
The Bigger the Better? Towards EMG-Based Single-Trial Action Unit
Recognition of Subtle Expressions
Dennis K
¨
uster
1 a
, Rathi Adarshi Rammohan
1 b
, Hui Liu
1 c
, Tanja Schultz
1 d
and
Rainer Koschke
2 e
1
Cognitive Systems Lab, University of Bremen, Bremen, Germany
2
AG Software Engineering, University of Bremen, Bremen, Germany
Keywords:
Action Units, Electromyography, Facial Action Coding System, EMG, sEMG, fEMG, Subtle Expressions,
Pattern Recognition, Machine Learning.
Abstract:
Facial expressions are at the heart of everyday social interaction and communication. Their absence, such as in
Virtual Reality settings, or due to conditions like Parkinson’s disease, can significantly impact communication.
Electromyography (EMG)-based facial action unit recognition (AUR) offers a sensitive and privacy-preserving
alternative to video-based methods. However, while prior research has focused on peak intensity action units
(AUs), there has been a lack of research on EMG-based AURs for lightweight recording of subtle expressions
at multiple muscle sites. This study evaluates EMG-based AUR for both low- and high-intensity expressions
across eight AUs using two types of mobile electrodes connected to the Biosignal Plux system. The results
of four subjects indicate that even limited data may be sufficient to train reasonably accurate AUR models.
Larger snap-on electrodes performed better for peak-intensity AUs, but smaller electrodes resulted in higher
performance for low-intensity expressions. These findings suggest that EMG-based AUR is viable for subtle
expressions from short data segments and that smaller electrodes hold promise for future applications.
1 INTRODUCTION
Even if the face is not a proverbial “window to the
soul”, the notion that facial expressions play a key
role in everyday nonverbal communication can be
dated back to Charles Darwin’s seminal work on “The
Expression of Emotions in Man and Animal” (Dar-
win, 1872; Kappas et al., 2013). On the downside,
however, this means that a lack of facial expressive-
ness can be a serious impediment to communication.
For example, Parkinson’s disease (PD) is character-
ized by hypomimia, and people with PD often ex-
perience reduced facial expressions (Sonawane and
Sharma, 2021), as well as an impaired ability to rec-
ognize and discriminate between different facial ex-
pressions (Mattavelli et al., 2021). In fact, automated
facial expression recognition may even be able to help
a
https://orcid.org/0000-0001-8992-5648
b
https://orcid.org/0000-0002-8538-727X
c
https://orcid.org/0000-0002-6850-9570
d
https://orcid.org/0000-0002-9809-7028
e
https://orcid.org/0000-0003-4094-3444
diagnose PD (Jin et al., 2020).
However, we do not need to be afflicted by a con-
dition such as PD to understand the negative impact
of diminished or obscured facial expressions. In some
situations, such as when talking on the phone, we may
already be used to the absence of visual cues. In other
instances, for example, when wearing a face mask,
such as those widely used during the recent COVID-
19 pandemic, listening to a speaker (Giovanelli et al.,
2021) and recognizing their facial expressions may be
impaired (Grahlow et al., 2022). Perhaps more impor-
tantly, even when the ability to discriminate between
expressions remains, perceived interpersonal close-
ness and mimicry may be reduced (Kastendieck et al.,
2022).
EMG-based AUR becomes particularly relevant
when interacting through immersive devices, such
as virtual reality (VR) headsets. Recent research
on avatar-mediated virtual environments underscores
that facial expressions may play a more critical role
than bodily cues in fostering interpersonal attraction
and liking (Oh Kruzic et al., 2020). However, VR
headsets inherently obstruct half of the face, posing
100
Küster, D., Rammohan, R. A., Liu, H., Schultz, T. and Koschke, R.
The Bigger the Better? Towards EMG-Based Single-Trial Action Unit Recognition of Subtle Expressions.
DOI: 10.5220/0013389300003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 100-110
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

a challenge for conventional video-based AUR sys-
tems (Wen et al., 2022). Potential approaches towards
bridging this gap include the use of add-ons such
as integrated eye-tracking and facial-tracking devices
(Schuetz and Fiehler, 2022) or incorporating infrared
light sources and cameras to develop visual databases
for training visual AUR while users wear VR headsets
(Chen and Chen, 2023). However, while these ap-
proaches may help to address the VR use case, EMG-
based AUR could still outperform such approaches
due to its superior sensitivity and time resolution (Vel-
danda et al., 2024).
Regardless of the camera type or additional track-
ing capabilities, vision-based AUR and facial expres-
sion analysis have relied on the Facial Action Cod-
ing System (FACS) since the 1970s (Ekman et al.,
2002). FACS itself was upon earlier foundational
work by (Hjortsj
¨
o, 1969), who cataloged facial con-
figurations (Barrett et al., 2019) originally depicted
in Duchenne’s research (Duchenne and Cuthbertson,
1990). The system classifies facial expressions into
44 Action Units (AUs), each representing specific,
independently controlled facial muscle movements.
Unlike basic emotions (Ekman, 1999), AUs are purely
descriptive and avoid interpretive labels (Zhi et al.,
2020). FACS has therefore been the nearly univer-
sally accepted standard for behavioral research on fa-
cial expressions and 3D emotion modeling (van der
Struijk et al., 2018). However, vision-based AUR
using FACS faces several substantial challenges that
could be addressed by EMG-based AUR.
1.1 EMG-Based Automatic Action Unit
Recognition (AUR)
AUR aims to automatically identify the facial muscle
movements associated with emotions, expressions,
and communicative intentions (Crivelli and Fridlund,
2019) by analyzing action units such as nose wrin-
kling (AU9), eyebrow-raising (AU1, AU2), or lip
corner pulling (AU12). Historically, AUR relied on
labor-intensive manual annotation of videos by certi-
fied FACS experts, a process requiring over an hour
to label just one minute of video (Bartlett et al., 2006;
Zhi et al., 2020).
Today, advancements in automatic affect recog-
nition have introduced tools ranging from early sys-
tems like the Computer Expression Recognition Tool-
box (CERT) (Littlewort et al., 2011) to modern open-
source software such as OpenFace (Baltrusaitis et al.,
2018) and LibreFace (Chang et al., 2024), enabling
cost-effective and efficient analysis of facial activity
in research settings (K
¨
uster et al., 2020). While many
tools have traditionally focused on detecting prototyp-
ical expressions tied to basic emotion theories (BETs)
(Ortony, 2022; Crivelli and Fridlund, 2018), growing
interest in facial AUR highlights its objectivity as a re-
search tool independent of BET- or other theoretical
frameworks (K
¨
uster et al., 2020).
