
From Controlled to Free-Living Contexts: Expanding the Monitoring
of Motor Symptoms in Parkinson’s Disease with Wearable mHealth
Technologies
María Centeno-Cerrato
1a
, Carlos Polvorinos-Fernández
1b
, Luis Sigcha
2c
,
Guillermo de Arcas
1d
, César Asensio
3e
, Juan Manuel López
4f
and Ignacio Pavón
1g
1
Instrumentation and Applied Acoustics Research Group, Mechanical Engineering Department,
ETS Ingenieros Industriales, Universidad Politécnica de Madrid, Madrid, Spain
2
Department of Physical Education and Sports Science, Health Research Institute, & Data-Driven
Computer Engineering (D2iCE) Group, University of Limerick, Limerick, Ireland
3
Instrumentation and Applied Acoustics Research Group, Department of Audiovisual Engineering and Communications,
ETS. de Ingeniería y Sistemas de Telecomunicación, Universidad Politécnica de Madrid, Madrid, Spain
4
Instrumentation and Applied Acoustics Research Group, Department of Physical Electronics, Electrical Engineering and
Applied Physics, ETS. de Ingeniería y Sistemas de Telecomunicación, Universidad Politécnica de Madrid, Madrid, Spain
Keywords: Wearables, Supervised Monitoring, Free-Living Monitoring,
Accelerometer, Gyroscope.
Abstract: This study examines the application of wearable mobile health (mHealth) technologies, specifically
smartwatches equipped with inertial sensors, for the monitoring of Parkinson’s disease (PD). The aim is to
investigate how the integration of the Monipar tool, designed to monitor supervised exercises, with the
BioCliTe system, which continuously collects data during free-living activities, can improve the assessment
of motor fluctuations and disease progression. The study proposes a set of free-living activities which can
serve as characteristic indicators for assessing motor symptoms. By combining structured exercises with
everyday tasks, this approach provides a more comprehensive evaluation of PD, capturing motor symptoms
in both controlled and real-world environments. The research seeks to advance disease monitoring and patient
care through more accurate tracking and the development of personalized treatment strategies.
1 INTRODUCTION
Parkinson's disease (PD) is a chronic, progressive
neurological disorder caused by the loss of
dopaminergic neurons, resulting in a significant
reduction in the production of dopamine —a key
neurotransmitter involved in the regulation of
movement and motor control. PD manifests through
a wide range of symptoms, categorized into two main
groups: motor symptoms (e.g., resting tremor,
bradykinesia, muscle rigidity, postural and gait
disturbances or dyskinesias) and non-motor
a
https://orcid.org/0009-0007-0113-3007
b
https://orcid.org/0000-0002-4594-9477
c
https://orcid.org/0000-0002-9968-5024
d
https://orcid.org/0000-0003-1699-7389
e
https://orcid.org/0000-0003-3265-3244
f
https://orcid.org/0000-0001-7847-8707
g
https://orcid.org/0000-0003-0970-0452
symptoms (e.g., sleep disorders, depression,
cognitive impairment, and dementia in advanced
stages). Despite advances in research, PD remains
incurable, and its progression is inevitable.
(Armstrong & Okun, 2020).
The most used scale to measure PD progression is
the Movement Disorder Society-Sponsored Revision
of the Unified Parkinson's Disease Rating Scale
(MDS-UPDRS), which evaluates symptoms and
mental health through questionnaires and clinician-
scored tests (Goetz et al., 2008).
Centeno-Cerrato, M., Polvorinos-Fernández, C., Sigcha, L., de Arcas, G., Asensio, C., López, J. M. and Pavón, I.
From Controlled to Free-Living Contexts: Expanding the Monitoring of Motor Symptoms in Parkinson’s Disease with Wearable mHealth Technologies.
DOI: 10.5220/0013386000003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 2: HEALTHINF, pages 1037-1044
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
1037

The clinical management of PD presents a
significant challenge due to the fluctuating nature of
its symptoms. Medical consultations are commonly
scheduled at intervals of six to twelve months,
resulting in long gaps without detailed patient
evaluation and treatment adjustment. Consequently,
many patients can experience a deterioration of
symptoms before their next clinical appointment
(Rodríguez-Martín et al., 2022). There is a growing
need for objective and continuous monitoring
systems to assess disease progression and refine
treatment strategies. This has led to the integration of
technological tools aimed at improving both short-
and long-term monitoring, as well as optimizing
overall disease management.
Mobile health (mHealth) technologies and
wearable devices allow continuous, accurate data
collection in a simple manner without causing
discomfort to the user. These devices, often equipped
with sensors (typically inertial or bioelectrical),
enhance monitoring quality while offering patients an
accessible and convenient solution (Polvorinos-
Fernández et al., 2024).
It is essential to ensure these sensors reliably operate
within the relevant amplitude and frequency ranges
for accurate treatment evaluation of the patients (Ru
et al., 2022).
To obtain a more comprehensive and objective
view, it is important to collect data from patients in a
wide range of environments: during the performance
of pre-defined guided exercises and during the
execution of activities of daily living.
