Loinc® Indianapolis, IN: Regenstrief Institute, Inc. Logical
Observation Identifiers Names and Codes (LOINC).
Lundberg, S. M. and Lee, S.-I. (2017). A unified approach
to interpreting model predictions. In Proceedings of
the 31st International Conference on Neural Informa-
tion Processing Systems, NIPS’17, page 4768–4777,
Red Hook, NY, USA. Curran Associates Inc.
Lundberg, S. M., Nair, B., Vavilala, M. S., Horibe, M.,
Eisses, M. J., Adams, T., Liston, D. E., Low, D. K.-
W., Newman, S.-F., Kim, J., et al. (2018). Explain-
able machine-learning predictions for the prevention
of hypoxaemia during surgery. Nature Biomedical En-
gineering, 2(10):749.
Majnari
´
c, L. T., Babi
ˇ
c, F., O’Sullivan, S., and Holzinger, A.
(2021). AI and big data in healthcare: Towards a more
comprehensive research framework for multimorbid-
ity. Journal of Clinical Medicine, 10(4):766. Num-
ber: 4 Publisher: Multidisciplinary Digital Publishing
Institute.
Martins, C., Neves, B., Teixeira, A. S., Froes, M., Sarmento,
P., Machado, J., Magalh
˜
aes, C., Silva, N., Silva, M.,
and Leite, F. (2024). Identifying subgroups in heart
failure patients with multimorbidity by clustering and
network analysis. BMC Medical Informatics and De-
cision Making, 24(1):95.
Mueller, O., Rentsch, K., Nickel, C., and Bingisser, R.
(2021). Disposition decision support by labora-
tory based outcome prediction. Journal of Clinical
Medicine, 10:939.
Navickas, R., Petric, V.-K., Feigl, A. B., and Seychell, M.
(2016). Multimorbidity: What Do We Know? What
Should We Do? Journal of Comorbidity, 6(1):4–11.
Nwanosike, E. M., Conway, B. R., Merchant, H. A., and
Hasan, S. S. (2022). Potential applications and perfor-
mance of machine learning techniques and algorithms
in clinical practice: A systematic review. Interna-
tional Journal of Medical Informatics, 159:104679.
Obermeyer, Z., Powers, B., Vogeli, C., and Mullainathan,
S. (2019). Dissecting racial bias in an algorithm
used to manage the health of populations. Science,
366(6464):447–453.
OHDSI (2014). Achilles for data characterization. (ac-
cessed: 13.01.2023).
OHDSI (2021). Whiterabbit for etl design. (accessed:
13.01.2023).
Plebani, M. and Lippi, G. (2016). Improving diagnosis and
reducing diagnostic errors: The next frontier of labo-
ratory medicine. Clinical Chemistry and Laboratory
Medicine, 54(7):1117–1118.
Rodrigues, A. M., Greg
´
orio, M. J., Sousa, R. D., Dias, S. S.,
Santos, M. J., Mendes, J. M., Coelho, P. S., Branco,
J. C., and Canh
˜
ao, H. (2018). Challenges of ageing in
portugal: Data from the EpiDoC cohort. Acta Medica
Portuguesa, 31(2):80–93.
Romana, G. Q., Kislaya, I., Salvador, M. R., Cunha-
Goncalves, S., Nunes, B., and Dias, C. (2019). Mul-
timorbidity in portugal: results from the first national
health examination survey. Acta M
´
edica Portuguesa,
32(1).
Sciences, O. H. D. and Informatics(OHDSI) (2023). Stan-
dardized data: The omop common data model. (ac-
cessed: 22.11.2023).
Shamout, F., Zhu, T., and Clifton, D. (2020). Machine
learning for clinical outcome prediction. IEEE Re-
views in Biomedical Engineering, PP:1–1.
Wald, A. (1943). Tests of statistical hypotheses concerning
several parameters when the number of observations
is large. Transactions of the American Mathematical
Society, 54:426–482.
WHO (2016). Multimorbidity Technical Series on Safer
Primary Care Multimorbidity: Technical Series on
Safer Primary Care. page 28.
Wians, F. H. (2009). Clinical laboratory tests: Which, why,
and what do the results mean? Laboratory Medicine,
40(2):105–113.
Williams, T. B., Garza, M., Lipchitz, R., Powell, T., Baghal,
A., Swindle, T., and Sexton, K. W. (2022). Cultivating
informatics capacity for multimorbidity: A learning
health systems use case. Journal of Multimorbidity
and Comorbidity, 12. Publisher: SAGE Publications.
Multimorbidity in Heart Failure Patients: Application of Machine Learning Algorithms to Predict Imminent Health Outcomes
339