However, efforts to evaluate and compare AUR
platforms (Krumhuber et al., 2021) have often been
constrained by a limited number of publicly available
databases, such as those referenced in (Chang et al.,
2024). As a result, performance estimates for these
tools may be overly optimistic, particularly in sponta-
neous and noisy field recording conditions where ac-
curacy tends to degrade (Krumhuber et al., 2021). A
more in-depth analysis of raw movement data at the
level of facial landmarks could help overcome these
challenges and provide significant benefits for video-
based AUR (Zinkernagel et al., 2019).
To date, AUR research has remained predomi-
nantly vision-based. Although camera-based AUR
has demonstrated reliable accuracy under controlled
recording conditions, advances in EMG-based meth-
ods for recording facial expressions have yet to be
fully integrated into AUR research (Veldanda et al.,
2024). This is despite a well-established body of
emotion research utilizing facial EMG (fEMG) (Box-
tel, 2001; Wingenbach, 2023; Tassinary et al., 2007)
and the development of robust laboratory guidelines
(Fridlund and Cacioppo, 1986; Tassinary et al., 2007)
and placement schemes for high-resolution EMG
(Guntinas-Lichius et al., 2023).
However, we argue that this state-of-the-art is be-
ginning to change. Some recent work has exam-
ined the use of inertial measurement units (IMUs) for
AUR, yielding promising early results (Verma et al.,
2021). Other work has already integrated EMG elec-
trodes into a VR-compatible device (Gjoreski et al.,
2022). In our work, we have demonstrated encourag-
ing pilot results, showing that EMG can provide re-
liable and real-time-capable data and models to clas-
sify four distinct AUs (Veldanda et al., 2024). In a
similar approach, (Kołodziej et al., 2024) Similarly,
(Kołodziej et al., 2024) used EMG to classify six dis-
crete emotion categories, employing both a support
vector machine (SVM) model and a k-nearest neigh-
bor (KNN) classifier.
1.2 Methodological Challenges and
Opportunities
EMG-based AUR offers a solution to several chal-
lenges that are difficult to overcome with camera-
based AUR alone (Veldanda et al., 2024). On a tech-
nical level, camera-based AUR is influenced by fac-
tors such as the visibility of specific AUs, viewing an-
The Bigger the Better? Towards EMG-Based Single-Trial Action Unit Recognition of Subtle Expressions
101

gles, and the databases used for training and valida-
tion. Cross-database evaluations often rely on posed
datasets, which may not reflect real-world conditions
(Namba et al., 2021a; Namba et al., 2021b; Zhi et al.,
2020).
Spontaneous facial expressions, while of greater
interest to emotion researchers (Krumhuber et al.,
2021), pose additional challenges. Spontaneous ex-
pressions are typically more subtle, dynamic, and
complex, often involving co-occurring AUs (Vel-
danda et al., 2024). However, the greater variabil-
ity inherent in spontaneous expressions makes it diffi-
cult for classifiers to accurately process less standard-
ized data (Krumhuber et al., 2023; Zhi et al., 2020).
On a conceptual level, facial expression research also
deals with issues such as inconsistent emotion mea-
surement and the interpretation of AUs within their
physical and social contexts. In particular, there is of-
ten only poor agreement between physiological mea-
sures and self-reported emotional experiences (Kap-
pas et al., 2013; Mauss and Robinson, 2009).
Advances in multimodal emotion recognition us-
ing machine learning appear promising but have
rarely incorporated high-resolution facial EMG
data, which could improve sensitivity compared to
webcam-based methods (Schuller et al., 2012; Stein-
ert et al., 2021). Here, EMG-based AUR could help to
pave the way for a more robust and fine-grained study
of facial expressions - in particular when studying fa-
cial expressions that are more spontaneous and sub-
tle. However, facial electromyography as a method
has always been limited with respect to the number
of available electrodes as well as concerning the is-
sue of cross-talk (van Boxtel et al., 1998; Tassinary
et al., 2007). That is, when recording from only a
small number of electrode positions, the source of the
signal can be difficult to determine by conventional
statistical measures, as neighboring muscle sites may
produce a very similar, albeit weaker, signal than the
targeted muscle site of interest. A simple and time-
tested approach towards addressing this issue in the
laboratory is to place electrodes on several different
sites, and design experiments in such a way that there
are clear predictions on which muscles should be ac-
tivated - or to include an unobtrusive camera record-
ing to exclude “noise” from unintended muscle acti-
vations. However, this latter approach effectively sac-
rifices much of the potential advantages of otherwise
privacy-preserving EMG by introducing a camera for
artifact checking. Furthermore, a camera-based cor-
rection is again limited to the visible signal, thus again
voiding the inherent advantage of EMG to detect sig-
nals below the visible threshold.
One way to address this challenge is to increase
the number of electrodes used. However, while cam-
era technology has made significant strides in improv-
ing spatial resolution, high-density EMG recordings
remain costly and constrained by the practical limits
of electrode placement on the human face. In their re-
cent effort to establish a high-resolution EMG record-
ing scheme, (Guntinas-Lichius et al., 2023) utilized
small, reusable pediatric surface electrodes with an
Ag/AgCl disc diameter of just 4 mm. This enabled
simultaneous bipolar recordings from 19 muscle po-
sitions to compare two different electrode placement
schemes. However, while such a setup provides ex-
cellent coverage, it is likely to be impractical for most
laboratories, which typically lack the resources for
high-density EMG. Furthermore, the large number
and sheer weight of the electrodes may hinder partic-
ipants’ ability to perform facial expressions naturally.
Therefore, a key goal for advancing EMG-based AUR
is to harness machine learning to disambiguate sig-
nals using only a small number of electrodes. This
would help identify the specific muscles responsible
for a given AU while maintaining signal clarity. At
the same time, we aim to build on the strengths of
EMG to capture even subtle or invisible facial muscle
activity.
1.3 The Present Work
In this paper, we aim to advance recent EMG-based
AUR models to include automatic recognition of sub-
tle facial expressions, which are characterized by a
low intensity of the expression. As EMG has been
the gold standard for the high-precision recording of
facial expressions in the psychophysiological labora-
tory for decades (Fridlund and Cacioppo, 1986; Win-
genbach, 2023), even a relatively small amount of
data may be sufficient to train initial models. Ad-
ditionally, we address the question of whether small
and more lightweight electrodes may be more suitable
for recording and building models for subtle expres-
sions despite their smaller diameters. We, therefore,
aim to examine a custom-built variant of the popu-
lar mobile Biosignal Plux EMG sensor to facilitate
placement of electrodes at the distances that allow a
closer and more accurate placement (Fridlund and Ca-
cioppo, 1986) correspond to the requirements of es-
tablished guidelines. While the vast majority of AUR
research to date has been conducted on video data,
our research aims to leverage EMG to pave the ground
for a growing number of privacy-preserving AUR use
cases.