Furthermore,
these data can be used to develop digital biomarkers
that facilitate the quantification of patients' motor
status (Polvorinos-Fernández et al., 2024).
These biomarkers could play a crucial role in
personalizing treatment and improving patients'
quality of life (Mahadevan et al., 2020). The
acquisition of data under both laboratory and free-
living conditions is crucial for evaluating the validity
and utility of novel biomarkers. Controlled laboratory
environments enable the generation of robust and
reliable biomarkers, whereas data collected in free-
living settings are indispensable for determining
whether these biomarkers retain their reliability and
relevance in real-world contexts.
This study explores the use of inertial sensors to
monitor patients with PD. All smartwatches
employed for the measurements conducted in the
study are equipped with an LSM6DS0 sensor, which
incorporates a triaxial accelerometer and a triaxial
gyroscope. The sampling frequency was configured
in every device at 50 Hz.
The precise number and placement of sensors on
the body remain subjects of debate; however, it is
generally recommended to minimize the number of
sensors to optimize usability and comfort, while
ensuring the integrity of the data (Monje et al., 2019).
In accordance with this, a decision was made to
sacrifice some data quantity to enhance usability by
placing the smartwatch on only one hand, specifically
on the wrist most affected by symptoms. This
approach ensures the capture of data related to motor
performance and physical activity, while prioritizing
user comfort and ease of use.
The study highlights the importance of collecting
movement data in both supervised and unsupervised
contexts.
For this purpose, the mHealth tool BioCliTe
(Digital Biomarkers for Motor Status Assessment of
Parkinson's Disease Patients for Clinical and
Therapeutic Application) was used to continuously
capture motion signals during free-living activities
and guided exercises. The guided exercises were
performed using the Monipar tool (Sigcha et al.,
2023), which provides instructions via a smartphone,
while the smartwatch synchronously records data
from the embedded inertial sensors.
2 MONITORING GUIDED
ACTIVITIES: Monipar
Monipar is a technological solution developed as part
of the TECAPARK project (TECAPARK),
specifically designed to monitor the execution of
specific guided activities selected from the MDS-
UPDRS. These activities are intended to be
performed in supervised contexts, ensuring controlled
and accurate execution.
Monipar consists of two modules: a smartphone
application that guides the user, and a wearable
module in the form of a smartwatch, designed for
real-time data collection. The mobile application,
which uses an interactive interface, provides
comprehensive guidance through both visual
elements and audio prompts. This approach ensures
that users execute the exercises accurately, reducing
performance variability.
In addition, the application transmits activation
and status data from the smartphone to the
smartwatch, facilitating the automatic labelling of the
recorded signals. Simultaneously, the smartwatch
collects data via the integrated inertial sensors during
each exercise, which is then stored in the device's
local database. (Sigcha et al., 2023).
Monipar is structured around a set of exercises
based on Part III of the MDS-UPDRS scale (Goetz et
al., 2008), which assess different aspects of motor
function. These exercises are strategically sequenced
to ensure a comprehensive assessment (Sigcha et al.,
2023). Figure 1 illustrates the instructions displayed
in Monipar's exercise routine.
WHC 2025 - Special Session on Wearable HealthCare
1038
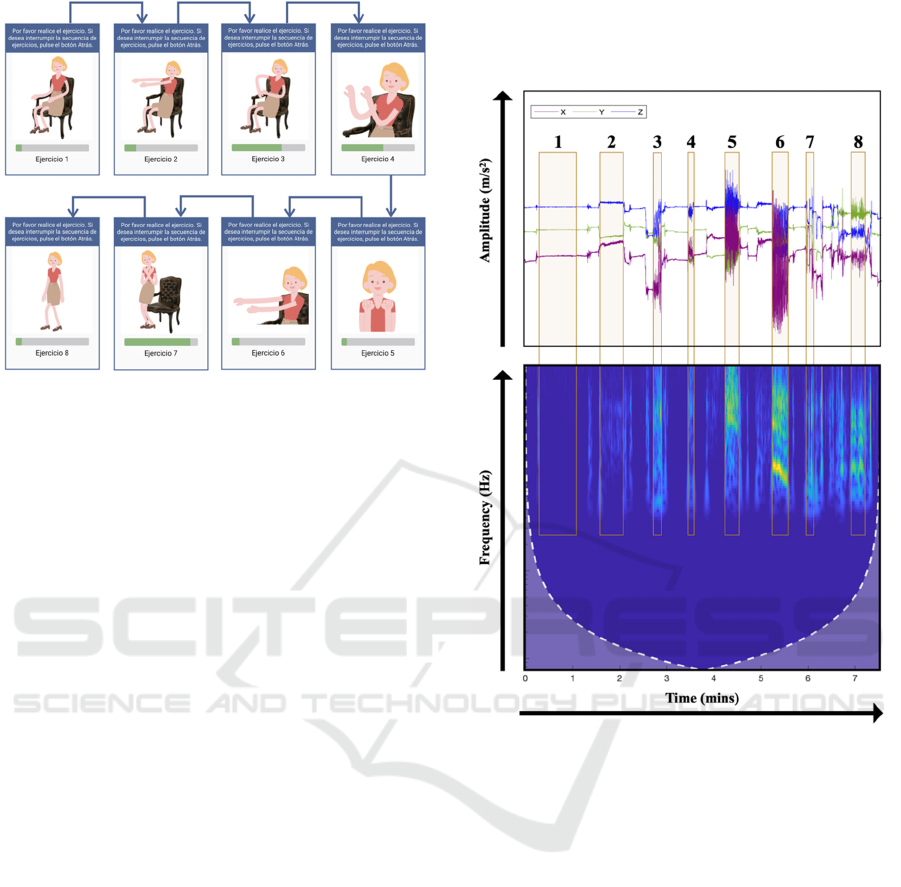
Figure 1: Visual Instructions for Monipar's exercise routine.