To examine these questions, we use a newly
recorded dataset of fEMG sensor data to predict a
subset of eight AUs in both high and low expression
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
102

intensity, as well as neutral, yielding a total of 17
distinct classes. The current work thus extends upon
our recent work studying peak expression intensities
of four AUs (Veldanda et al., 2024).
2 METHODOLOGY
To evaluate the performance of the two electrodes,
we propose a framework that utilizes fEMG data syn-
chronized with video recordings of facial expressions
at both high and low intensities from four partici-
pants. We extract time-series features using the Time-
Series Feature Extraction Library (TSFEL) (Baran-
das et al., 2020) to train a set of standard machine-
learning models (RF, SVM, GNB, KNN). The best-
performing model is then selected for further analy-
sis.
2.1 Data Collection
The framework for fEMG dataset collection, includ-
ing the synchronization with concurrent video record-
ings was adapted from the approach used in the study
by Veldanda and colleagues (Veldanda et al., 2024).
In this current study, data were recorded in single tri-
als for each type and intensity of AU. Four partici-
pants (three female, one male) were recruited, with a
mean age of 28.25 years (SD = 2.98). The task in-
volved imitating facial expressions presented as stim-
ulus videos via a customized graphical user interface
(GUI) as in the Figure 1. The stimulus videos were
sourced from the MPI Video Database (Kleiner et al.,
2004), which provides accurate portrayals of AU ac-
tivations.
Figure 1: Graphical User Interface (GUI) for the data col-
lection.
One of our primary objectives was to detect sub-
tle facial expressions. To this end, participants were
instructed to first hold the target facial expressions at
a maximum (high) intensity and then repeat the same
expression at a subtle (low) intensity. Both, the fEMG
signals and corresponding video recordings were cap-
tured for a duration of 5 seconds for each expression
to obtain short data segments featuring the same tar-
get expression and intensity.
The recording setup was adapted from the study
by Veldanda and colleagues (Veldanda et al., 2024),
with a desktop PC to display stimuli, a webcam, and
an fEMG acquisition system. A bipolar recording
configuration was used, comprising three channels
covering upper facial regions (Lateral Frontalis, Cor-
rugator Supercilii, Medial Frontalis) and three addi-
tional channels covering lower facial regions (Zygo-
maticus Major, Levator Labii Superioris, Mentalis).
In total, we considered nine AUs, which additionally
include neutral expressions, as listed in Table 1.
Table 1: Selected actions units for the pilot study.
Action Unit Action
AU1 Inner Brow Raiser
AU2 Outer Brow Raiser
AU4 Brow Lowerer
AU9 Nose Wrinkler
AU12 Lip Corner Puller
AU17 Chin Raiser
AU20 Lip stretcher
AU24 Lip Pressor
AU0 Neutral Expression
Another important objective of this study was to
compare the performance of two types of electrodes
in recognizing the action units. The original EMG
sensors (PLUX Biosignals
1
), with a diameter of 24
mm (in Figure 2a) and a hub, were used as part of
our recording setup. In addition, a modified version
of these sensors with lightweight adapters was em-
ployed, allowing the use of small Ag/AgCl electrodes
with a diameter of only 5 mm (in Figure 2b). The
smaller size allows electrode placement according to
the guidelines of the Society for Psychophysiologi-
cal Research (Fridlund and Cacioppo, 1986), which
recommend maintaining the center-to-center distance
between electrodes within 1 cm. Notably, this config-
uration ensured that both types of electrodes could be
compared with the same settings, software, and am-
plifiers.
During data collection, one type of electrode (big,
small) was placed in the upper region of the face and
the other type on the lower region, respectively, as in
Figure 3. At the end of a session, the electrode po-
sitions were swapped for the next session. To ensure
a balanced design, the sequence of electrode place-
ments was counterbalanced across the four partici-
1
www.pluxbiosignals.com
The Bigger the Better? Towards EMG-Based Single-Trial Action Unit Recognition of Subtle Expressions
103

(a) (b)
Figure 2: Original EMG sensor with 24mm diameter snap-
on EMG electrodes (a), and modified EMG sensor with
5mm diameter Ag/AgCl EMG electrode (b).
Figure 3: Electrode placement.
pants.
The video recordings and EMG data were syn-
chronized using the Lab Streaming Layer (LSL) pro-
tocol. The sampling frequency of the fEMG signals
was set to 2,000 Hz. Facial expression segments from
the EMG signals were extracted based on video times-
tamps, ensuring proper alignment between the modal-
ities.
2.2 Feature Extraction
Traditionally, time-series data are filtered to remove
noise before extracting domain-specific features for
machine learning classification. This process can
be both complex and time-consuming. However,
the Time-Series Feature Extraction Library (TSFEL)
(Barandas et al., 2020) provides a comprehensive, au-
tomated pipeline for efficient feature extraction across
multiple domains, including temporal, statistical, and
spectral feature sets. In our study, we segmented the
raw EMG data into 100 ms windows with a 20% over-
lap and utilized all the feature sets provided by TS-
FEL.
2.3 Classification
We evaluated the performance of both electrode types
for recognizing high and low-intensity AUs using the
following popular machine learning classifiers:
1. Random Forest (RF)
2. Support Vector Machine (SVM)
3. Gaussian Naive Bayes (GNB)
4. k-Nearest Neighbors (KNN)
All models were obtained from the scikit-learn li-
brary (Pedregosa et al., 2011) and employed with their
default hyperparameters. Training was carried out us-
ing the time-series features extracted from the TSEFL
library.
3 RESULTS
3.1 Comparison of Classification
Models
The machine learning models were trained to rec-
ognize all AUs for both types of electrodes. Their
performance was evaluated based on overall ac-
curacy, using 4-fold leave-one-out cross-validation
(LOOCV) approach, in which data from three partic-
ipants formed the training set in each fold. The mean
accuracies are presented in Table 2.
Table 2: Comparison of the performance of machine learn-
ing models.
Model Mean accuracy
RF 0.38
SVM 0.32
GNB 0.36
KNN 0.25
The overall decrease in performance across all
models can be attributed to the short data segments
represented by the small number of trials. Never-
theless, consistent with prior work (Veldanda et al.,
2024), the Random Forest classifier performed best.
As indicated by the confusion matrix of the RF in
Figure 4, patterns of interest do emerge, prompting
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
104
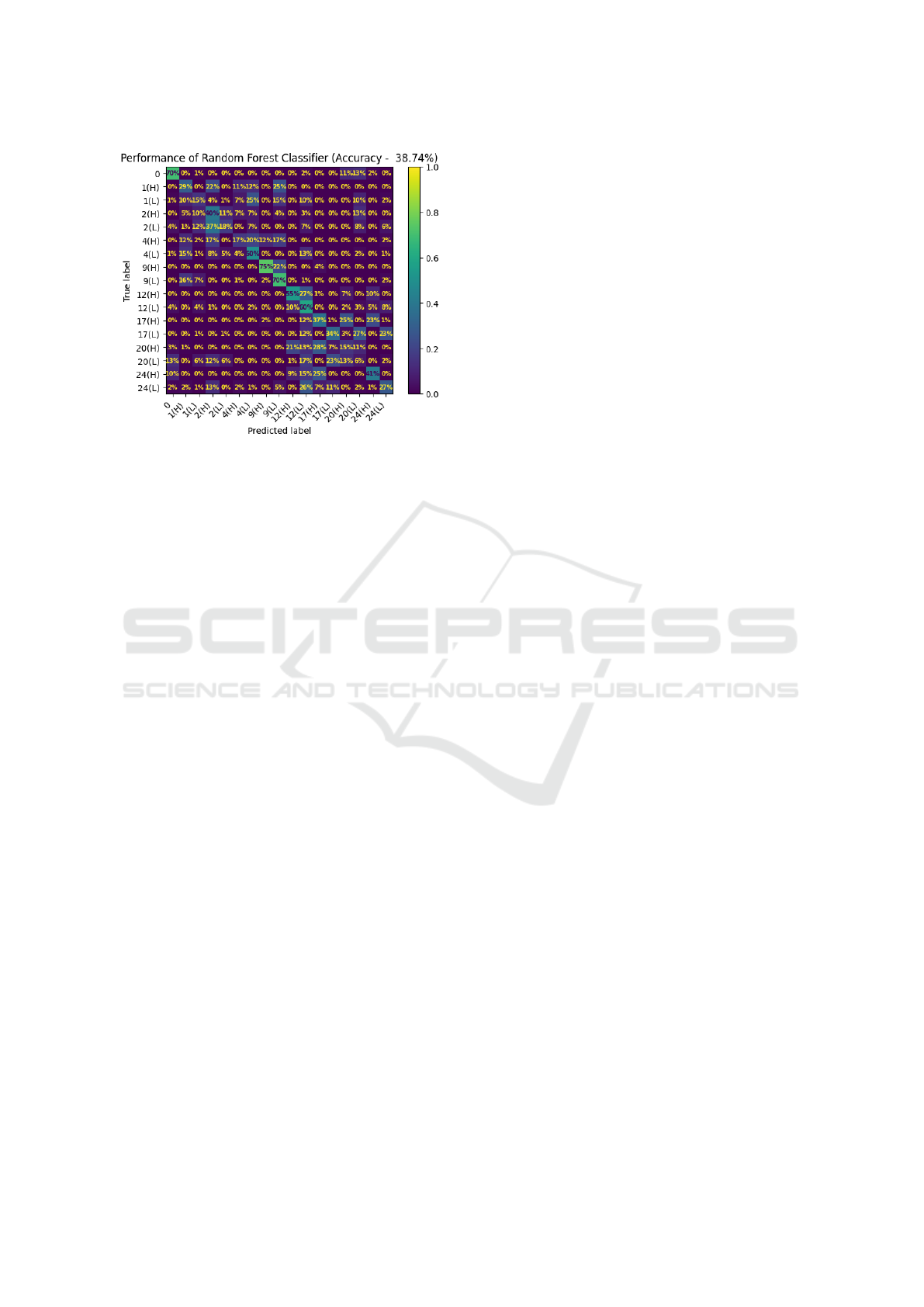
Figure 4: Confusion matrix illustrating classification per-
formance for all action units across both electrode types.
The labels in the confusion matrix indicate action units and
their intensities: H = High, L = Low.
further investigation in subsequent analyses. As ex-
pected, this overall recognition performance is sub-
stantially lower than previous work examining five
classes of AUs (Veldanda et al., 2024). However, the
results are encouraging when considering the vastly
greater number of 17 classes, the inclusion of subtle
expressions, and the much smaller amount of training
data per subject.
3.2 Impact of Electrode Size and
Expression Intensity
To gain more insight into the observed differences, we
performed a cell-wise, two-tailed two-proportion Z-
test on the confusion matrices generated for different
electrode sizes and intensities. Since multiple statisti-
cal comparisons were carried out, we applied a Bon-
ferroni correction to each of the 81 individual com-
parisons to reduce the likelihood of Type I errors.
Figure 5 illustrates the differences in proportions
associated with electrode size and expression inten-
sity. Asterisks indicate significant differences at p <
.0001. As illustrated by the results of the comparison
between big and small electrodes (panel a), models
based on the laboratory-grade 5 mm small electrodes
overall performed dramatically better (76 %) than the
big snap-on electrodes for correctly detecting the ab-
sence of an AU. Here, models run on the data for the
big electrodes more often falsely predicted the pres-
ence of another expression, such as a movement of the
lip stretcher (AU20). When considering the overall
comparison between high- and low-intensity expres-
sions (panel b), a complex pattern of confusions was
observed, in particular with respect to different types
of eyebrow movements. Here, e.g., a low intensity of
lowering the eyebrows (AU4) was significantly bet-
ter recognized than the high-intensity version of the
same AU, whereas the opposite pattern was observed
for raising the outer eyebrow. Considering the coun-
teractive nature of both of these muscles, this pattern
of results appears less surprising. Nevertheless, con-
sidering the complexity of these confusion patterns,
we decided to further split the data by conditions to
examine whether more systematic performance dif-
ferences could be found.
Figure 6 shows the corresponding confusion ma-
trices for the split of the data by electrode size and ex-
pression intensity of the AUs, which can be regarded
as a 2x2 factorial design. Again, an RF classifier was
trained and tested on these four conditions, and the re-
sulting confusion matrices were analyzed using a chi-
square test of independence. Before the analysis, each
confusion matrix was treated as a contingency table,
and any columns with zero totals were removed.
We found a significant performance advantage of
7.4% for using the bigger snap-on electrodes when
classifying high-intensity expressions, (χ
2
(61) =
1453.12, p < .0001), as well as a significant advan-
tage of 5.81% for the smaller electrodes compared
to big electrodes when classifying low-intensity ex-
pressions, (χ
2
(76) = 2691.09, p < .0001). Simulta-
neously, models on the data from big electrodes per-
formed significantly better for high vs. low-intensity
expressions, yielding 8.66% better recognition per-
formance for high-intensity expressions, (χ
2
(67) =
2113.65, p < .0001). Finally, models on small elec-
trodes performed significantly better on low-intensity
expressions than high-intensity expressions, with a
4.55% increment for low-intensity AUs over high-
intensity AUs, (χ
2
(72) = 2364.60, p < .0001). This
pattern of results appears to correspond to a disordi-
nal (crossed) interaction effect, wherein both types of
electrodes showed substantial performance gains for
these two different types of expressions. These re-
sults suggest that small laboratory electrodes may be
more suitable for subtle expressions, whereas the big-
ger snap-on electrodes may be able to more robustly
detect peak intensity expressions.