Exercise 1, Resting Tremor Assessment (Item
3.17), quantifies the amplitude of tremor when
the limbs are at rest.
Exercise 2, Postural Tremor Assessment (Item
3.15), assesses the presence of tremor when the
patient maintains a fixed posture.
Exercise 3, Repetitive Arm Extension
Movement. To complement the items of the
MDS-UPDRS scale, an additional exercise was
incorporated, involving repeated forward arm
extensions and bringing the hands to the chest.
Exercise 4, Finger Tapping (Thumb-Index)
(Item 3.4), assesses bradykinesia and fine
motor coordination by repetitively tapping the
thumb and index finger.
Exercise 5, Rapid Hand Movements (Item 3.5),
measures the patient’s ability to perform rapid,
repetitive hand movements, providing data on
motor agility and bradykinesia.
Exercise 6, Upper Limb Pronation-Supination
(Item 3.6), assesses bradykinesia and motor
symptoms, by measuring the pronation and
supination movements of the hands.
Exercise 7, Rising from a Chair (Item 3.9),
assesses the patient’s ability to rise from a
seated position, reflecting postural control.
Exercise 8, Gait Analysis (Item 3.10), assesses
gait quality, enabling the identification of
typical symptoms, such as freezing of gait.
Strategically timed rest periods were integrated
between each exercise, ensuring optimal performance
and minimizing the impact of fatigue on the data
collected. The Monipar protocol has a total duration
of 8 minutes. The duration of each exercise within the
protocol varies, depending on factors such as the
nature of the activity, its level of difficulty, and other
relevant considerations, all aimed at achieving the
most effective results for each specific task.
Figure 2: Example of an accelerometer signal recorded by
Monipar, including labelled data. The upper section shows
the temporal signal obtained from the three axes (time vs
amplitude), while the lower section shows the scalogram of
the combined signal from all three axes (time vs frequency)
(Sigcha et al., 2023).
3 TRANSITION FROM Monipar
TO BioCLiTe
Currently, Monipar provides valuable data on PD
motor status in controlled, supervised environments.
However, to ensure a comprehensive and precise
evaluation of the disease progression and treatment
efficacy, monitoring guided exercises must be
combined with monitoring free-living activities,
which do not require supervision. This integration
offers several significant advantages:
More Realistic Representation of Motor
Status. Guided exercises in controlled
environments, such as those performed with
Monipar, allow for the evaluation of specific
From Controlled to Free-Living Contexts: Expanding the Monitoring of Motor Symptoms in Parkinson’s Disease with Wearable mHealth
Technologies
1039

movements under standardized conditions,
which is useful for measuring specific
parameters of motor function. However, these
exercises do not always reflect the demands
and variations encountered in daily life. Free-
living activities, such as walking, eating, or
dressing, provide a more realistic perspective
on how the disease affects functionality in
everyday contexts. The integration of both
types of monitoring can provide a holistic
assessment of motor function, capturing both
the accuracy of movements in defined
exercises and the motor performance in real-
life situations.
Detection of Motor Fluctuations and "Off"
Periods. PD is characterized by motor
fluctuations, particularly in advanced stages,
where patients experience "on" periods (with
good motor control) and "off" periods (with
significant impairment). Continuous
monitoring during free-living activities permits
the tracking of these fluctuations, information
that may not be apparent during guided
exercises. This allows for a more accurate
assessment of motor function, facilitating
better adjustment to medication and other
treatments (Mantri et al., 2021).
More Comprehensive Data for Longitudinal
Analysis. Collecting data in different contexts
(guided and free-living) provides a richer and
more diverse dataset for longitudinal analysis.
This allows the observation of long-term
patterns, a more detailed assessment of disease
progression, and the effectiveness of
treatments. In addition, the diversity of data can
help to develop more robust predictive models
regarding disease progression.
In summary, the combination of guided exercises
and free-living activities in the monitoring of PD
patients provides a more comprehensive, accurate and
personalized view of their motor status.