4 DISCUSSION
The present results suggest that EMG-based AUR
may be suitable for detecting a large number of dif-
ferent AUs - even with relatively little training data
and a default RF baseline model. Notably, how-
ever, electrode positions in the lower and upper face
The Bigger the Better? Towards EMG-Based Single-Trial Action Unit Recognition of Subtle Expressions
105
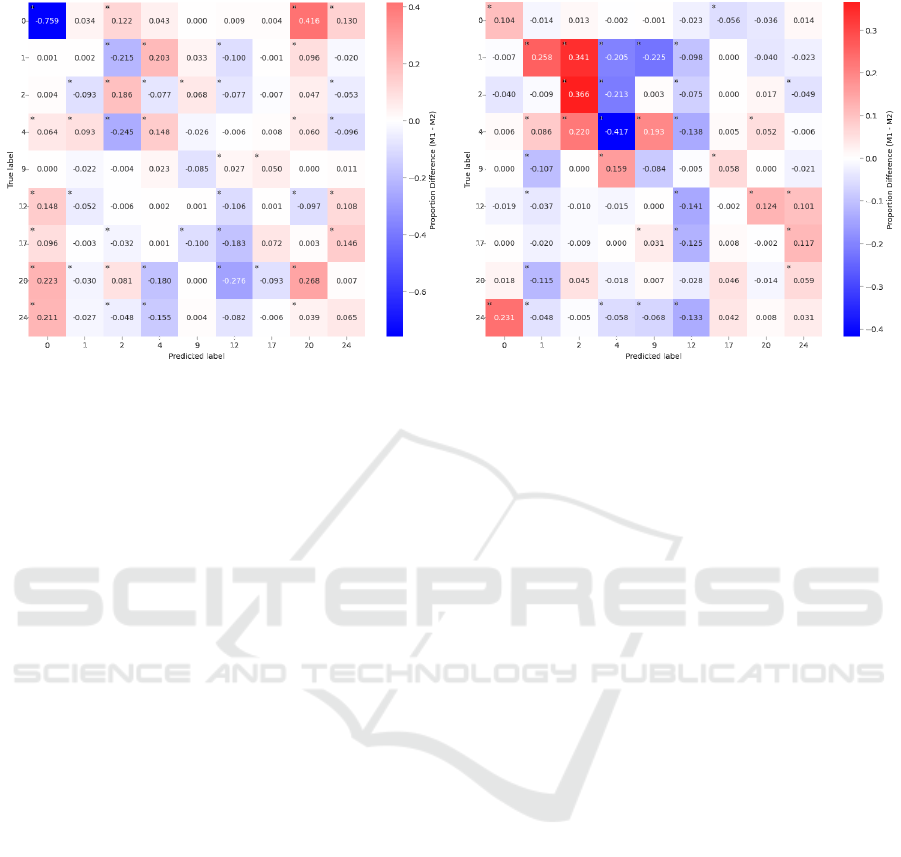
(a) Big vs. small sensors
(b) High vs. low intensity AUs
Figure 5: Comparison of differences in proportions for sensor size (a) and AU intensity (b). Red indicates greater proportions
for big electrodes (a) or high intensity (b). Blue indicates greater proportions for small electrodes (a) or low intensity (b).
showed patterns of confusions suggesting that the
models faced substantial challenges in distinguishing
AUs that are physically close to each other. E.g.,
AU1, AU2, and AU4 all describe different types of
eyebrow-related movements, whereas AU12, AU17,
AU20, and AU24 all involve movements around the
mouth region. Perhaps unsurprisingly, these two clus-
ters of AUs showed a lot of confusions amongst the
respective AUs, as these signals are likely to have
involved substantial amounts of cross-talk. In con-
trast, the nose wrinkler (AU9), which is generally a
relatively difficult AU to produce for laypeople, was
recognized exceptionally well. Here, we speculate
that this may have been the case because AU9 is suf-
ficiently independent from both clusters, while still
close enough to at least two of the electrode pairs to
receive valid signals.
Another key finding of this work is that the two
different electrode types appeared to suit different ex-
pression intensities. Here, the larger recording sur-
face of the original single-use electrodes may be bet-
ter able to differentiate the relative intensity of large
muscle contractions at nearby sites. Conversely, the
smaller electrodes may have allowed subjects to retain
a better “feeling” for very fine-grained intensity dif-
ferences, with less cross-talk - whereas moving mus-
cles underneath the bigger electrodes could have re-
quired more effort and, possibly, more unwanted co-
activation of neighboring muscle sites. This interpre-
tation appears to be supported by the larger number
of erroneous “neutral” labels for low-intensity expres-
sions recorded by the big electrodes.
While the present results are encouraging, some
limitations remain for the current pilot data set. First,
the present study was still based on a very small
number of participants, who performed the minimum
number of expressions to train the present initial ma-
chine learning models. Here, we are presently collect-
ing a more substantial data set with several repetitions
of each of the 17 different AU classes examined in the
present work. We expect that this expanded data set
will provide a basis for better-performing models than
the current baseline. Second, we have not yet con-
ducted a formal statistical test of the apparent inter-
action between electrode type and AUR performance
for low vs. high-intensity expressions. Here, we had
expected a more clear-cut decision for one or the other
type of electrodes, and we regard the apparent interac-
tion between both factors as an exploratory finding at
this stage. In our future work with a larger dataset, we
plan to submit this hypothesis to a robust generalized
linear mixed model test, with the subject as a random
factor. Third, several different approaches could still
be attempted to improve and further analyze the cur-
rent model results. However, the RF classification has
consistently emerged as the best model already in our
previous work, and this study has only aimed to pro-
vide initial results for a proof of concept for EMG-
based AUR for low-intensity expressions as well as
the comparison of small laboratory and big snap-on
electrodes with the same base system.