Consequently, there is a need to move from Monipar
(monitoring under controlled conditions) to BioCliTe
(monitoring also under free-living conditions).
4 MONITORING OF
FREE-LIVING AND GUIDED
ACTIVITIES: BioCLiTe
BioCliTe provides a technological solution that
evolves from the Monipar tool, adapting and
extending its functionalities to increase both the scope
and accuracy of monitoring in contexts beyond the
clinical environment.
While Monipar was limited to recording data
exclusively during guided exercises in a controlled
environment, BioCliTe provides continuous
movement data collection, extending monitoring
throughout the entire day (without the need for
supervision). This approach facilitates the collection
of movement data while patients are performing tasks
that reflect their daily routines.
In addition to providing data from the
accelerometer and gyroscope, BioCliTe also supports
the real-time tracking of the participant’s physical
activity, including total steps, walking steps, running
steps, speed, distance, and calories burned.
BioCliTe not only focuses on monitoring free-
living activities but also preserves the core
functionality of Monipar by integrating guided
exercises into daily tasks. This allows patients to
carry out these exercises autonomously in their home
environment, without the need for direct supervision.
Previously, the Monipar mobile application
activated the smartwatch to initiate activity
measurement; however, due to the transition to
BioCliTe, this functionality has been updated. The
mobile application now records the time intervals
during which activities are performed, generating a
time-stamped data file that tracks the signal's status
throughout the activity, enabling the automatic
labelling of movement data captured by the
smartwatch.
The battery life of the smartwatch is a limitation
to consider. Each smartwatch has a different battery
capacity, making it necessary to evaluate how many
hours of continuous data recording it can support.
According to the studies conducted, the commercial
smartwatches used in the experiments are capable of
measuring acceleration and angular velocity for up to
five consecutive hours. During the remaining hours
of the day, the smartwatch only recorded data related
to physical activity.
The system was designed to allow flexibility in
the initiation and termination of measurements taken
through the accelerometer and gyroscope, adapting to
the individual needs of the patient. The recording
period can be adjusted according to the patient's
schedule, allowing guided activities to be performed
at the most convenient times.
In Figure 3, acceleration data (recorded over a
period longer than 1 hour) shows clear regions where
tremor is present. This extended monitoring period
allows for a more comprehensive assessment
compared to the previous functionality of Monipar,
which only evaluated movement during guided
exercises. In addition to the Monipar exercises, the
participant was asked to perform a free-living activity
(cooking – beating a mixture).
WHC 2025 - Special Session on Wearable HealthCare
1040
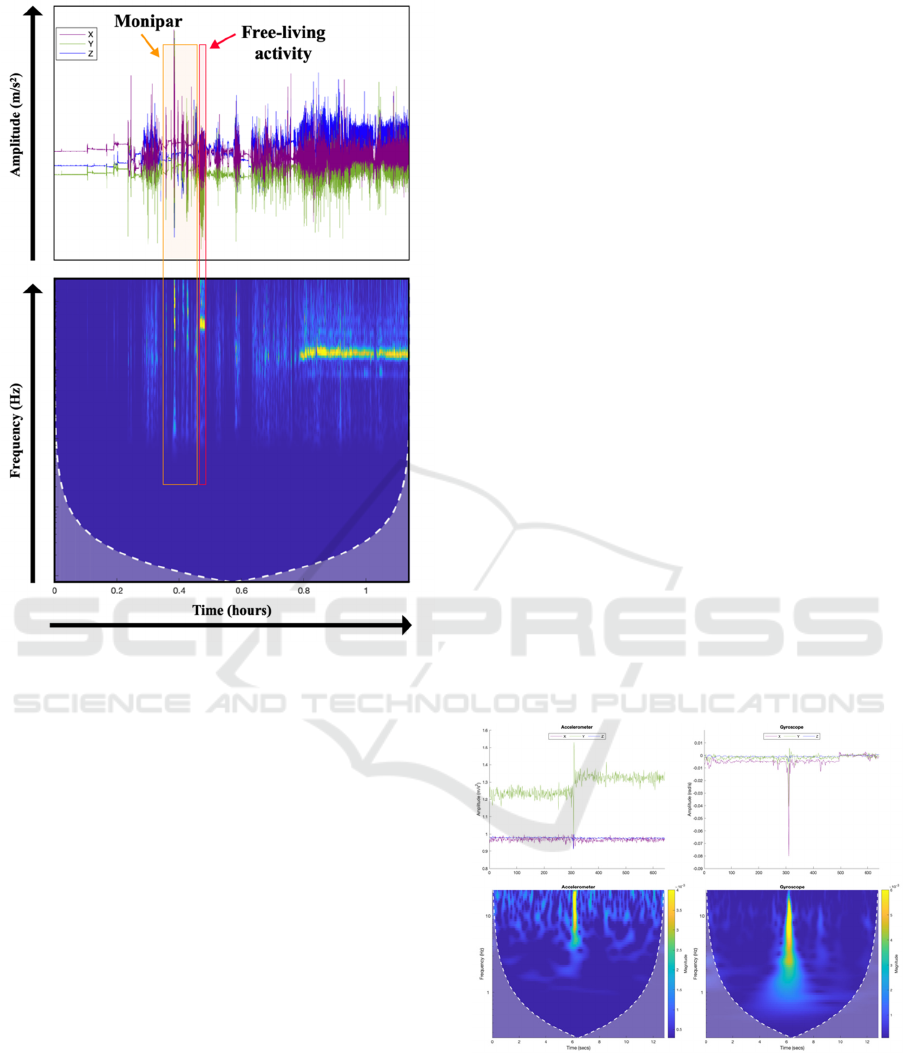
Figure 3: Example of an accelerometer signal recorded by
BioCliTe, including labelled data.
4.1 Proposed Free-Living Activities
Certain routine activities performed in daily living
have been recognized as effective in the monitoring
process, helping to characterize PD motor symptoms,
such as tremor and bradykinesia.
Due to the current limitations in recognizing
patterns associated with each specific free-living
activity, the first stage is to manually label these
exercises. To accomplish this, it is proposed to
develop a mobile application like Monipar, where the
user is guided through the task using visual and/or
auditory instructions, while also marking the precise
start and end points of each activity.
The data files would be stored locally on the
mobile device, and by cross-referencing this
information with the data recorded by the
smartwatch, it will be possible to identify which
sections of the signal correspond to each specific
activity. After having gained a thorough
understanding of the patterns and characteristics
associated with the signals from each proposed free-
living activity (using artificial intelligence
techniques) the objective is to eliminate the need for
manual labelling. In this context, the artificial
intelligence system will autonomously recognize the
activity occurring at any given moment.
Several daily activities have been identified as
suitable for monitoring motor symptoms, including
walking, standing up and sitting down, writing or
drawing, cooking, typing and brushing teeth.
A series of controlled laboratory experiments
were conducted, involving 20 healthy participants, to
thoroughly evaluate each of the proposed activities.
During these tests, participants performed the tasks
while their movements were tracked by the inertial
sensors embedded in a smartwatch. The data
collected allowed an in-depth analysis of how these
activities could function as reliable indicators of
motor symptom progression when monitored over
extended periods of time, in environments where
constant supervision is not required.
It is noteworthy that the experiments involving
patients with PD have not yet started, and the current
findings provide a preliminary foundation for
subsequent research involving PD individuals.
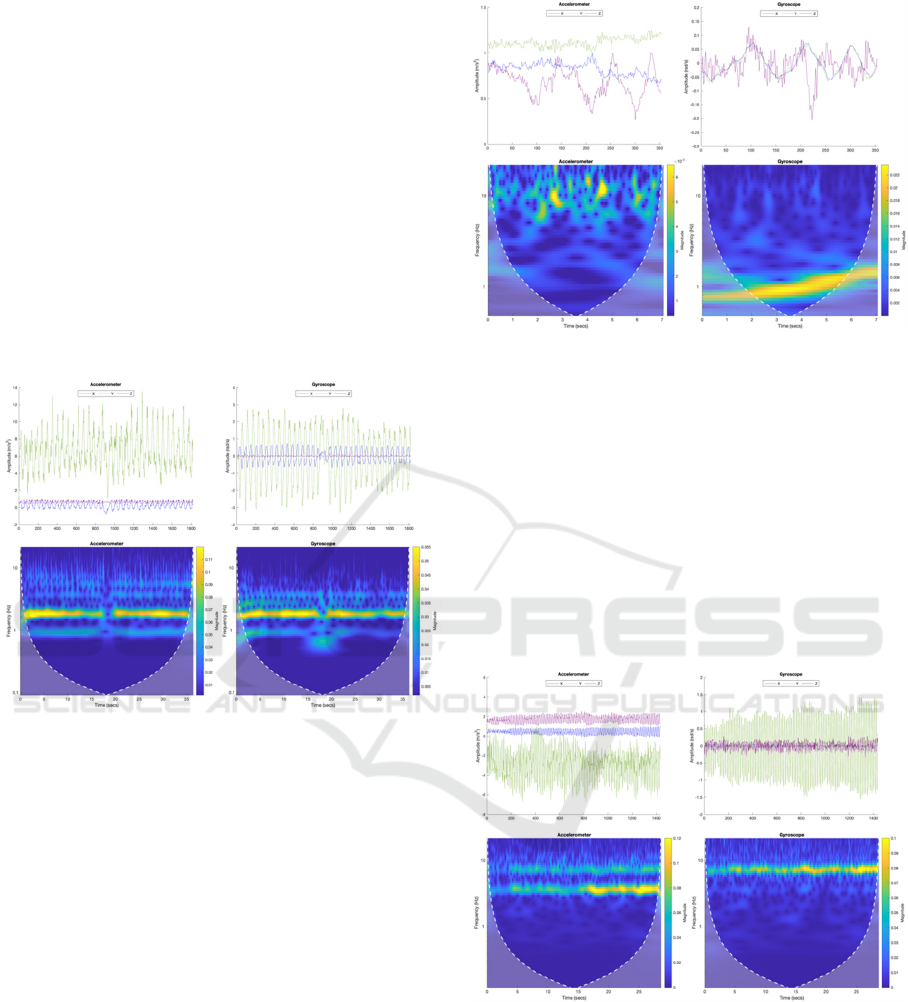
4.1.1 Standing up and Sitting down
Inertial sensors can capture the duration and fluidity
of transitions from a seated to a standing position,
providing valuable data to assess the impact of PD on
functional mobility in daily activities. Figure 4
illustrates a significant amplitude increase in
accelerometer and gyroscope signals corresponding
to the participant's standing up activity.