5 CONCLUSION
The present results are consistent with the notion that
surface EMG is capable of detecting even very sub-
tle muscle activity for EMG-based AUR - and that
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
106
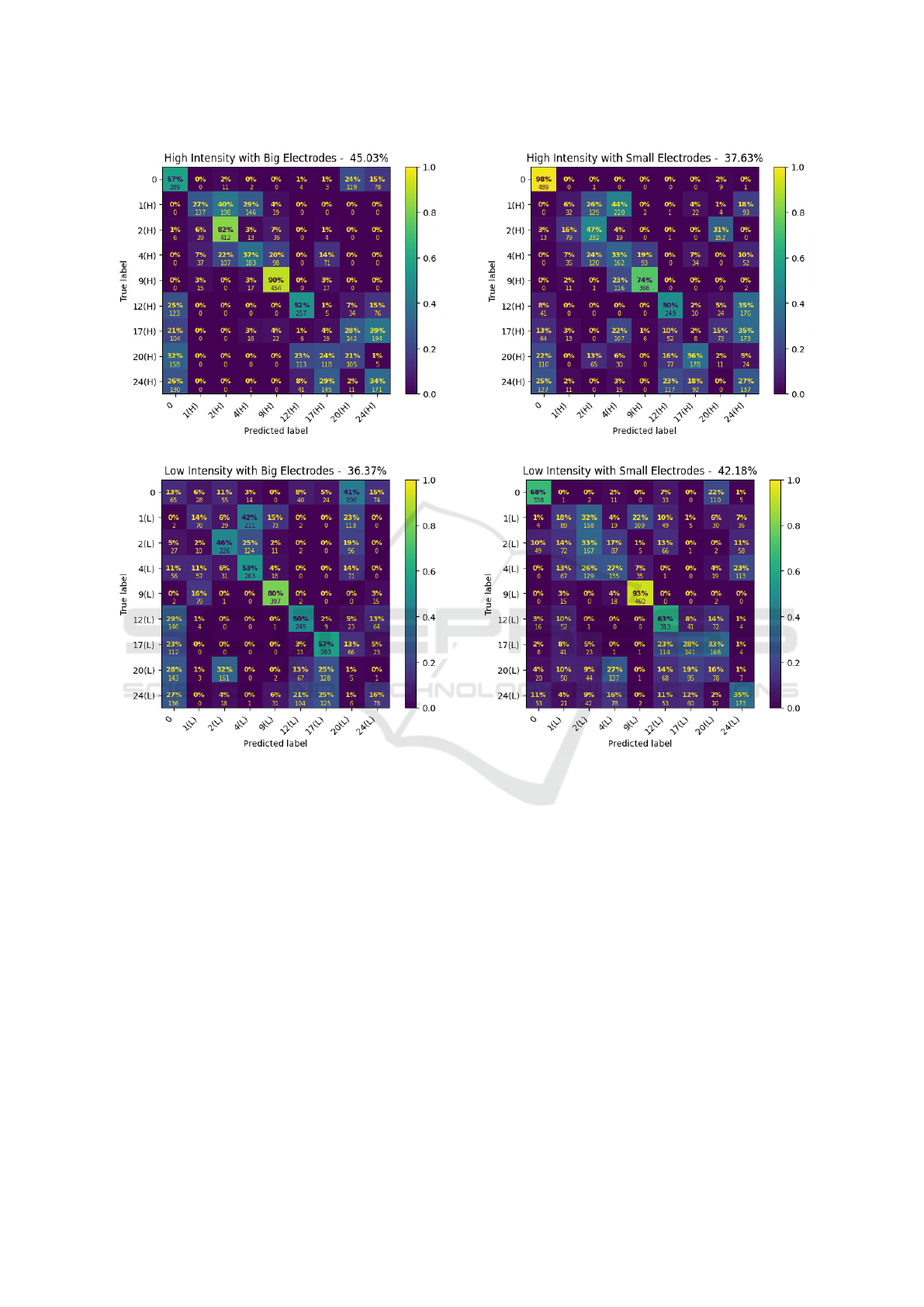
Figure 6: Confusion matrices under the four conditions.
with little relative degradation in performance com-
pared to peak intensity expressions. To the best of
our knowledge, this is the first study to have success-
fully detected low-intensity expressions via EMG-
based AUR. Intriguingly, different types of electrodes
may be more suitable for different use cases - even if
they are attached to the same base amplifiers. This
is consistent with findings from previous studies that
have demonstrated that the control of human facial
muscles is a complex process (Cattaneo and Pavesi,
2014), which is influenced by substantial anatomical
variations (D’Andrea and Barbaix, 2006) as well as
differences in signal strength across muscle regions
(Schultz et al., 2019).
In future work, we aim to extend the current eval-
uation with further electrode types, while also varying
the targeted electrode placement. Notably, the tradi-
tional placement guidelines (Fridlund and Cacioppo,
1986) were designed almost 40 years ago, with the
purpose of better comparability of studies for sta-
tistical analyses across laboratories. When consid-
ering the relatively recent advent of advanced ma-
chine learning methods and current work involving
high-resolution facial EMG (Guntinas-Lichius et al.,
2023), this raises the question if there could be a more
fine-grained adaptation of effective electrode place-
ments for individual subjects. Indeed, while we had
expected to see a more clearcut advantage of the more
accurately placed smaller electrodes, our present re-
sults suggest that the optimal electrode type- and
placement for the training EMG-based AUR systems
may differ from the original guidelines that aimed
to optimize comparability of mean activity between
muscle recording sites. Considering the limited sam-
ple size of the present study, there is a clear need
for further validation with a larger sample size and
The Bigger the Better? Towards EMG-Based Single-Trial Action Unit Recognition of Subtle Expressions
107

a substantially greater number of trials for each AU.
This would allow conducting more robust hypothesis-
guided statistical tests, in particular with regard to the
present exploratory finding of an apparent interaction
between sensor size and expression intensity on AUR
performance.
Recording schemes for training machine learning
models might benefit more from signals that are cor-
related with a particular AU, while simultaneously be-
ing as distinctive as possible from signals from other
AUs. That is, instead of maximizing the mean sig-
nal strength at a recording site, EMG-based AUR
may benefit from a somewhat more distal and indi-
vidualized electrode placement. Here, another po-
tential application on the horizon for real-time EMG-
based AUR systems could be the development of au-
tomated placement guidance for subject-tailored op-
timal placement of recording electrodes. Finally, a
more distal electrode placement would likewise be
a requirement for the development of EMG-based
AUR devices, e.g., for applications in VR, since cur-
rent prototypes with inbuilt electrodes (Gjoreski et al.,
2022) may still be too expensive and unwieldy for the
majority of potential applications. Depending on the
use case, the performance of AUR under laboratory
conditions could just be a starting point. For instance,
Ag/AgCl electrodes may oxidize over time, prompt-
ing considerations about whether electrodes in end-
user devices should be cleaned or replaced. Together,
these findings call for more research into EMG-based
AUR, with the ultimate aim of building biosignals
adaptive cognitive systems (Schultz and Maedche,
2023) that are designed to provide privacy-preserving
AUR-capabilities across a broad range of fields for
applications, from the diagnosis of Parkinson’s dis-
ease to immersive avatar-mediated communication in
VR.
ACKNOWLEDGEMENTS
This research work was funded by a grant of the
Minds, Media, Machines High Profile Research Area
at the University of Bremen (MMM-Seed Grant
No.005). We furthermore gratefully acknowledge the
contributions of our students, Romina Razeghi Osk-
ouei and Ferdinand Rohlfing in conducting the study.
REFERENCES
Baltrusaitis, T., Zadeh, A., Lim, Y. C., and Morency, L.-
P. (2018). OpenFace 2.0: Facial Behavior Analysis
Toolkit. In 2018 13th IEEE International Conference
on Automatic Face & Gesture Recognition (FG 2018),
pages 59–66, Xi’an. IEEE.
Barandas, M., Folgado, D., Fernandes, L., Santos, S.,
Abreu, M., Bota, P., Liu, H., Schultz, T., and Gamboa,
H. (2020). TSFEL: Time Series Feature Extraction
Library. SoftwareX, 11:100456.
Barrett, L. F., Adolphs, R., Marsella, S., Martinez, A. M.,
and Pollak, S. D. (2019). Emotional Expressions Re-
considered: Challenges to Inferring Emotion From
Human Facial Movements. Psychological Science in
the Public Interest: A Journal of the American Psy-
chological Society, 20(1):1–68.
Bartlett, M. S., Littlewort, G. C., Frank, M. G., Lainscsek,
C., Fasel, I. R., and Movellan, J. R. (2006). Auto-
matic Recognition of Facial Actions in Spontaneous
Expressions. Journal of Multimedia, 1(6):22–35.
Boxtel, A. (2001). Optimal signal bandwidth for the record-
ing of surface EMG activity of facial, jaw, oral, and
neck muscles. Psychophysiology, 38(1):22–34.
Cattaneo, L. and Pavesi, G. (2014). The facial motor sys-
tem. Neuroscience & Biobehavioral Reviews, 38:135–
159.
Chang, D., Yin, Y., Li, Z., Tran, M., and Soleymani, M.
(2024). LibreFace: An Open-Source Toolkit for Deep
Facial Expression Analysis. pages 8205–8215.
Chen, X. and Chen, H. (2023). Emotion recognition us-
ing facial expressions in an immersive virtual reality
application. Virtual Reality, 27(3):1717–1732.
Crivelli, C. and Fridlund, A. J. (2018). Facial Displays Are
Tools for Social Influence. Trends in Cognitive Sci-
ences, 22(5):388–399. Publisher: Elsevier.
Crivelli, C. and Fridlund, A. J. (2019). Inside-Out: From
Basic Emotions Theory to the Behavioral Ecology
View. Journal of Nonverbal Behavior, 43(2):161–194.
Darwin, C. (1872). The expression of the emotions in man
and animals. Oxford University Press, New York,
1998 ed. edition.
Duchenne, G.-B. and Cuthbertson, R. A. (1990). The mech-
anism of human facial expression. Studies in emotion
and social interaction. Cambridge University Press
; Editions de la Maison des Sciences de l’Homme,
Cambridge [England] ; New York : Paris.
D’Andrea, E. and Barbaix, E. (2006). Anatomic research
on the perioral muscles, functional matrix of the max-
illary and mandibular bones. Surgical and Radiologic
Anatomy, 28(3):261–266.
Ekman, P. (1999). Basic emotions. In Handbook of cog-
nition and emotion, pages 45–60. John Wiley & Sons
Ltd, Hoboken, NJ, US.
Ekman, P., Friesen, W. V., and Hager, J. C. (2002). Facial
action coding system: the manual. Research Nexus,
Salt Lake City, Utah.
Fridlund, A. J. and Cacioppo, J. T. (1986). Guidelines for
Human Electromyographic Research. Psychophysiol-
ogy, 23(5):567–589.
Giovanelli, E., Valzolgher, C., Gessa, E., Todes-
chini, M., and Pavani, F. (2021). Unmasking
the difficulty of listening to talkers with masks:
lessons from the covid-19 pandemic. i-Perception,
12(2):2041669521998393.
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
108

Gjoreski, M., Kiprijanovska, I., Stankoski, S., Mavridou,
I., Broulidakis, M. J., Gjoreski, H., and Nduka, C.
(2022). Facial EMG sensing for monitoring af-
fect using a wearable device. Scientific Reports,
12(1):16876. Publisher: Nature Publishing Group.
Grahlow, M., Rupp, C. I., and Derntl, B. (2022). The impact
of face masks on emotion recognition performance
and perception of threat. 17(2):e0262840. Publisher:
Public Library of Science.
Guntinas-Lichius, O., Trentzsch, V., Mueller, N., Hein-
rich, M., Kuttenreich, A.-M., Dobel, C., Volk, G. F.,
Graßme, R., and Anders, C. (2023). High-resolution
surface electromyographic activities of facial muscles
during the six basic emotional expressions in healthy
adults: a prospective observational study. Scientific
Reports, 13(1):19214. Publisher: Nature Publishing
Group.
Hjortsj
¨
o, C.-H. (1969). Man’s Face and Mimic Language.
Studentlitteratur, Lund, Sweden.
Jin, B., Qu, Y., Zhang, L., and Gao, Z. (2020). Diagnosing
parkinson disease through facial expression recogni-
tion: Video analysis. 22(7):e18697. Company: Jour-
nal of Medical Internet Research Distributor: Journal
of Medical Internet Research Institution: Journal of
Medical Internet Research Label: Journal of Medical
Internet Research Publisher: JMIR Publications Inc.,
Toronto, Canada.
Kappas, A., Krumhuber, E., and K
¨
uster, D. (2013). Facial
behavior, pages 131–165.
Kastendieck, T., Zillmer, S., and Hess, U. (2022). (un)mask
yourself! effects of face masks on facial mimicry
and emotion perception during the COVID-19 pan-
demic. 36(1):59–69. Publisher: Routledge eprint:
https://doi.org/10.1080/02699931.2021.1950639.
Kleiner, M., Wallraven, C., Breidt, M., Cunningham, D. W.,
and B
¨
ulthoff, H. H. (2004). Multi-viewpoint video
capture for facial perception research. In Workshop on
Modelling and Motion Capture Techniques for Virtual
Environments (CAPTECH 2004), Geneva, Switzer-
land.
Kołodziej, M., Majkowski, A., and Jurczak, M. (2024).
Acquisition and Analysis of Facial Electromyo-
graphic Signals for Emotion Recognition. Sensors,
24(15):4785. Number: 15 Publisher: Multidisci-
plinary Digital Publishing Institute.
Krumhuber, E. G., K
¨
uster, D., Namba, S., and Skora,
L. (2021). Human and machine validation of 14
databases of dynamic facial expressions. Behavior Re-
search Methods, 53(2):686–701.
Krumhuber, E. G., Skora, L. I., Hill, H. C. H., and Lander,
K. (2023). The role of facial movements in emotion
recognition. Nature Reviews Psychology, 2(5):283–
296.
K
¨
uster, D., Krumhuber, E. G., Steinert, L., Ahuja, A.,
Baker, M., and Schultz, T. (2020). Opportunities and
challenges for using automatic human affect analy-
sis in consumer research. Frontiers in neuroscience,
14:400.