Figure 4: Signal recorded during the activity of standing up
(healthy participant). Upper panels show the time-domain
signals; lower panels the scalograms; left column
corresponds to accelerometer data; right column to
gyroscope data.
From Controlled to Free-Living Contexts: Expanding the Monitoring of Motor Symptoms in Parkinson’s Disease with Wearable mHealth
Technologies
1041
4.1.2 Gait
It is recommended to identify periods when the
participant is walking during free-living activities for
having observation in unsupervised environments.
There is no need to develop a new tool to manually
label walking events, as this can be achieved by cross-
referencing physical activity data (steps or speed)
with information from the accelerometer and
gyroscope. Gait is a key indicator of PD progression,
as difficulties such as "freezing of gait" can occur
outside of controlled environments. Measurements
taken during everyday activities like walking indoors
or outdoors allow for the detection of subtle changes
in mobility (Figure 5) (Borzì et al., 2023)
(Polvorinos-Fernández et al., 2024).
Figure 5: Signal recorded during the activity of gait (healthy
participant).
4.1.3 Writing or Drawing
Activities related to writing and drawing, particularly
the task of tracing a spiral, are common methods for
assessing motor symptoms in patients with PD. These
daily living tasks, which require continuous and fluid
movements of the hands and wrists, are effective for
detecting tremors, which appear as irregular or
discontinuous strokes. Inertial sensors placed on the
wrist can record deviations in acceleration and
angular velocity, providing accurate data on the
presence of tremors (Figure 6). Furthermore, these
activities are well-suited for assessing bradykinesia
symptoms, characterized by a progressive reduction
in the size of letters or strokes when drawing. With
these inertial sensors, it could be possible to capture
the decrease in movement amplitude and the
progressive slowing of motion of the upper limbs of
the patients (Thomas Kollamkulam, 2017).
Figure 6: Signal recorded during the activity of drawing a
spiral (healthy participant).
4.1.4 Brushing Teeth
Tooth brushing is a common daily activity that
involves repetitive and structured movements. PD
tremors may clearly manifest while holding the
toothbrush, leading to irregular oscillations that can
be detected by inertial sensors as fluctuations in
acceleration and angular velocity. In addition,
bradykinesia becomes evident in the reduced speed of
tooth brushing, reflecting a decrease in the amplitude
and speed of repetitive movements (Figure 7).
Figure 7: Signal recorded during the activity of brushing
teeth (healthy participant).
4.1.5 Cooking
Cooking activities present a valuable framework for
assessing motor function in people with PD because
they replicate the complex, coordinated movements
required in daily life. Routine tasks such as beating a
mixture, stirring a pot or a cup of coffee, or cutting
WHC 2025 - Special Session on Wearable HealthCare
1042
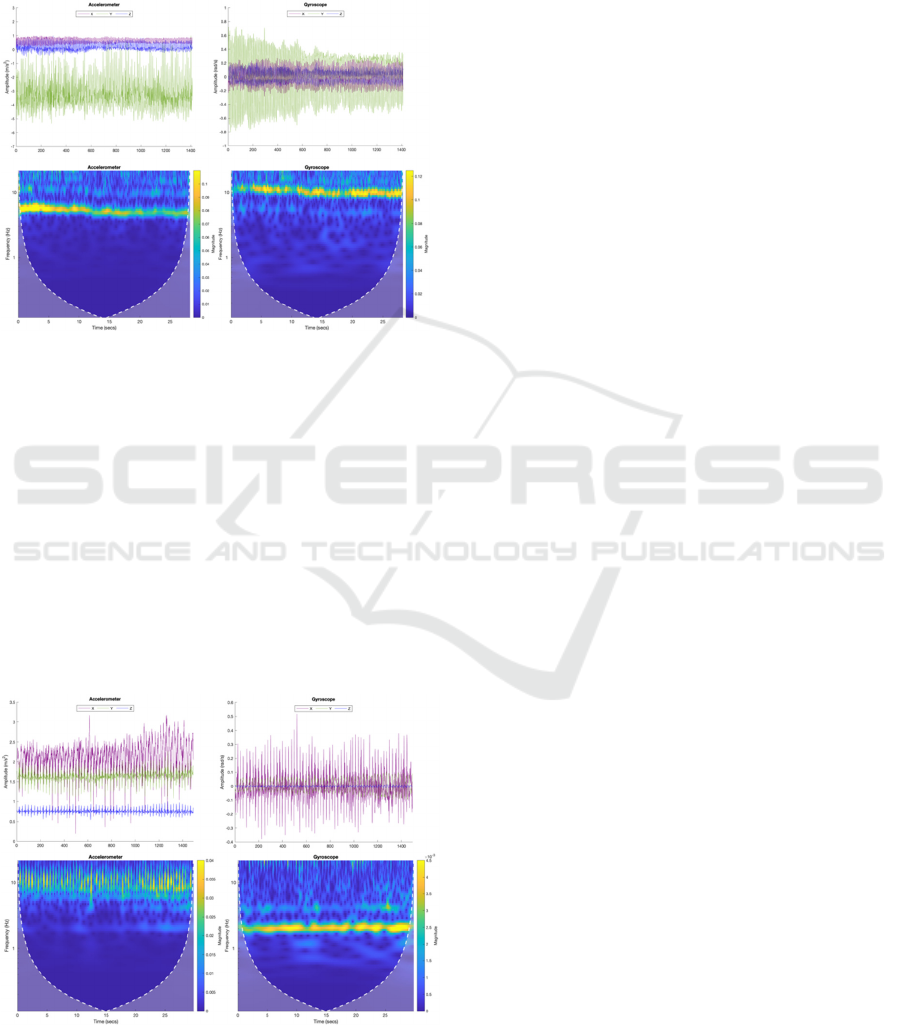
and chopping ingredients, involve rapid, repetitive,
and synchronized movements of the arm and wrist
(Figure 8). These tasks require controlled movements
and precise regulation of force, making them
particularly well-suited for assessing motor
impairments (tremor, bradykinesia and rigidity).
Figure 8: Signal recorded during the activity of beating a
mixture (healthy participant).
4.1.6 Typing
Patients with PD often experience motor symptoms
when typing, which can affect both the accuracy and
speed of their keystrokes. By analysing the variations
in hand movement, the sensors detect patterns
indicative of bradykinesia, such as reduced
movement amplitude or slower motion, as well as
tremor-related irregularities, like involuntary,
rhythmic motions. The changes in acceleration and
angular velocity can be valuable in assessing the
severity and progression of PD symptoms (Figure 9).
Figure 9: Signal recorded during the activity of typing
(healthy participant).
5 CONCLUSIONS
This study highlights the critical role of mHealth
technologies, such as Monipar and BioCliTe, in
improving the assessment of PD. Using wearable
devices, specifically smartwatches, can allow
accurate, continuous, and non-invasive monitoring of
motor symptoms, contributing to a deeper
understanding of the disease’s progression.
Monipar provides a structured framework for the
guided execution of standardized exercises derived
from the MDS-UPDRS scale, enabling controlled
assessments in supervised settings. Building on this
foundation, BioCliTe extends the monitoring
capabilities to free-living activities, allowing for
continuous data collection in unsupervised, real-
world environments. This dual approach—combining
guided exercises with daily activity monitoring—
provides a comprehensive assessment of motor
function, potentially bridging the gap between
clinical assessments and the challenges patients face
in their everyday lives.
To improve the understanding of motor
impairments in unsupervised settings, several free-
living activities have been proposed for analysis,
including gait, standing up and sitting down, writing
or drawing, cooking, typing, and brushing teeth.
These activities replicate hand movements commonly
performed in everyday life, allowing for the detection
of tremor, bradykinesia, rigidity, and other motor
symptoms under real life conditions (Polvorinos-
Fernández, Sigcha, Pablo, et al., 2024). Incorporating
data from these activities complements traditional
guided exercises and enriches the dataset available for
clinical evaluation. However, challenges remain in
accurately recognizing patterns of free-living
activities and ensuring reliable classification using
smartwatch data alone. To perform automatic
labeling effectively, a large and robust database is
required. Additionally, the complexity of monitoring
a wide range of daily activities necessitates the
development of advanced analytical tools, including
machine learning and data fusion techniques, to
improve activity recognition and symptom detection.
One of the key limitations of this study is the
absence of PD patients in the trial sample, which
restricts the ability to directly evaluate the
effectiveness of the technologies in monitoring PD-
specific motor symptoms, particularly in relation to
the proposed activities. Another limitation is the
reliance on smartwatches for data collection, as
factors such as device calibration, user behaviour, and
environmental conditions can introduce variability
and affect the accuracy of the measurements.
Additionally, the battery life of the smartwatch may
impact the continuous monitoring of motor
From Controlled to Free-Living Contexts: Expanding the Monitoring of Motor Symptoms in Parkinson’s Disease with Wearable mHealth
Technologies
1043

symptoms, which could influence the completeness
of the data collected throughout the day. These factors
must be considered in future studies to improve the
generalizability and applicability of the findings to
the PD population.