Littlewort, G., Whitehill, J., Wu, T., Fasel, I., Frank, M.,
Movellan, J., and Bartlett, M. (2011). The computer
expression recognition toolbox (CERT). In 2011 IEEE
International Conference on Automatic Face & Ges-
ture Recognition (FG), pages 298–305.
Mattavelli, G., Barvas, E., Longo, C., Zappini, F., Ottaviani,
D., Malaguti, M. C., Pellegrini, M., and Papagno, C.
(2021). Facial expressions recognition and discrimi-
nation in parkinson’s disease. 15(1):46–68.
Mauss, I. B. and Robinson, M. D. (2009). Measures of emo-
tion: A review. Cognition & Emotion, 23(2):209–237.
Namba, S., Sato, W., Osumi, M., and Shimokawa, K.
(2021a). Assessing Automated Facial Action Unit De-
tection Systems for Analyzing Cross-Domain Facial
Expression Databases. Sensors, 21(12):4222.
Namba, S., Sato, W., and Yoshikawa, S. (2021b). Viewpoint
Robustness of Automated Facial Action Unit Detec-
tion Systems. Applied Sciences, 11(23):11171.
Oh Kruzic, C., Kruzic, D., Herrera, F., and Bailenson, J.
(2020). Facial expressions contribute more than body
movements to conversational outcomes in avatar-
mediated virtual environments. Scientific Reports,
10(1):20626.
Ortony, A. (2022). Are All “Basic Emotions” Emotions? A
Problem for the (Basic) Emotions Construct. Perspec-
tives on Psychological Science, 17(1):41–61.
Pedregosa, F., Varoquaux, G., Gramfort, A., Michel, V.,
Thirion, B., Grisel, O., Blondel, M., Prettenhofer,
P., Weiss, R., Dubourg, V., Vanderplas, J., Passos,
A., Cournapeau, D., Brucher, M., Perrot, M., and
Duchesnay, E. (2011). Scikit-learn: Machine learning
in Python. Journal of Machine Learning Research,
12:2825–2830.
Schuetz, I. and Fiehler, K. (2022). Eye tracking in virtual
reality: Vive pro eye spatial accuracy, precision, and
calibration reliability. Journal of Eye Movement Re-
search, 15(3). Number: 3.
Schuller, B., Valster, M., Eyben, F., Cowie, R., and Pantic,
M. (2012). AVEC 2012: the continuous audio/visual
emotion challenge. In Proceedings of the 14th ACM
international conference on Multimodal interaction,
ICMI ’12, pages 449–456, New York, NY, USA. As-
sociation for Computing Machinery.
Schultz, T., Angrick, M., Diener, L., K
¨
uster, D., Meier,
M., Krusienski, D. J., Herff, C., and Brumberg, J. S.
(2019). Towards restoration of articulatory move-
ments: Functional electrical stimulation of orofacial
muscles. In 2019 41st Annual International Confer-
ence of the IEEE Engineering in Medicine and Biol-
ogy Society (EMBC), pages 3111–3114.
Schultz, T. and Maedche, A. (2023). Biosignals meet Adap-
tive Systems. SN Applied Sciences, 5(9):234.
Sonawane, B. and Sharma, P. (2021). Review of automated
emotion-based quantification of facial expression in
parkinson’s patients. 37(5):1151–1167.
Steinert, L., Putze, F., K
¨
uster, D., and Schultz, T. (2021).
Audio-visual recognition of emotional engagement of
people with dementia. In Interspeech, pages 1024–
1028.
Tassinary, L. G., Cacioppo, J. T., and Vanman, E. J. (2007).
The Skeletomotor System: Surface Electromyogra-
phy. In Cacioppo, J. T., Tassinary, L. G., and Berntson,
The Bigger the Better? Towards EMG-Based Single-Trial Action Unit Recognition of Subtle Expressions
109

G., editors, Handbook of Psychophysiology, pages
267–300. Cambridge University Press, Cambridge, 3
edition.
van Boxtel, A., Boelhouwer, A., and Bos, A. (1998).
Optimal EMG signal bandwidth and interelec-
trode distance for the recording of acoustic,
electrocutaneous, and photic blink reflexes.
Psychophysiology, 35(6):690–697. eprint:
https://onlinelibrary.wiley.com/doi/pdf/10.1111/1469-
8986.3560690.
van der Struijk, S., Huang, H.-H., Mirzaei, M. S., and
Nishida, T. (2018). FACSvatar: An Open Source
Modular Framework for Real-Time FACS based Fa-
cial Animation. In Proceedings of the 18th Interna-
tional Conference on Intelligent Virtual Agents, IVA
’18, pages 159–164, New York, NY, USA. Associa-
tion for Computing Machinery.
Veldanda, A., Liu, H., Koschke, R., Schultz, T., and K
¨
uster,
D. (2024). Can electromyography alone reveal facial
action units? a pilot emg-based action unit recog-
nition study with real-time validation:. In Proceed-
ings of the 17th International Joint Conference on
Biomedical Engineering Systems and Technologies,
page 142–151, Rome, Italy. SCITEPRESS - Science
and Technology Publications.
Verma, D., Bhalla, S., Sahnan, D., Shukla, J., and Parnami,
A. (2021). ExpressEar: Sensing Fine-Grained Fa-
cial Expressions with Earables. Proceedings of the
ACM on Interactive, Mobile, Wearable and Ubiqui-
tous Technologies, 5(3):1–28.
Wen, L., Zhou, J., Huang, W., and Chen, F. (2022). A
Survey of Facial Capture for Virtual Reality. IEEE
Access, 10:6042–6052. Conference Name: IEEE Ac-
cess.
Wingenbach, T. S. H. (2023). Facial EMG – Investigating
the Interplay of Facial Muscles and Emotions. In Bog-
gio, P. S., Wingenbach, T. S. H., da Silveira Co
ˆ
elho,
M. L., Comfort, W. E., Murrins Marques, L., and
Alves, M. V. C., editors, Social and Affective Neuro-
science of Everyday Human Interaction: From The-
ory to Methodology, pages 283–300. Springer Inter-
national Publishing, Cham.
Zhi, R., Liu, M., and Zhang, D. (2020). A comprehensive
survey on automatic facial action unit analysis. The
Visual Computer, 36(5):1067–1093.
Zinkernagel, A., Alexandrowicz, R. W., Lischetzke, T., and
Schmitt, M. (2019). The blenderFace method: video-
based measurement of raw movement data during fa-
cial expressions of emotion using open-source soft-
ware. Behavior Research Methods, 51(2):747–768.
BIODEVICES 2025 - 18th International Conference on Biomedical Electronics and Devices
110