The integration of wearable devices into these
mHealth solutions can offer significant advantages,
including real-time detection of motor fluctuations,
improved tracking of disease progression, and more
personalized treatment strategies. These technologies
represent a transformative step in PD management,
providing clinicians with detailed, patient-specific
insights. Future research will focus on optimizing
data analysis algorithms to enhance the accuracy and
reliability of symptom detection in diverse real-world
scenarios.
ACKNOWLEDGEMENTS
This paper is part of the BIOCLITE research project
PID2021-123708OB-I00, funded by MCIN/AEI/
10.13039/501100011033/FEDER, EU.
REFERENCES
Armstrong, M., & Okun, M. (2020). Diagnosis and
Treatment of Parkinson Disease: A Review. Jama, 323,
548. https://doi.org/10.1001/jama.2019.22360
Borzì, L., Sigcha, L., & Olmo, G. (2023). Context
Recognition Algorithms for Energy-Efficient Freezing-
of-Gait Detection in Parkinson’s Disease. Sensors,
23(9), 4426.
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T.,
Fahn, S., Martinez-Martin, P.,…LaPelle, N. (2008).
Movement Disorder Society-sponsored revision of the
Unified Parkinson's Disease Rating Scale (MDS-
UPDRS): Scale presentation and clinimetric testing
results. Movement Disorders, 23(15), 2129-2170.
https://doi.org/https://doi.org/10.1002/mds.22340
Mahadevan, N., Demanuele, C., Zhang, H., Volfson, D.,
Ho, B., Erb, M., & Patel, S. (2020). Development of
digital biomarkers for resting tremor and bradykinesia
using a wrist-worn wearable device. npj Digital
Medicine, 3, 5. https://doi.org/10.1038/s41746-019-
0217-7
Mantri, S., Lepore, M., Edison, B., Daeschler, M., Kopil,
C., Marras, C., & Chahine, L. (2021). The Experience
of OFF Periods in Parkinson’s Disease: Descriptions,
Triggers, and Alleviating Factors. Journal of Patient-
Centered Research and Reviews, 8, 232-238.
https://doi.org/10.17294/2330-0698.1836
Monje, M., Foffani, G., Obeso, J., & Sánchez-Ferro, A.
(2019). New Sensor and Wearable Technologies to Aid
in the Diagnosis and Treatment Monitoring of
Parkinson's Disease. Annual Review of Biomedical
Engineering, 21, 111-143. https://doi.org/10.1146/
annurev-bioeng-062117-121036
Polvorinos-Fernández, C., Pavón, I., & Sigcha, L. (2024).
Smartwatch gait dataset in simulated Parkinson's
disease restricted arm swing conditions (Zenodo.
https://doi.org/10.5281/zenodo.13884808
Polvorinos-Fernández, C., Sigcha, L. F., Borzí, L., Olmo,
G., Asensio, C., Lopez Navarro, J. M.,…Pavón, I.
(2024). Evaluating Motor Symptoms in Parkinson’s
Disease Through Wearable Sensors: A Systematic
Review of Digital Biomarkers. Applied Sciences, 14,
10189. https://doi.org/10.3390/app142210189
Polvorinos-Fernández, C., Sigcha, L. F., Pablo, L., Borzí,
L., Cardoso, P., Costa, N.,…Pavón, I. (2024, 01).
Evaluation of the Performance of Wearables’ Inertial
Sensors for the Diagnosis of Resting Tremor in
Parkinson’s Disease
Rodríguez-Martín, D., Cabestany, J., Pérez, C., Pie, M.,
Calvet, J., Samà Monsonís, A.,…Rodríguez-Molinero,
A. (2022). A New Paradigm in Parkinson's Disease
Evaluation With Wearable Medical Devices: A Review
of STAT-ON. Frontiers in Neurology, 13, 912343.
https://doi.org/10.3389/fneur.2022.912343
Ru, X., Gu, N., Shang, H., & Zhang, H. (2022). MEMS
Inertial Sensor Calibration Technology: Current Status
and Future Trends.
Micromachines, 13(6), 879.
Sigcha, L., Pavón, I., De Arcas, G., Costa, N., Costa, S.,
Arezes, P.,…Polvorinos, C. (2023). Monipar
Database: smartwatch movement data to monitor
motor competency in subjects with Parkinson's disease
(Zenodo. https://doi.org/10.5281/zenodo.8104853
Sigcha, L., Polvorinos-Fernández, C., Costa, N., Costa, S.,
Arezes, P., Gago, M.,…Pavón, I. (2023). Monipar:
movement data collection tool to monitor motor
symptoms in Parkinson’s disease using smartwatches
and smartphones [Original Research]. Frontiers in
Neurology, 14. https://doi.org/10.3389/
fneur.2023.1326640
TECAPARK. https://www.i2a2.upm.es/tecapark/
Thomas Kollamkulam, M. a. L. A. a. P. P. (2017).
Handwriting Analysis in Parkinson's Disease: Current
Status and Future Directions. Movement Disorders
Clinical Practice, 4. https://doi.org/10.1002/
mdc3.12552
WHC 2025 - Special Session on Wearable HealthCare
1044
